Stylocellidae Hansen & Sørensen, 1904
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3595.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:0E34F9DE-B76C-4197-94D0-5A08A1F7C534 |
|
DOI |
https://doi.org/10.5281/zenodo.5622714 |
|
persistent identifier |
https://treatment.plazi.org/id/296DA64C-7064-997C-DEAA-FC68A89FF904 |
|
treatment provided by |
Plazi |
|
scientific name |
Stylocellidae Hansen & Sørensen, 1904 |
| status |
|
Stylocellidae Hansen & Sørensen, 1904 View in CoL
Comments: Several morphological features in the family are distributed unevenly among the lineages, resulting in definitions for the genera that rest largely on statistical statements ( Table 1 View TABLE 1 ). A case may be made for a particular specimen as belonging to one genus or another based on the probability that a member of that genus would present a certain combination of characters. For example, having an anal gland pore could place a species in any genus (when considering undescribed species in Leptopsalis and Meghalaya with anal gland pores), but if Rambla’s organ is lacking, the ventral prosomal complex is large and flat, eyes are present, the fourth coxae tapered anteriorally, and the anterior sternal opisthosomal sulci are distinct and parallel, then it is most likely in Miopsalis and cannot be in Fangensis, Giribetia , Stylocellus , or Meghalaya. Known cheliceral characters clearly follow this distributional bias, states for any one aspect never being exclusive to one genus. My initial doubt over the role of the second ventral process is now replaced by a more complex picture: although a distinct second ventral process and a reduced first one is a synapomorphy of the family, a large first process and reduced second is more derived in Stylocellidae , with a number of exceptions. That is, although the ancestral stylocellid had the unique development of a second cheliceral process, the family appears to have generally reverted back to a condition common in the rest of Cyphophthalmi . A reduced first process may be a byproduct of troglomorphism, since mostly the troglobitic Miopsalis—M. globosa ( Schwendinger & Giribet, 2004) new comb., M. gryllospeca ( Shear, 1993) , and M. silhavyi ( Rambla, 1991) —have reduced first processes like troglobitic Fangensis ; but so do Giribetia gen. nov. and Stylocellus , which live in leaf litter, as well as the small, non-troglomorphic Miopsalis leakeyi ( Shear, 1993) new comb. Extensive granulation on the second article of the chelicera is mostly found in the early lineages, and having a small smooth ridge along the laterodistal area may be exclusive to Fangensinae subfam. nov. and Stylocellinae (not just Stylocellus , as I have seen it in one species of Meghalaya). Several species of Miopsalis also have the same heavy granulations down the second article, a feature that often comes with the other plesiomorphic traits, like anal gland pores, a large ventral prosomal complex, Rambla’s organ, a reduced first ventral cheliceral process, and claw-like chelicerae. About the latter, I have simply defined it as having a ratio of the third-to-second cheliceral article of 0.3 or greater, although species in Stylocellus , Meghalaya, and Leptopsalis with this ratio look far less claw-like than those in Fangensis and Giribetia gen. nov. This is because the latter have the second article’s widest point past halfway toward the distal end, and a curved distal end of the whole chelicera. However, keeping the definition just as the article ratio is clearer and sufficiently makes the point that claw-like chelicerae are more frequent in the early lineages, which also completely lack attenuate ones.
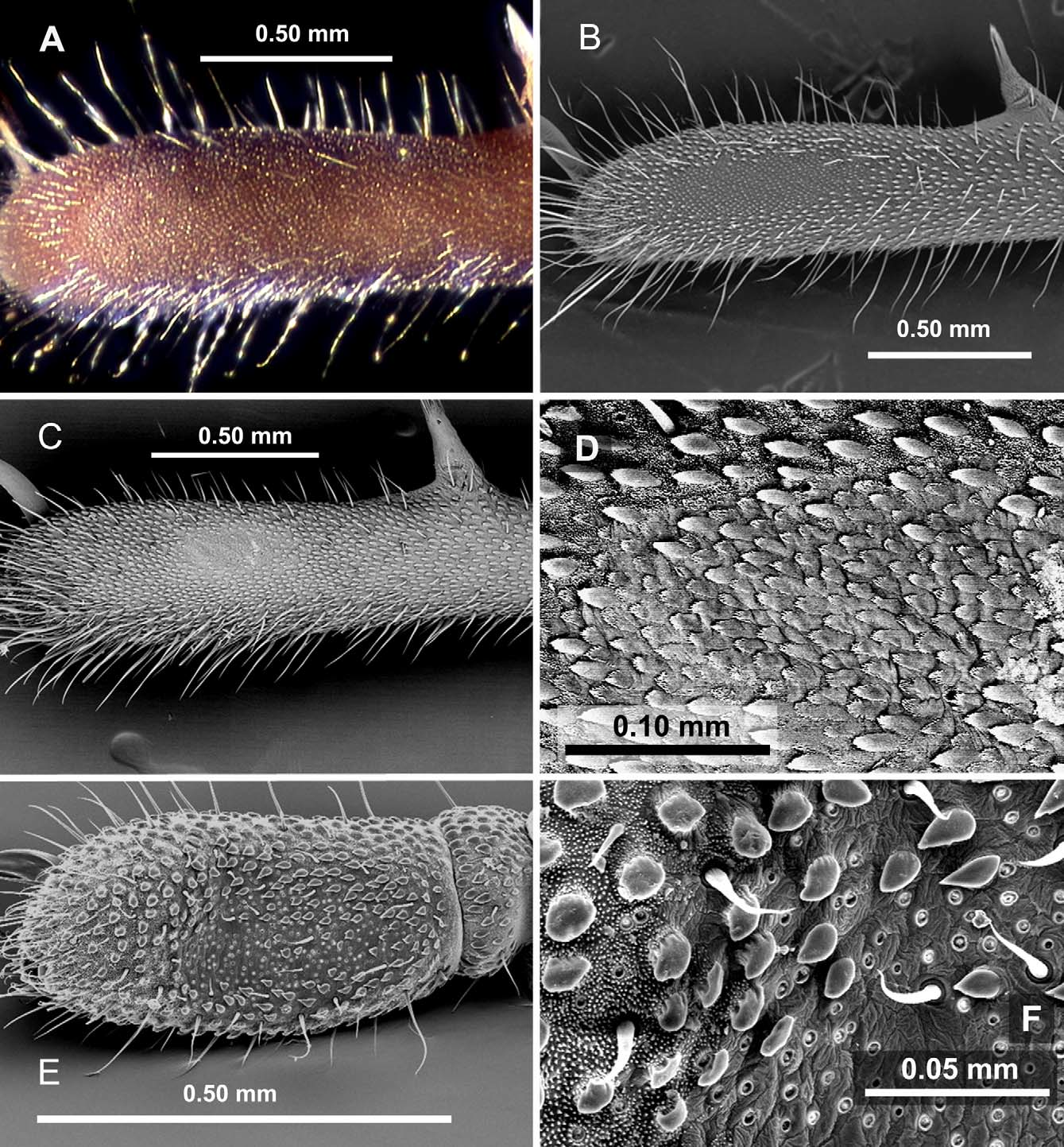
Rambla’s organ ( Fig. 3 View FIGURE 3 ) is also found in more than one lineage, although it is more restricted than anal gland pores, and its different states appear to be exclusive to each lineage. Appearing as a small, raised patch in Fangensis ( Schwendinger & Giribet 2005) , it is enormous in Giribetia gen. nov. and Meghalaya ( Fig. 3 View FIGURE 3 , E–F), although in the former it is more clearly demarcated, the larger tuberculate-granules and microgranules stopping more abruptly at its proximal border and large tuberculate-granules extending for only a short distance past its distal border. In Stylocellus Rambla’s organ is barely visible (keeping in mind that we can only examine it in one species, as the type of S. sumatranus is missing its fourth legs), but under a scanning electron microscope what can be seen as a shiny patch under a light microscope is recognized as an area where microgranules are missing and larger granules are reduced. In Miopsalis , where the first observation of this organ was made by Rambla in her description of M. silhavyi , the area is well-demarcated and small, not raised or depressed, and the microgranules, larger tuberculate-granules, and general cuticle combine to form a regular series of triangular projections that resemble scales ( Fig. 3 View FIGURE 3 , A–D). Although not noted in its original description ( Shear 1993), this form of Rambla’s organ can also be seen in the type of M. gryllospeca ( Fig. 3 View FIGURE 3 , A), and I have photographed it in three other large, cave-dwelling Miopsalis . These species differ in size and even the presence of anal gland pores, but their Rambla’s organ retains the same general appearance. Rambla’s organ is completely missing so far in Leptopsalis , even among those with anal gland pores.
Subfamiles included: Stylocellinae Hansen & Sørensen, 1904, Fangensinae subfam. nov., Leptopsalinae subfam. nov.
Distribution: Southeast Asia, from northeastern India to western New Guinea, including the Thai-Malay Peninsula, the Indo-Malay Archipelago, and the Philippine islands of Palawan and Mindanao.
TABLE 1. Current species of Stylocellidae and states for certain morphological features. Locality codes are as follows: BO = Borneo, CP = Central Thai-Malay Peninsula, EH = Eastern Himalayas, JA = Java, NG = New Guinea, NP = Northern Thai-Malay Peninsula, PI = Philippines, SP = Southern Thai-Malay Peninsula, SU = Sumatra, SW = Sulawesi. Cheliceral proportions are shown as the ratio of third article (mobile digit) to the second article, followed by “ C ” (“ clawlike ”) if the ratio is equal to or greater than 3.0, and “ A ” (“ attenuate ”) if less than or equal to 2.0.
| Species | Locality | Anal gland pore | Rambla's organ | First cheliceral process | Second cheliceral process | Cheliceral proportions |
|---|---|---|---|---|---|---|
| Fangensinae subfam. nov. | ||||||
| Fangensis cavernarus Schwendinger & Giribet, 2005 | NP | yes | small, distinct, raised | reduced | distinct | 0.34 (C) |
| Fangensis leclerci Rambla, 1994 | NP | yes | small, distinct, raised | reduced | distinct | 0.37 (C) |
| Fangensis spelaeus Schwendinger & Giribet, 2005 | NP | yes | small, distinct, raised | reduced | distinct | 0.35 (C) |
| Giribetia insulana ( Schwendinger & Giribet, 2005) | CP | yes | large, distinct, sunken | reduced | distinct | 0.39 (C) |
| Stylocellinae | ||||||
| Stylocellus lornei , sp. nov. | CP | yes | large, diffuse, flat | reduced | distinct | 0.30 (C) |
| Stylocellus sumatranus Westwood, 1874 | SU | yes | – | ? | ? | 0.34 (C) |
| Meghalaya annandalei Giribet, Sharma & Bastawade, 2007 | EH | – | large, diffuse, sunken | reduced | distinct | 0.32 (C) |
| Leptopsalinae subfam. nov. | ||||||
| Miopsalis collinsi ( Shear, 1993) | BO | yes | – | large | reduced | 0.14 (A) |
| Miopsalis globosa ( Schwendinger & Giribet, 2004) | SP | yes | – | reduced | distinct | 0.35 (C) |
| Miopsalis gryllospeca ( Shear, 1993) | BO | – | small, distinct, flat | absent | large | 0.33 (C) |
| Miopsalis kinabalu ( Shear, 1993) | BO | – | – | large | reduced | 0.25 |
| Miopsalis leakeyi ( Shear, 1993) | BO | – | – | reduced | large | 0.31 (C) |
| Miopsalis lionota ( Pocock, 1897) | BO | yes | small, distinct, flat | ? | ? | ? |
| Miopsalis mulu ( Shear, 1993) | BO | yes | – | distinct | distinct | 0.20 (A) |
| Miopsalis pocockii ( Hansen & Sørensen, 1904) | BO | ? | ? | large | distinct | 0.28 |
| Miopsalis pulicaria Thorell, 1890 | SP | ? | ? | distinct | reduced | 0.19 (A) |
| Miopsalis sabah ( Shear, 1993) | BO | yes | – | large | reduced | 0.21 (A) |
| Miopsalis silhavyi ( Rambla, 1991) | BO | – | small, distinct, flat | reduced | distinct | 0.32 (C) |
| Miopsalis tarumpitao ( Shear, 1993) | PI | – | – | large | reduced | 0.32 (C) |
| Leptopsalis beccarii Thorell, 1882 | SU | – | – | distinct | distinct | 0.20 (A) |
| Leptopsalis dumoga ( Shear, 1993) | SW | – | – | large | distinct | 0.35 (C) |
| Leptopsalis hillyardi ( Shear, 1993) | SW | – | – | distinct | distinct | 0.28 |
| Leptopsalis javana ( Thorell, 1882) | JA | – | – | large | reduced | 0.26 |
| Leptopsalis laevichelis ( Roewer, 1942) | SP | ? | ? | ? | ? | ? |
| Leptopsalis lydekkeri ( Clouse & Giribet, 2007) | NG | – | – | large | reduced | 0.28 |
...... continued on the next page
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Cyphophthalmi |
|
Family |