Stephanopis nana Machado, 2019
|
publication ID |
https://doi.org/10.3853/j.2201-4349.71.2019.1698 |
|
publication LSID |
lsid:zoobank.org:pub:7EDBAB7F-0E3B-47D7-AA29-0906728ADA05 |
|
persistent identifier |
https://treatment.plazi.org/id/462BB904-EA42-4B30-8908-02A3A250B823 |
|
taxon LSID |
lsid:zoobank.org:act:462BB904-EA42-4B30-8908-02A3A250B823 |
|
treatment provided by |
Felipe |
|
scientific name |
Stephanopis nana Machado |
| status |
sp. nov. |
Stephanopis nana Machado View in CoL sp. nov.
http: //zoobank.org/NomenclaturalActs/ 462BB904-EA42-4B30-8908-02A3A250B823
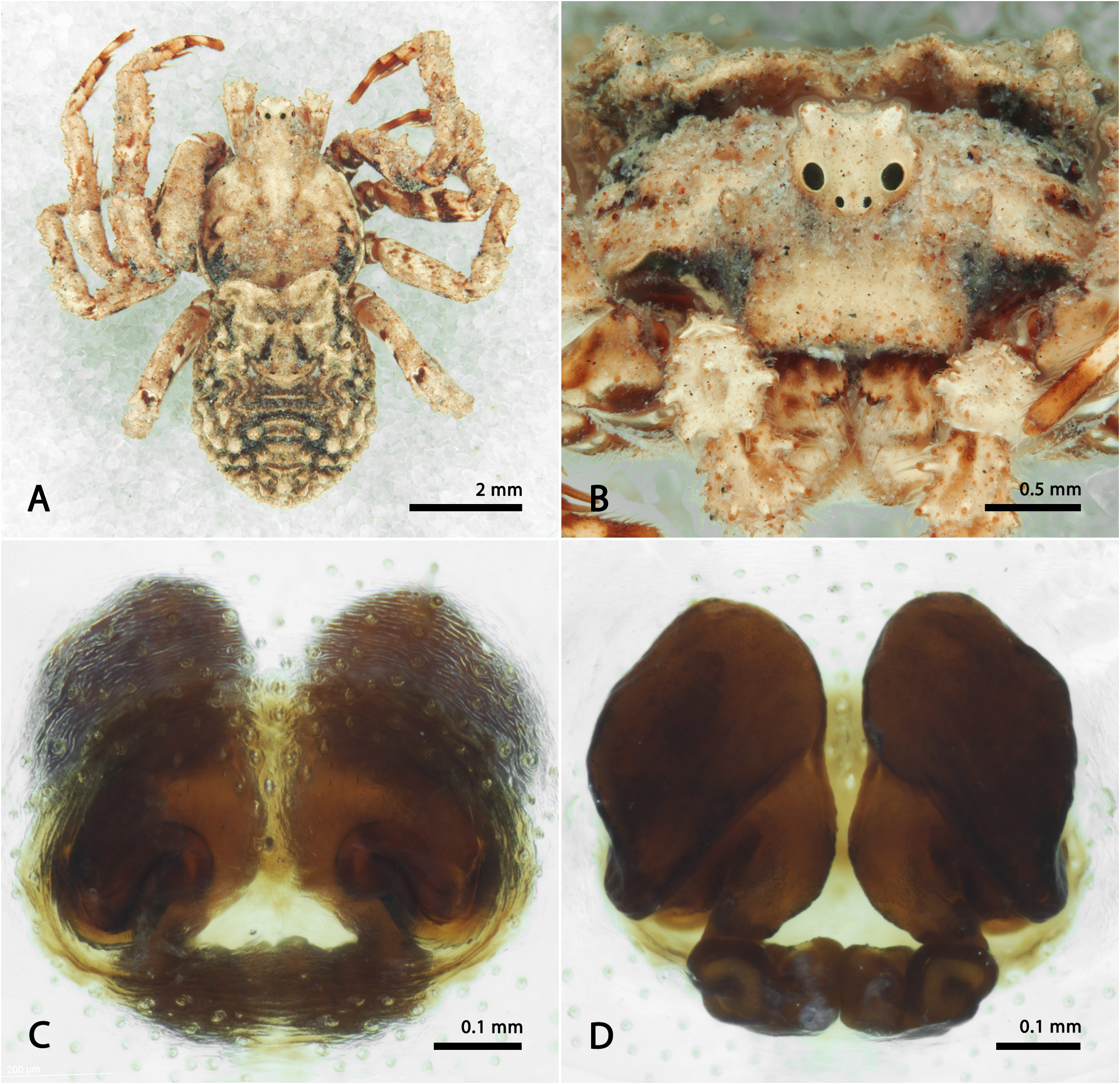
Fig. 36 View Figure 36
Holotype ♀, QM S14589 View Materials , Chinchilla , Queensland, Australia, 26°44'19.28"S 150°37'15.01"E, T. Adams, 19 February 1979 GoogleMaps . Paratypes: QM S80820, 1♀, Moolayember Creek NP, Queensland, Australia, 25°14.5'S 148°37.4'E, C. Burwell, 10 March 2006 GoogleMaps ; QM S80247 View Materials , 1♀, Little Wolfang Peak , Queensland, Australia, 22°33'S 147°49'43.32"E, Queensland Museum party, 5 March 2006 GoogleMaps .
Diagnosis. The females of S. nana sp. nov. are similar to those of S. altifrons and S. nigra , possessing a high clypeus, eyes located on an elevated ocular projection ( Fig. 36B View Figure 36 ) and opisthosoma with stout posterior setiferous tubercles ( Fig. 36A View Figure 36 ). However, S. nana sp. nov. is easily recognizable by its considerably smaller body size and position of the CO: females of S. nigra have CO disposed horizontally while in S. altifrons they are oblique but delimiting a “V-shape” on the atrium. In S. nana sp. nov. the anterior folds of the openings are directed towards each other in the middle of the plate ( Fig. 36C View Figure 36 ).
Description. Female (QM S14589 View Materials ): Anterior eye row strongly recurved and posterior procurve; prosoma and legs
predominantly pale-yellow with few brown or dark-brown spots ( Fig. 36A View Figure 36 ); opisthosoma concave at the anterior border, rugose, rounded, predominantly pale-yellow with dark-brown patches randomly distributed. Spermathecae small, coiled and preceded by large “lung-shaped” chambers ( Fig. 36D View Figure 36 ).
Measurements: eye diameters and interdistances: AME 0.05, ALE 0.15, PME 0.12, PLE 0.10, AME–AME 0.10, AME–ALE 0.09, PME–PME 0.17, PME–PLE 0.10, MOQ length 0.48, width 0.32; leg formula: 1-2-3-4: leg I—femur 2.97/ patella 1.75/ tibia 2.28/ metatarsus 1.70/ tarsus 0.90/ total 9.60; II—2.35/ 1.31/ 1.79/ 1.47/ 0.88/ 7.80; III—1.90/ 1.00/ 1.54/ 1.45/ 0.81/ 6.70; IV—1.92/ 0.89/ 1.39/ 1.40/ 0.73/ 6.33. Total body length 8.02; prosoma length 3.67, width 3.05; opisthosoma length 4.35; clypeus height 0.81; sternum length 1.56, width 1.38; gnathocoxae length 0.81, width 0.37; labium length 0.44, width 0.60.
Male: Unknown.
Etymology. The specific name is an adjective derived from the Ancient Greek that means “dwarf” and refers to the reduced size of these spiders, especially when compared to its similar congeneric species S. altifrons , S. carcinoides sp. nov. and S. nigra .
Distribution. Queensland, Australia ( Fig. 42 View Figure 42 ).
| QM |
Queensland Museum |
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |