Polypterus senegalus
|
publication ID |
https://doi.org/10.26028/cybium/2020-441-003 |
|
DOI |
https://doi.org/10.5281/zenodo.10881467 |
|
persistent identifier |
https://treatment.plazi.org/id/304B075D-686A-E85B-FCB5-8687FF56FCCE |
|
treatment provided by |
Felipe |
|
scientific name |
Polypterus senegalus |
| status |
|
Comparative morphology of finlet spines in Polypterus senegalus View in CoL ( Figs 8-11 View Figure 8 View Figure 9 View Figure 10 View Figure 11 )
This description was done with a complete series of 10 finlets for an adult male Polypterus senegalus of 260 mm TL and with additional comparative observations of other specimens.
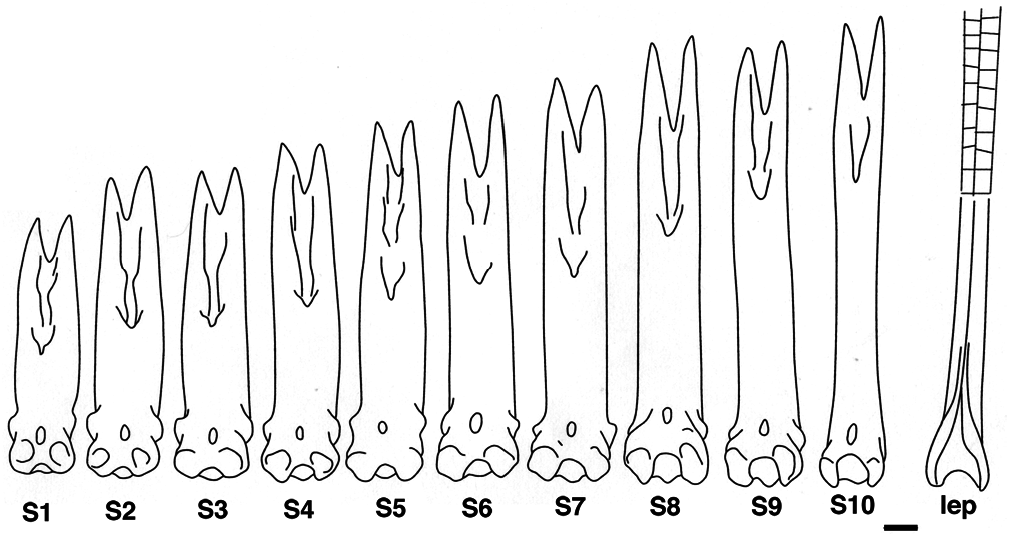
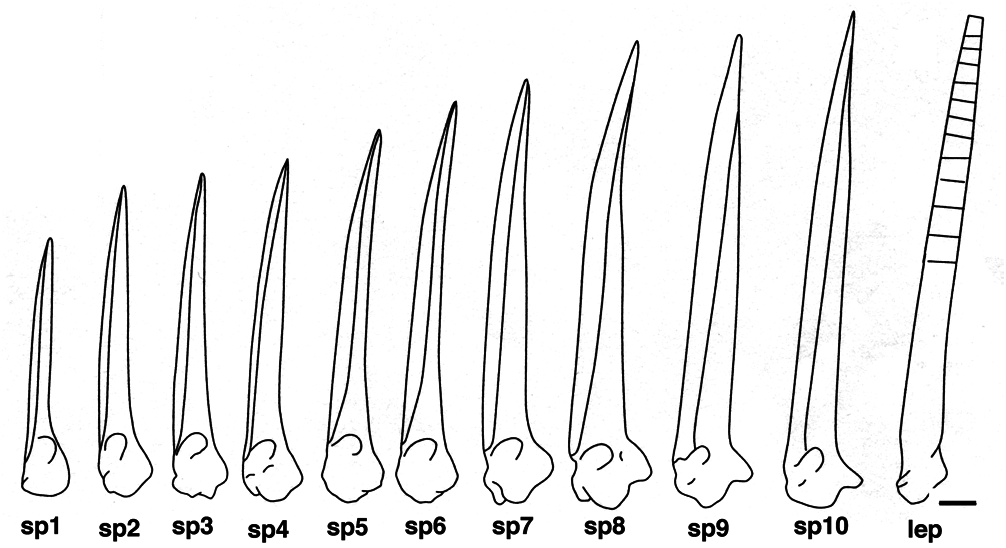
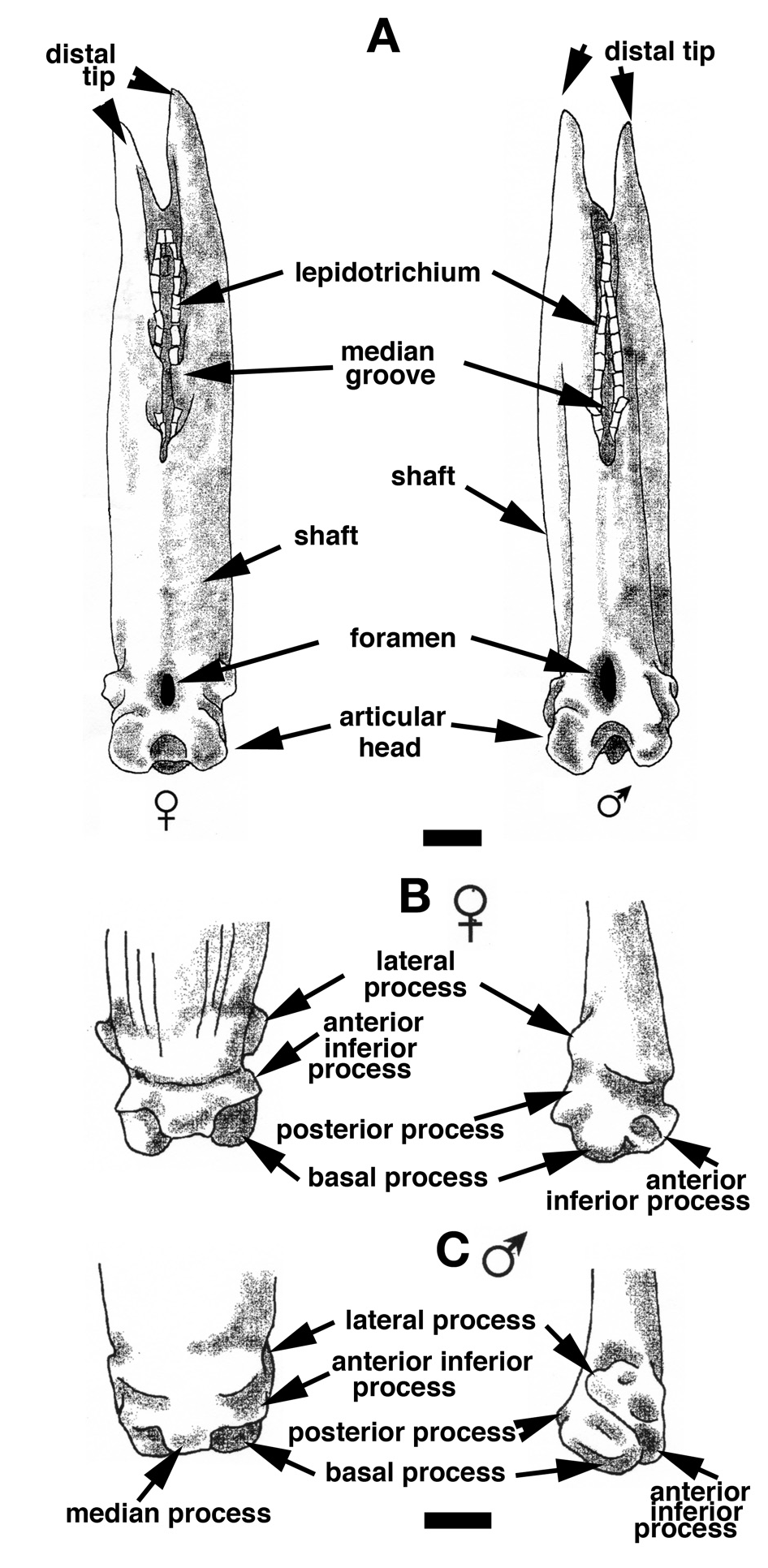
We observe an anterior-posterior gradient of spine height in Polypterus senegalus as for all the Polypterus species, though with a difference in largest to smallest spine height ratio ( Fig. 8 View Figure 8 ). Even if finlet differentiation is posterior-anterior oriented, the shape of the posterior spine, generated first, is more similar to the first lepidotrichium at the second part of the dorsal fin than to the anterior spines, i.e. more bent at its lower third versus larger for the most anterior spines ( Fig. 8 View Figure 8 : S10). In transverse section, the shaft of the first spine shows a nearly straight anterior margin ( Fig. 7B View Figure 7 ), even though it is more or less rounded for distal sections ( Fig. 7 View Figure 7 C-I) as it is for the posterior spines.
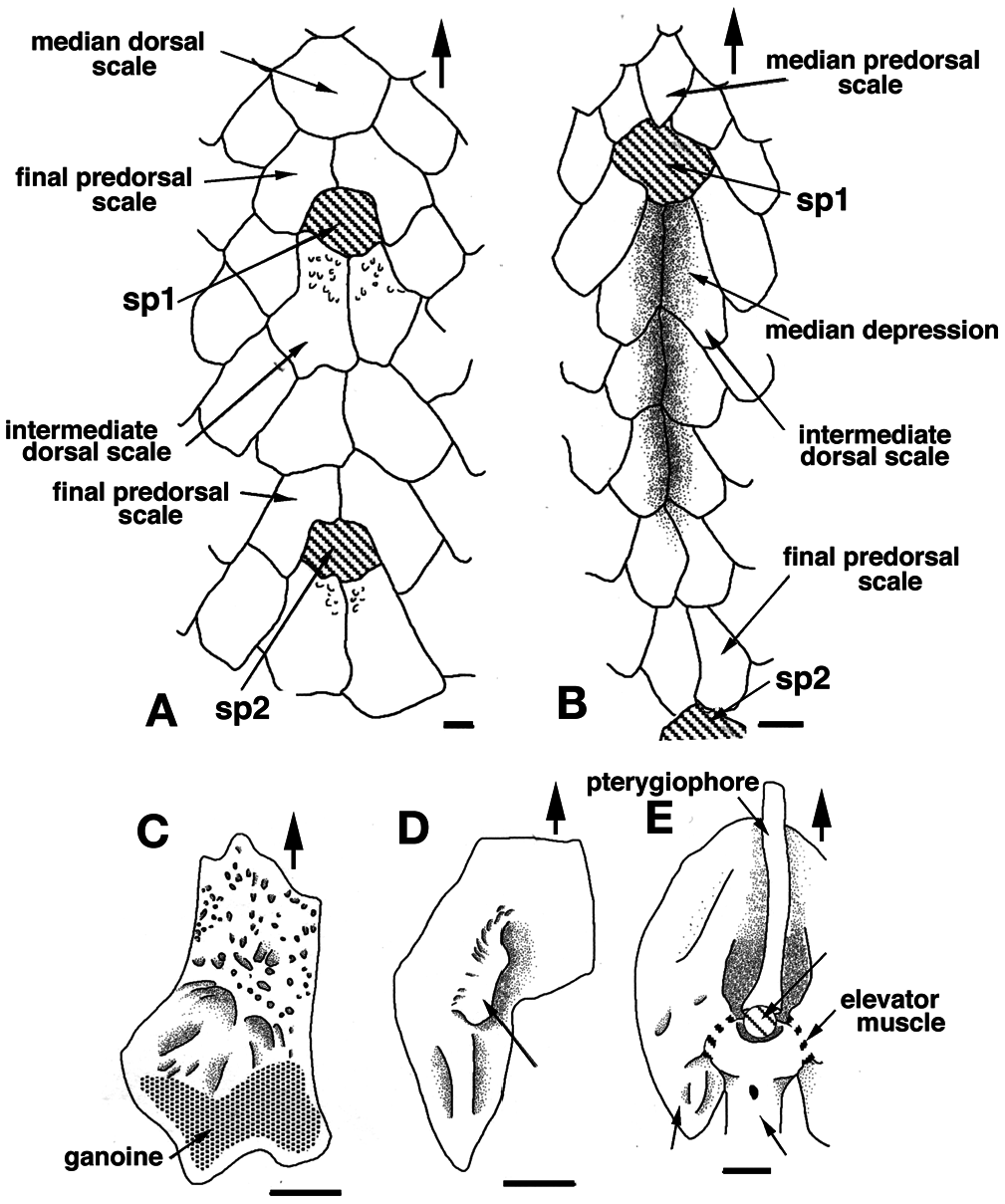
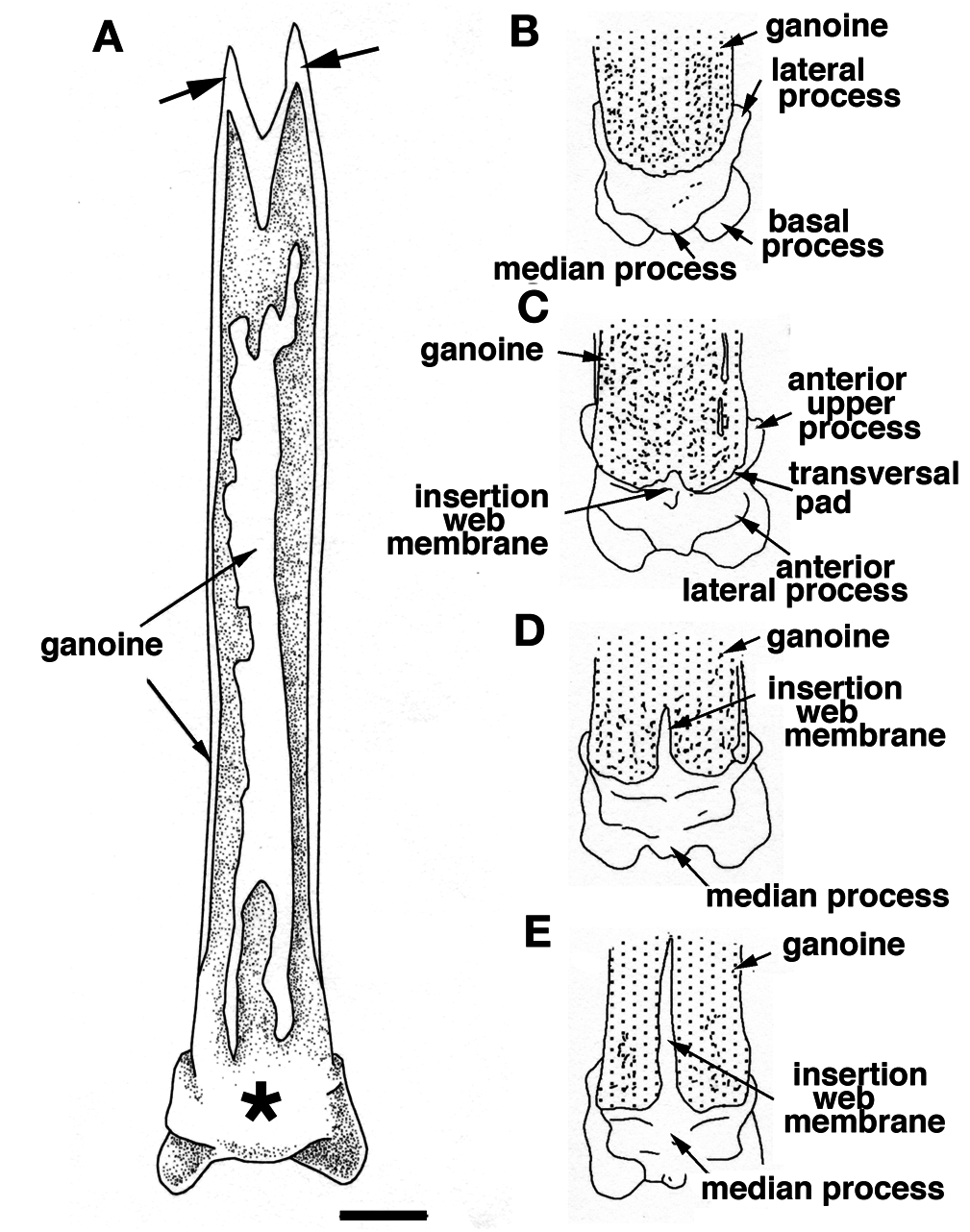
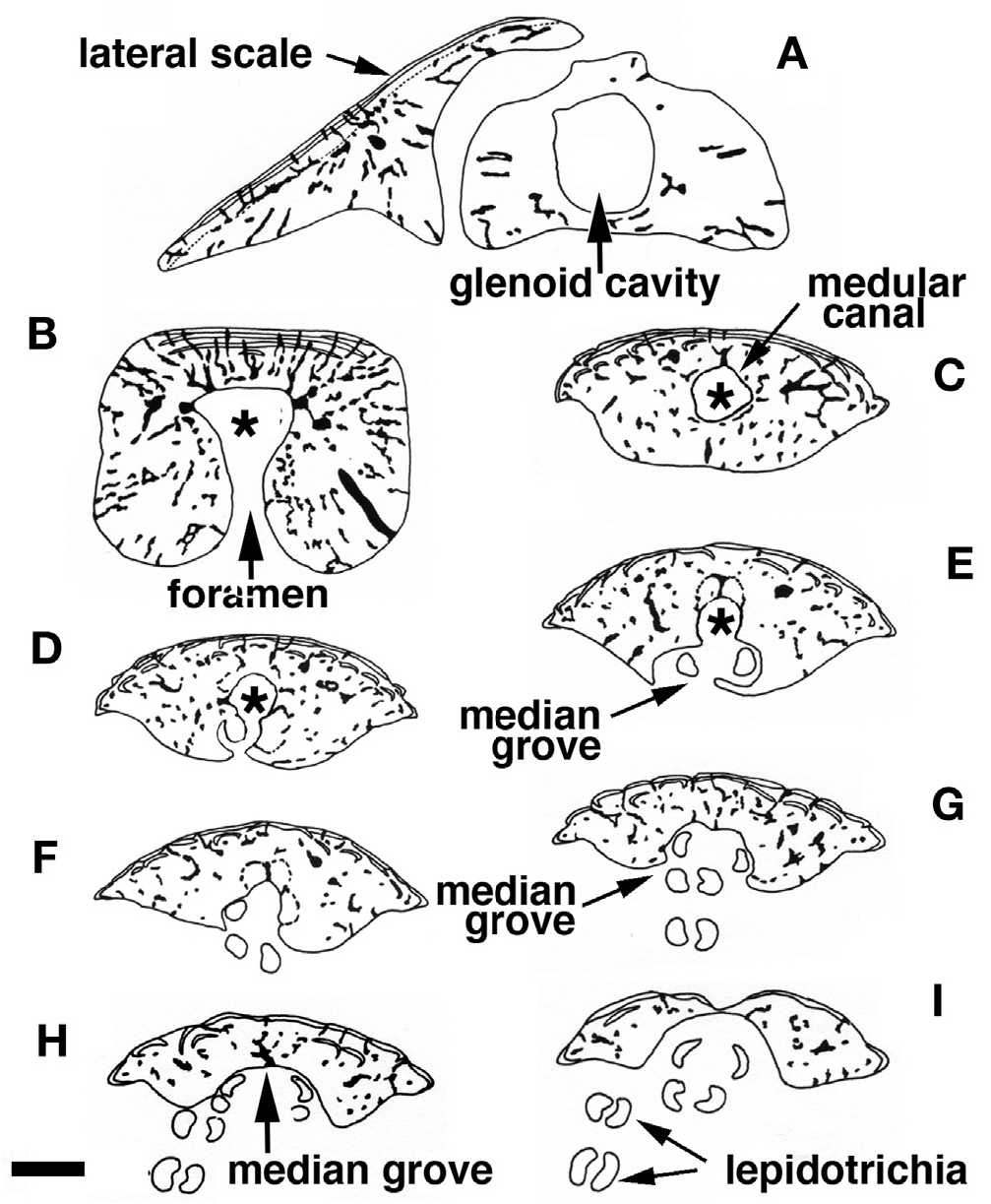
The anterior face of the finlet shaft is covered by a thin continuous ganoine layer ( Fig. 3 View Figure 3 B-E). It runs over the articular head downward to about its mid-height, ending at the superior transversal pad ( Fig. 3D, E View Figure 3 ). The transversal pad forms two anterior upper processes directly connected to the lateral processes ( Figs 3C View Figure 3 , 4C View Figure 4 ; and see “posterior face description”). It is responsible for the lock of the spine inbetween the paired scale at a 45° angle when the finlet erects. The pad is not formed from the ganoine layer; rather it is continually present even though the ganoine surface decreases from the first to the last spine ( Fig. 3 View Figure 3 B-E). The web is attached to the base of the following finlets for the most anterior (before the 6 th or 7 th finlet) and to the first quarter for the most posterior ( Fig. 3 View Figure 3 C-E).
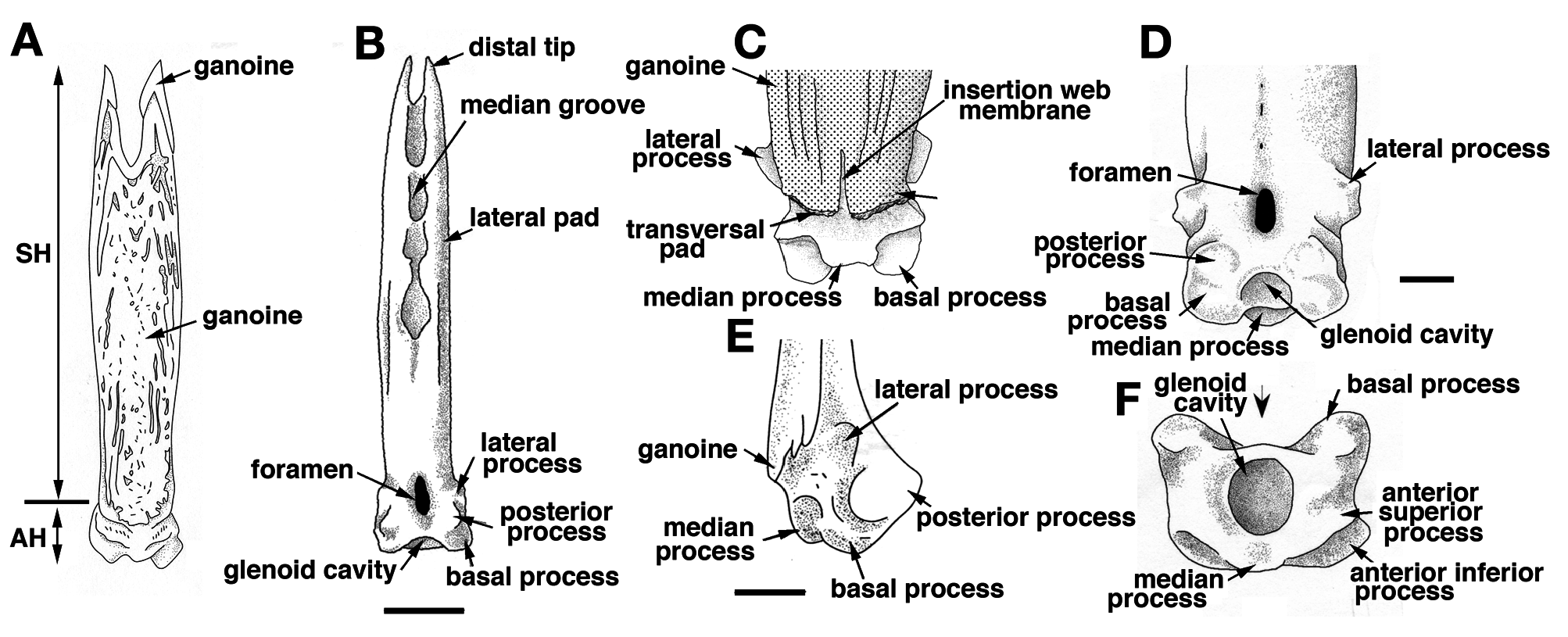
On its posterior face, the articular head is roughly square, due to the presence of two lateral and two basal processes ( Fig. 4D View Figure 4 ). The four processes allow the ligament connection to the scales and articulation of the finlet. The lateral processes are located on both sides, approximately at the level of the basal foramen (basal opening of the medullar canal of the finlet shaft; Fig. 4D View Figure 4 ). They are well developed on the first spine and are almost absent on the last ( Fig. 8 View Figure 8 : S10). The inclinator muscles present in Actinopterygians ( François, 1959) and attached to the analogue of the lateral processes are missing in polypterids ( Daget, 1950; Bartsch and Gemballa, 1992; Gemballa, 1995). The basal processes, which are the largest and the roundest of the processes, frame the glenoid cavity (articulation point with the pterygiophore; Figs 2E View Figure 2 , 4F View Figure 4 ), and are tied to the anterior processes of the two basal scales by two strong ligaments. Two posterior processes, significantly smaller and still individualized ( Fig. 4D View Figure 4 ), reach distally to the basal processes and lean against the anterior median margin of the basal scales while the finlet is relaxed on the back of the animal; they allow the attachment of the anterior section of the depressor muscles. The glenoid cavity of the first spine is small or not visible at all in a posterior view, due to its lower position ( Fig. 9 View Figure 9 ). In contrast, it becomes more and more visible in the following spines as its position rises ( Fig. 9 View Figure 9 ; see basal face).
The joint between the two half spines is composed of a basal median bony bridge, which isolates the basal foramen from the median groove ( Fig. 4B, D View Figure 4 ). In larva, the finlets are formed in a posterior-anterior sequence ( Sewertzoff, 1924; Bartsch and Gemballa, 1992). The ratio between the total height of the spine and the height of the basal bridge is nearly the same for all specimens (sex and size): it varies from 1.70 for a 216 mm TL specimen to 1.81 for a 380 mm TL specimen (maximal size observed so far): the spine growth rate is mostly constant.
The finlet spines of Polypterus senegalus are most nearly straight with a slight posterior-oriented curve at their distal extremity ( Fig. 10 View Figure 10 ). The ventral roundness of the articular head is a consequence of the development of the basal processes. Correlated to the pinnulae position and/or the specimen age, this roundness is posteriorly interrupted by the increase of a concavity separating the posterior processes from the basal processes. Indeed, in contrast to the posterior-anterior formation of the finlet shaft, the formation of the articular head is anterior-posterior oriented. Thus, the developmental stage of the articular head is more advanced at the first spine. The head of the ultimate spine is almost identical to the head of the closest following lepidotrichium: the posterior processes are well pronounced and the lateral processes are weakly distinguishable ( Figs 8-10 View Figure 8 View Figure 9 View Figure 10 ). Moreover, the shape of the last spine in adults is similar to the shape of the most anterior spine in juveniles with only a size difference: the adult’s last spine is larger than the juvenile’s first spine ( Figs 8 View Figure 8 , 10 View Figure 10 ).
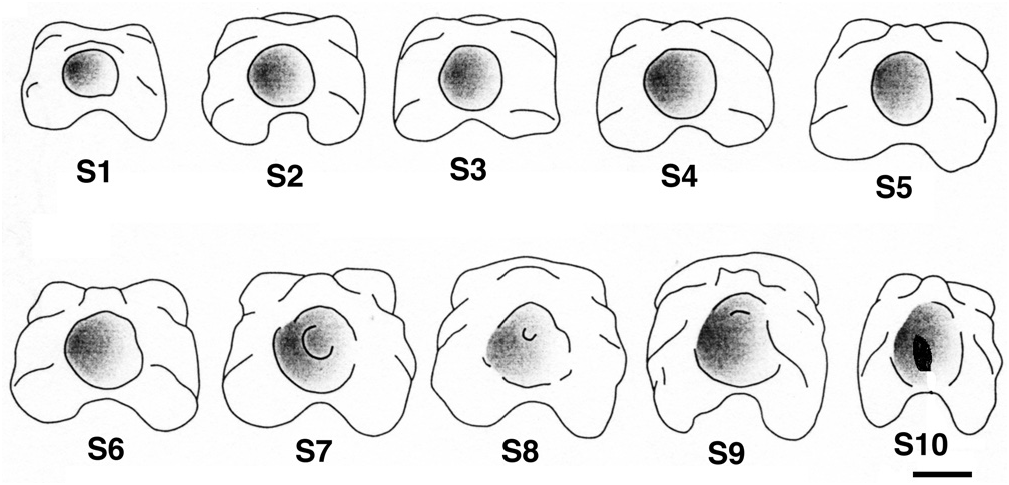
The basal surface of the articular head is more or less quadrangular shaped ( Figs 4F View Figure 4 , 9 View Figure 9 ) due to the basal processes and the nearly undifferentiated anterior upper and lateral processes. The first spine appears rectangular (anterior-posterior compression), but the last one looks more like a square ( Fig. 9 View Figure 9 ). This shape difference between the first and last spines can be observed in juveniles as well as in adults. This is quite unexpected because of the similarity of the processes of the juvenile first spines and of the adult last spines. It seems that only the spine position (relative to the posterior margin of the dorsal fin) is able to influence this shape more than the developmental stage.
At the first spine, the glenoid cavity is perfectly rounded ( Fig. 9 View Figure 9 ). As one moves posteriorly, its boundaries tend to disappear and its size enlarges. The ultimate spine shows a narrow anterior-posterior oriented slot at the bottom of the glenoid cavity ( Fig. 9 View Figure 9 : S10). It corresponds to an incomplete fusion of the two hemi-lepidotrichia at the origin of the spine. Interestingly, the two basal hemi-segments of the first lepidotrichium of the dorsal fin’s caudal section are not fused either.
The development of the basal surface of the spines varies in an anterior-posterior gradient. The last spine does not match this pattern and seems to be influenced by the proximity of the first non-modified lepidotrichium. This is probably the result of a particular development pathway: its articular head is the last of a set of spine series to differentiate, while its shaft is the first; this explains the large height of the shaft and the juvenile aspect of the articular head. Indeed, in spite of the fact that the ultimate spine shaft is formed first, the dichotomy of the lepidotrichium occurs only after the formation of all the caudal lepidotrichia of the dorsal fin, from the first to the last spine ( Sewertzoff, 1924).
The finlet spines of female and male Polypterus senegalus show several minor differences that do not affect the species description ( Fig. 11 View Figure 11 ). The lower-anterior region of the shaft spine (just above the articular head) is larger in females than in males. In posterior view, the shaft of the female spine is wide and regularly rounded, whereas the male’s is narrow and concave on the edges ( Fig. 11A View Figure 11 ). The lateral processes of all female spines are more individualized and are located at a more anterior position. In lateral view, the posterior processes appear more pronounced for females than for males, clearly breaking the roundness of the articular head base ( Fig. 11B, C View Figure 11 ). Finally, in basal view, the basal surface of the male spines appears from the first spines to be more squared than for females due to its narrower shaft and articular head.
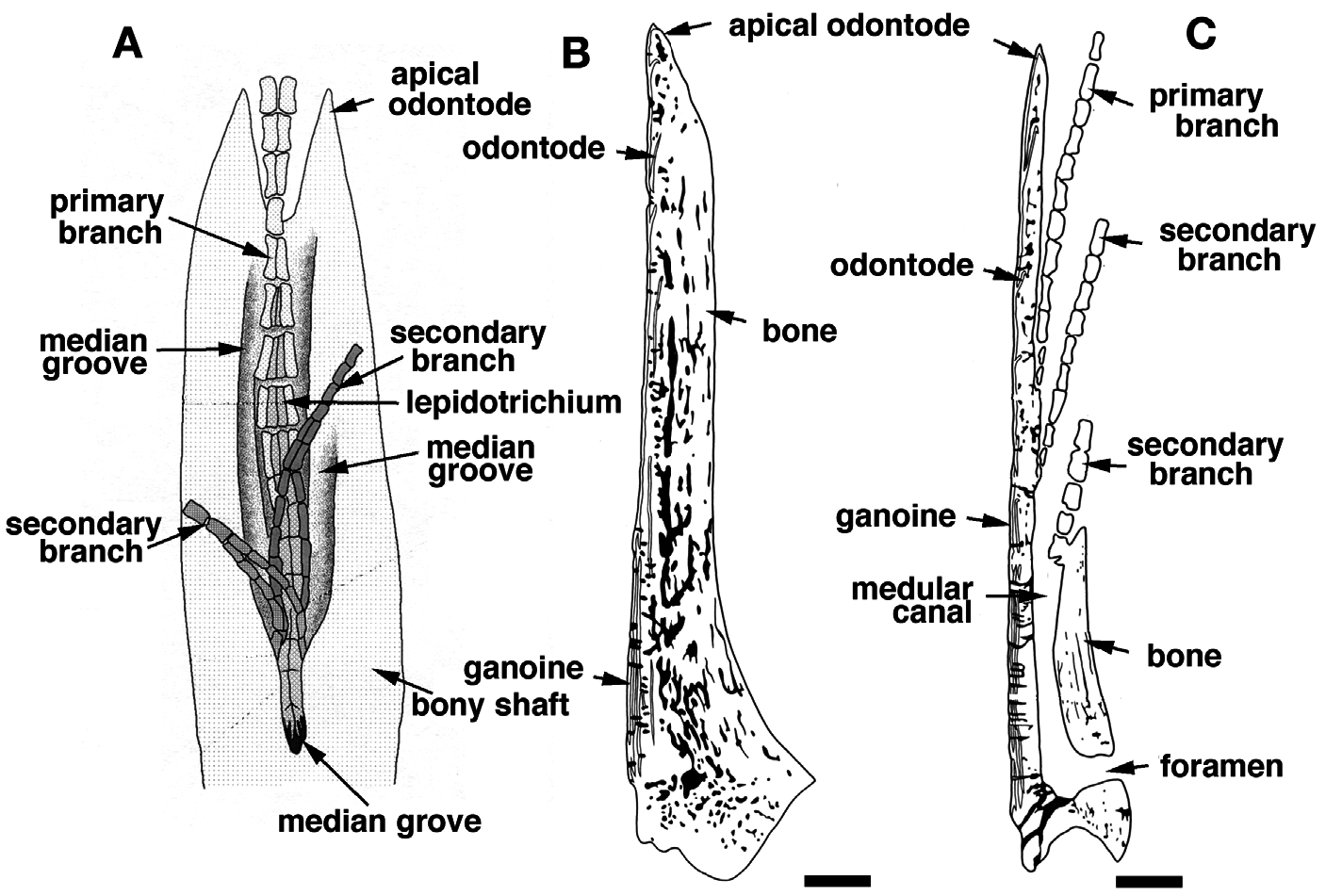
The spine of each pinnule is composed of a set of odontodes of variable size, fitted together and resting on a bony base ( Fig. 6B, C View Figure 6 ) (see Meunier, 1980; Meinke, 1982). The central medular cavity opens at the base of the ray at the level of the basal foramen ( Figs 7C View Figure 7 , 11A View Figure 11 ), and becomes first a tube, the medular canal, ( Fig. 7C, D View Figure 7 ) that quickly turns into a median groove on the posterior-distal section of the spine ( Fig. 7 View Figure 7 E-I). Each odontode is organized around this cavity and is composed of dentine covered on the anterior face by a unique stratified layer of ganoine. The ganoine layers of several odontodes can overlap locally, forming a multiple strata pattern ( Figs 6B View Figure 6 , 7B View Figure 7 ) ( Meunier, 1980). The spine growth essentially acts at the distal extremity by addition of neo-formed odontodes ( Meunier, 1980; Meinke, 1982). The spine thickens through the overlay of one or several older units by the base of new odontodes. This particular organization can be observed in transverse section: several levels of odontodes more or less aligned ( Fig. 7B View Figure 7 ). However, the histological structure of the spines is quite different from that of the scales ( Meunier, 1980). In the case of the finlet spines, the neo-formed odontodes are not totally fused, in contrast to the true so-called odonto-complexes of scales sensu Ørvig (1977).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
ParvPhylum |
Osteichthyes |
|
Order |
|
|
Family |
|
|
Genus |