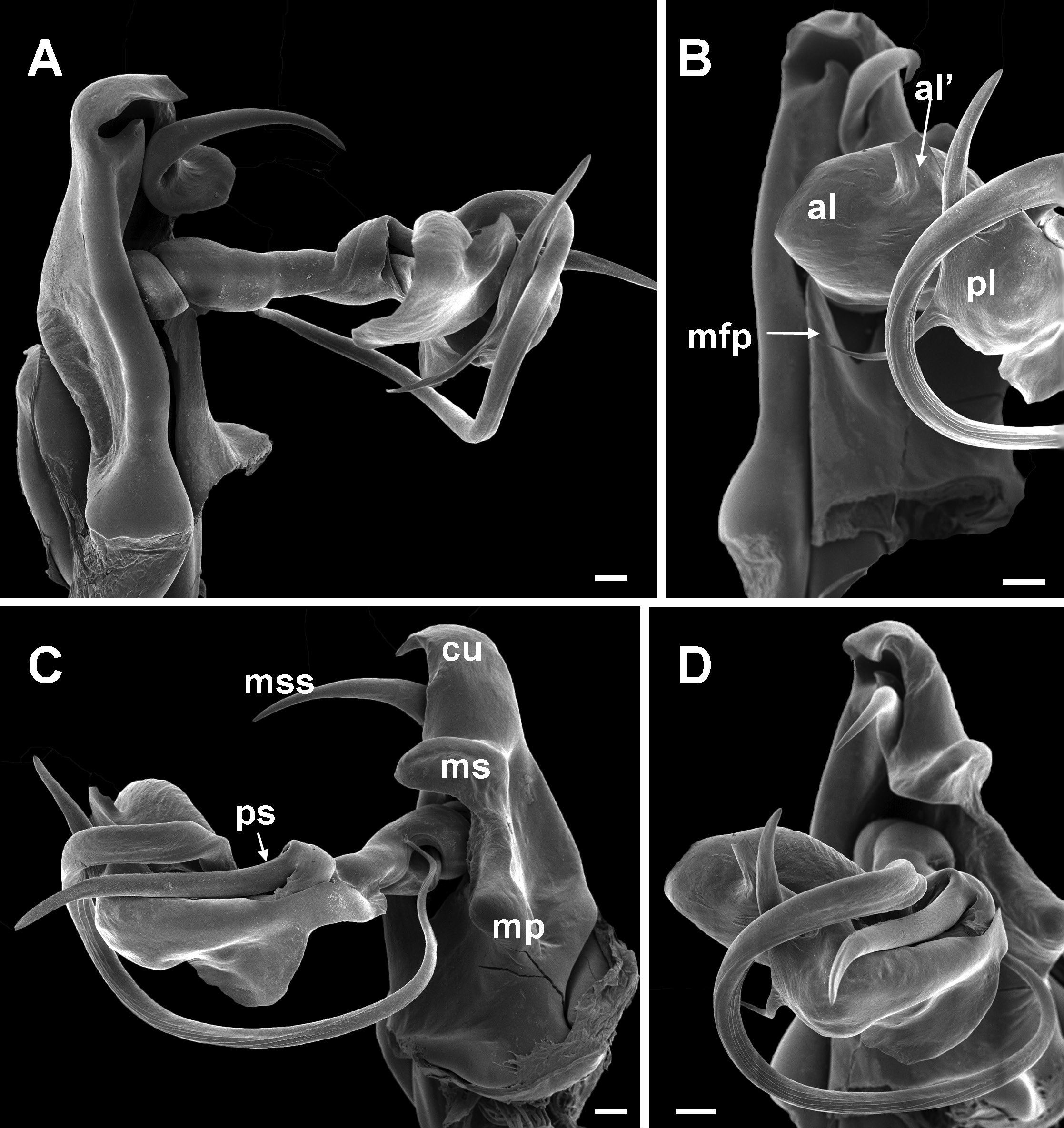
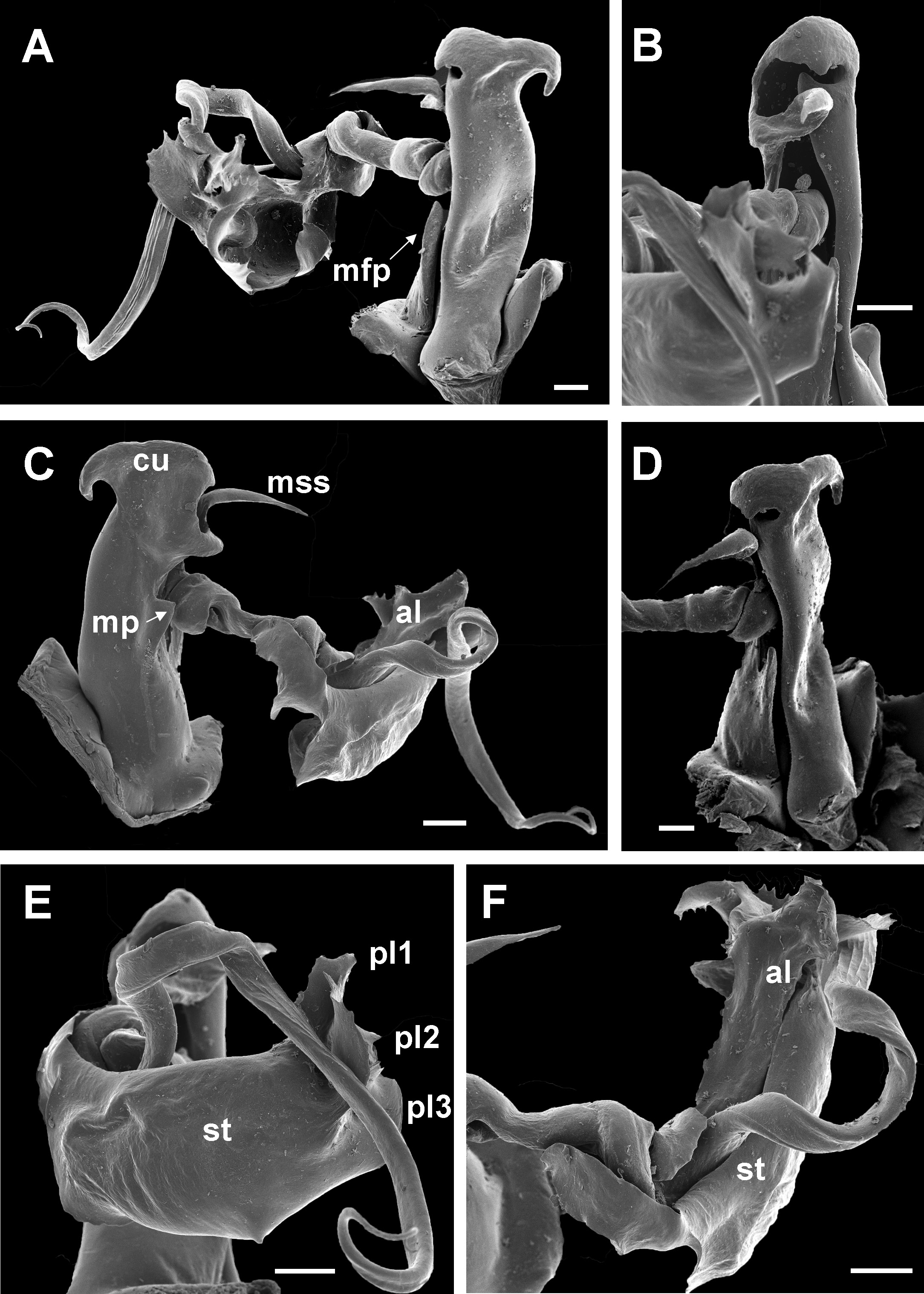
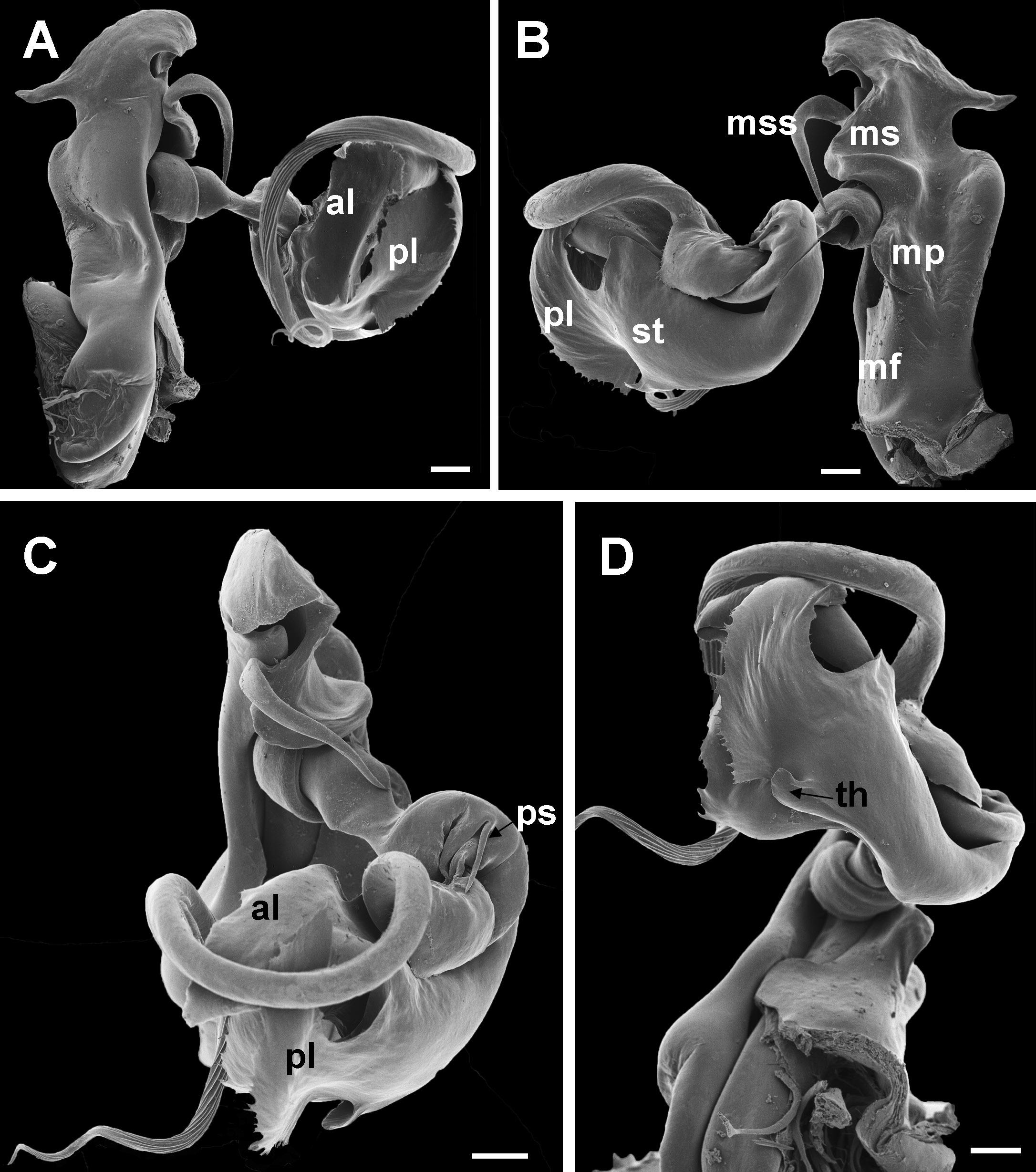
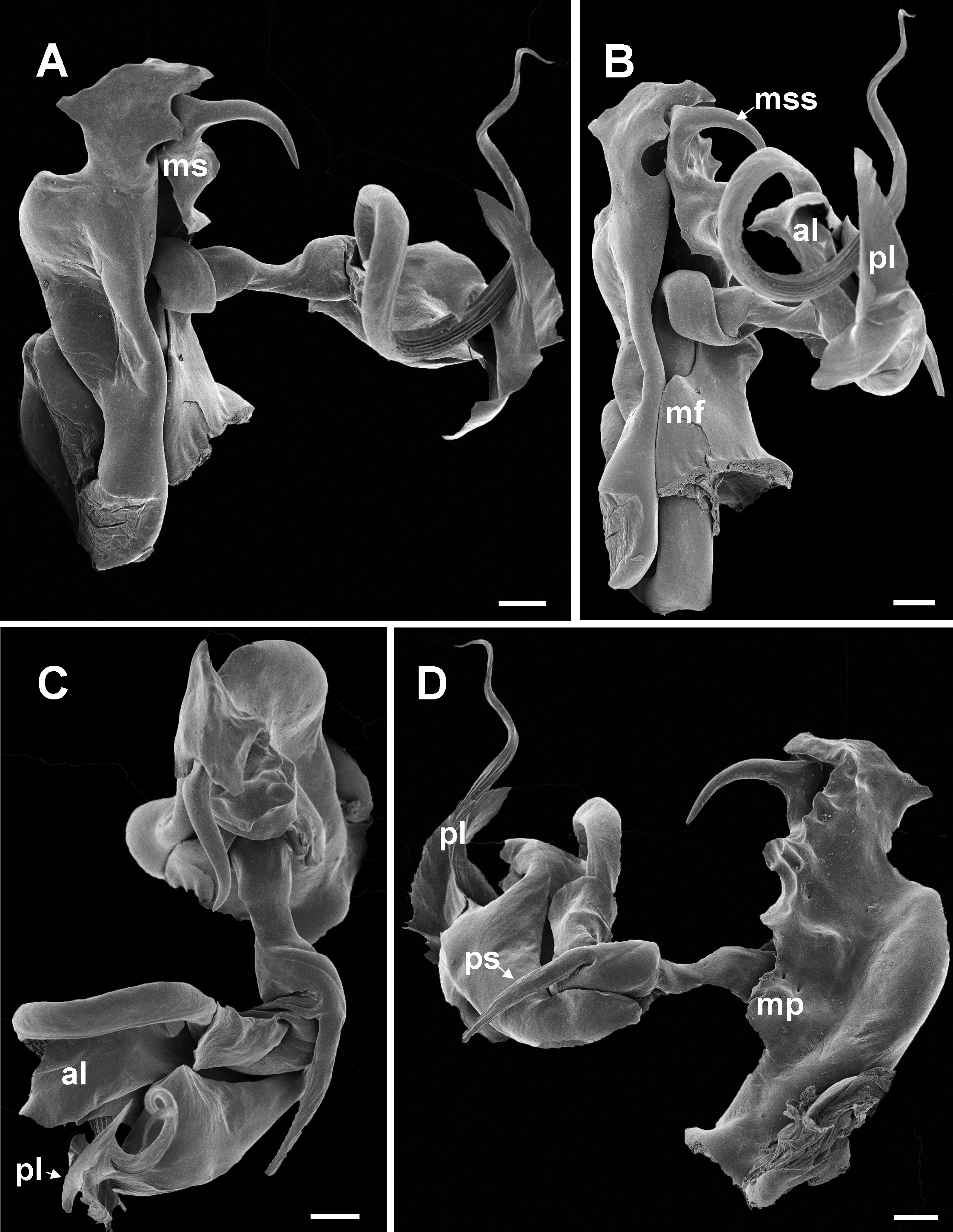
Chaleponcus dabagaensis, Kraus, 1958
|
publication ID |
https://doi.org/10.5852/ejt.2014.100 |
|
publication LSID |
lsid:zoobank.org:pub:B3E6C489-6D96-4AF5-A33D-EE8329A9321B |
|
DOI |
https://doi.org/10.5281/zenodo.14617429 |
|
persistent identifier |
https://treatment.plazi.org/id/334F2769-C12F-FFC0-1EEA-F728FED8FEEB |
|
treatment provided by |
Carolina |
|
scientific name |
Chaleponcus dabagaensis |
| status |
|
Key to species of the Chaleponcus dabagaensis View in CoL group
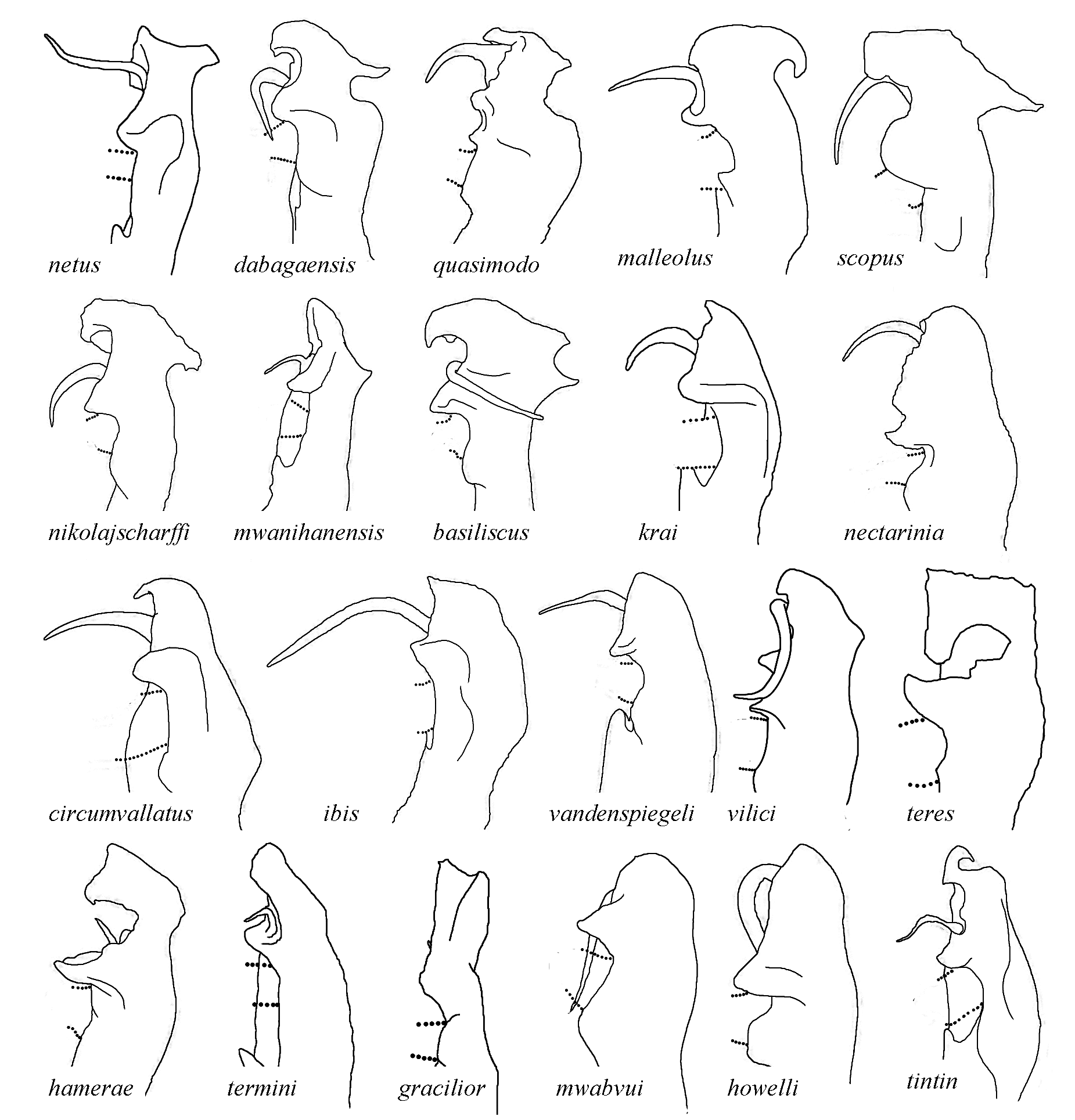
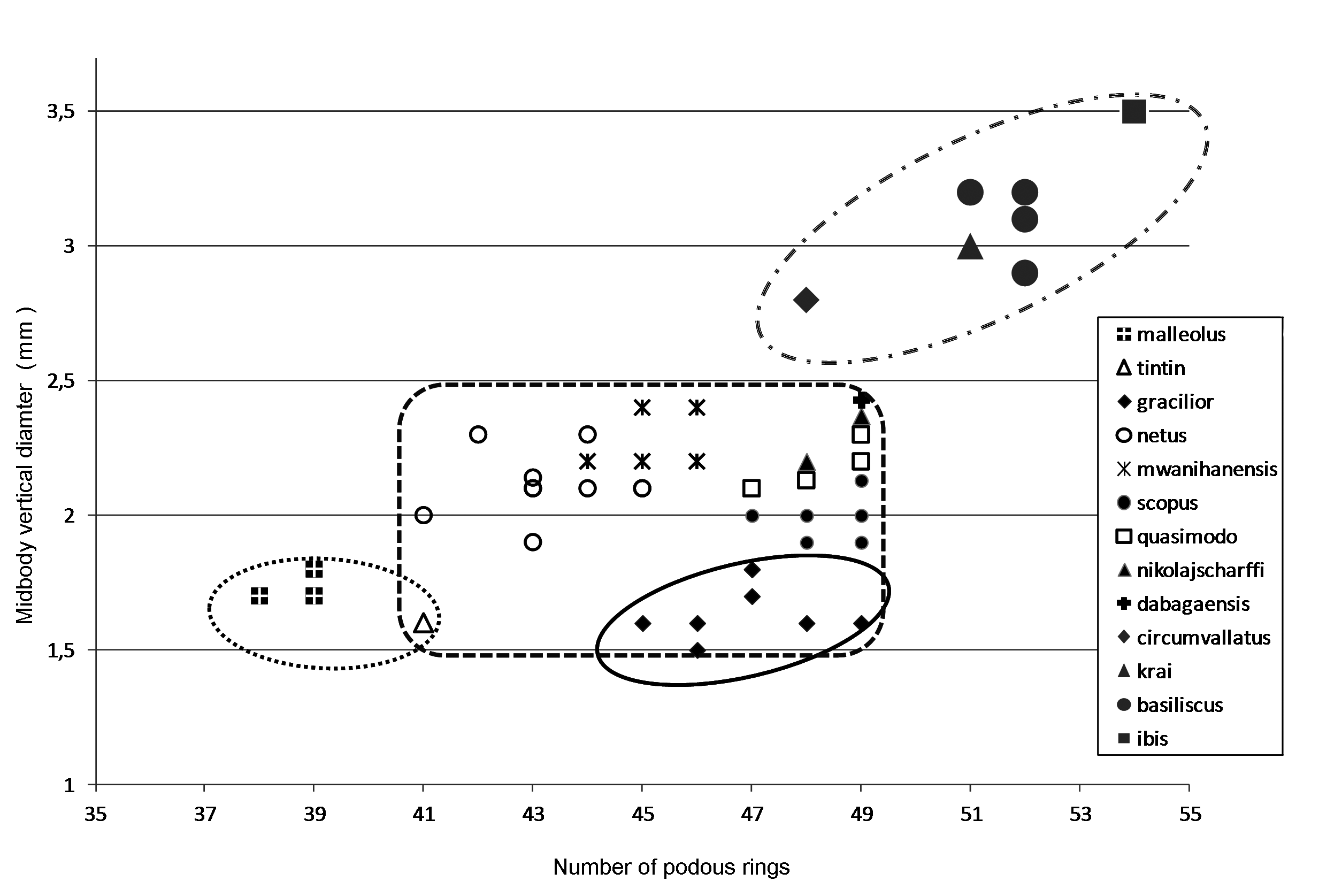
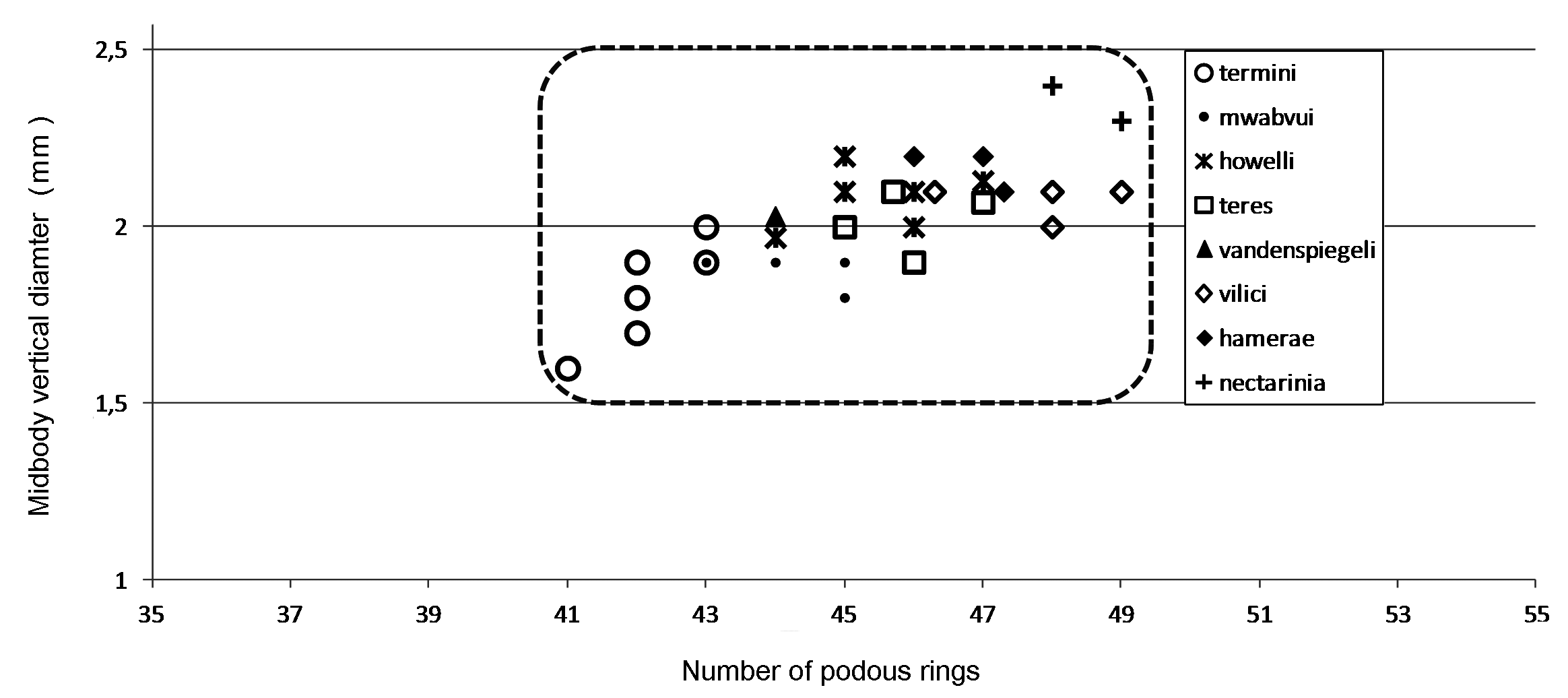
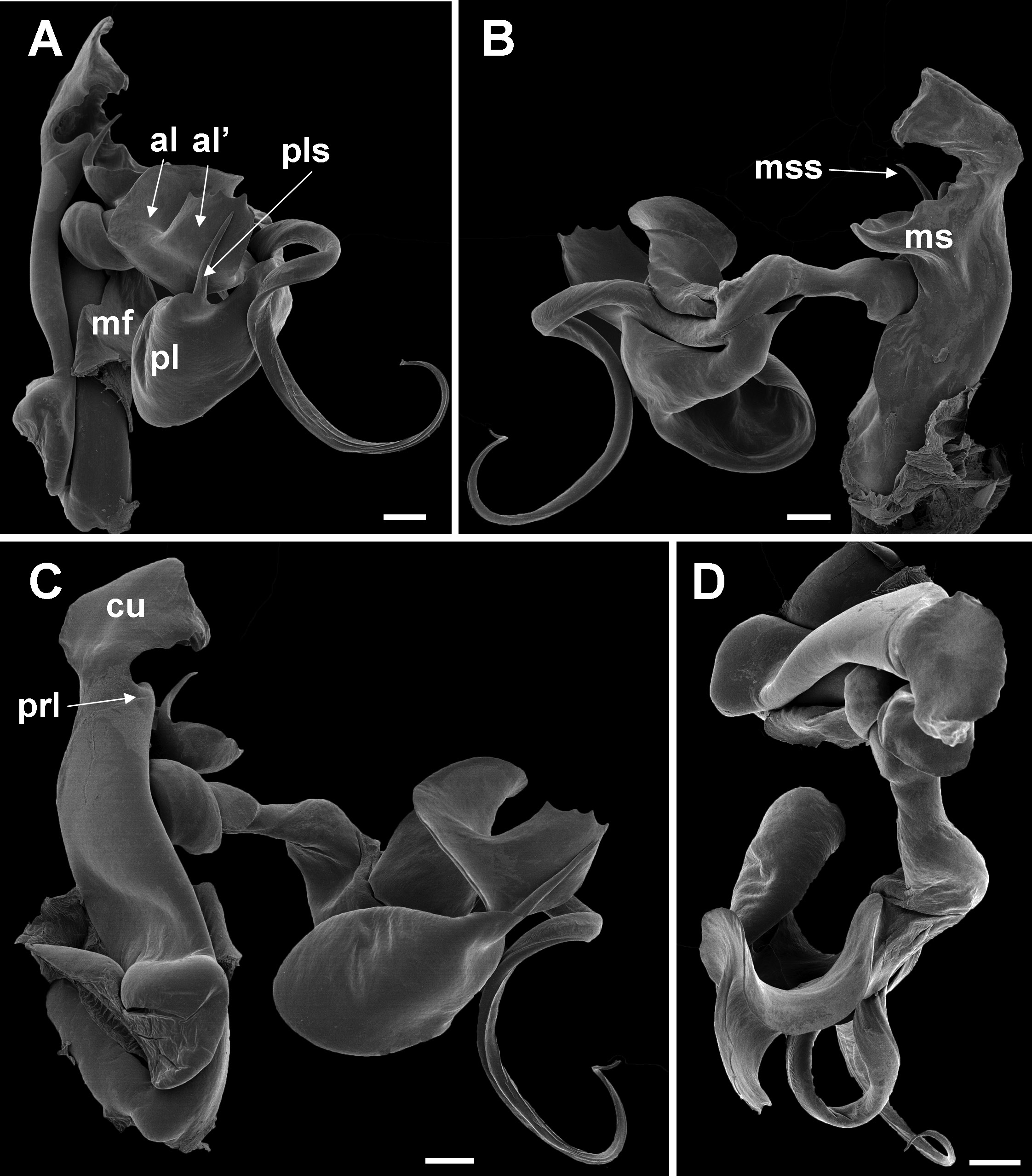
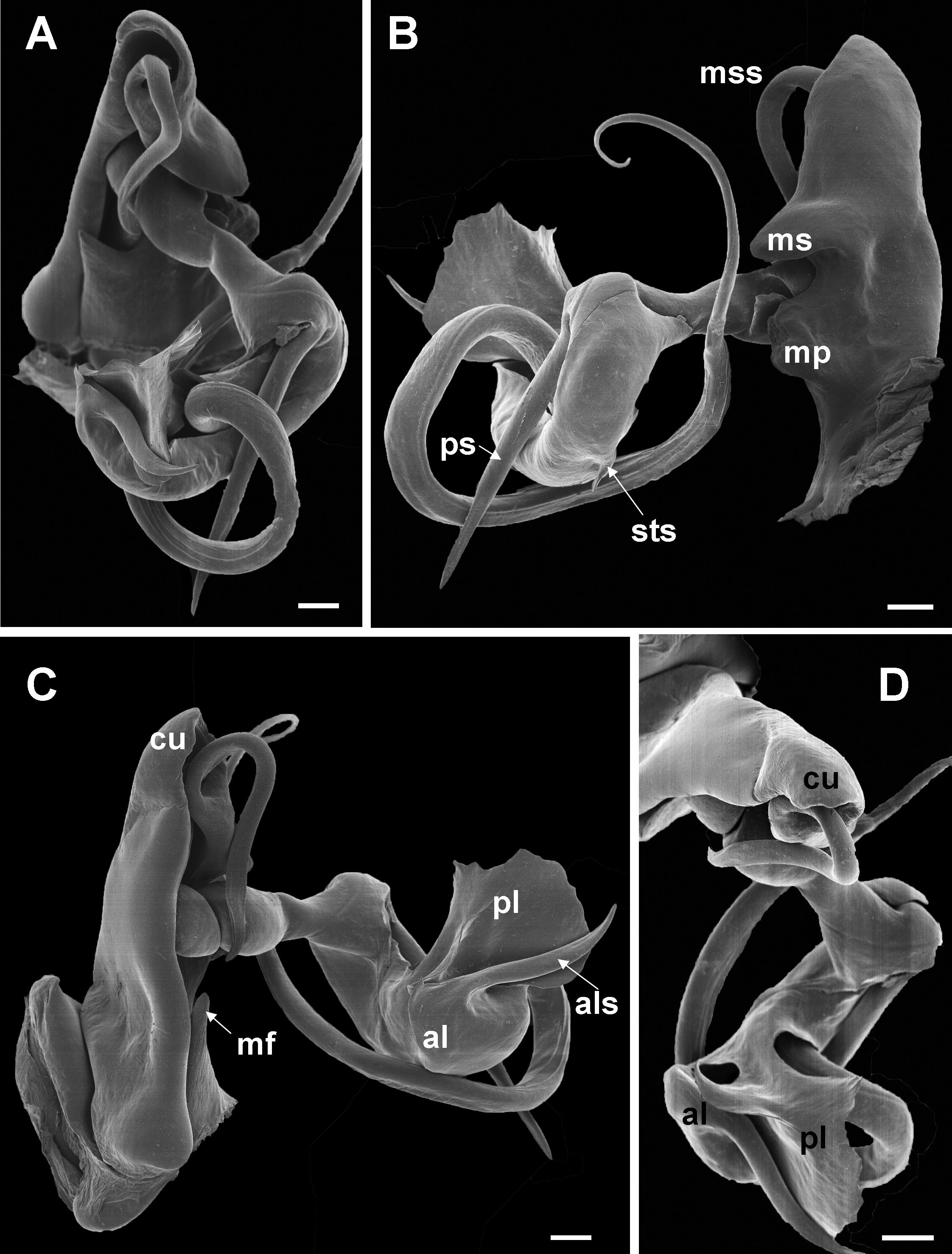
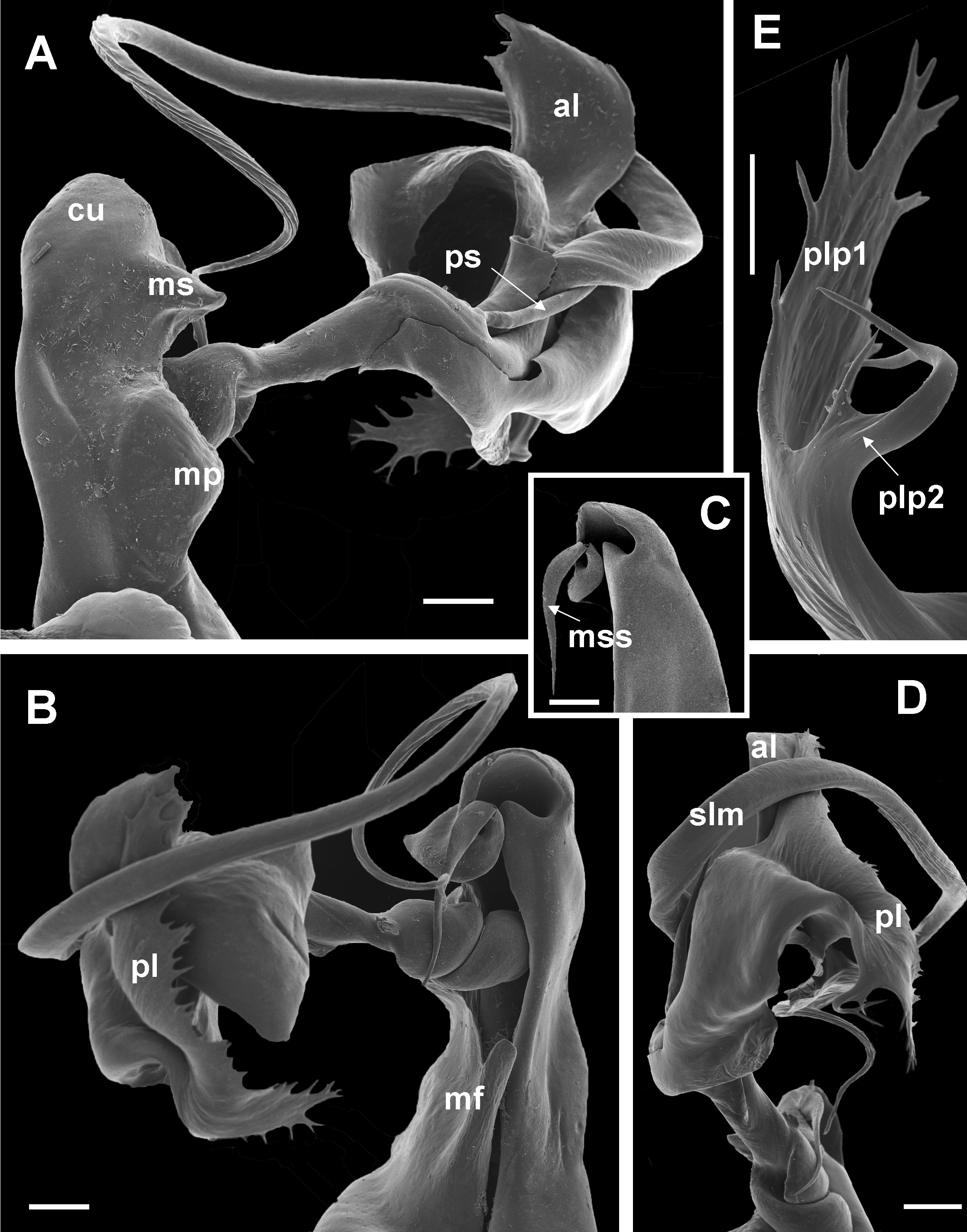
The 21 known species of the C. dabagaensis group are almost all quite distinctive in gonopod morphology. A key to the species is given below, but comparison with the gonopod coxa profiles in Fig. 9 View Fig will often lead directly to the right species. As new species of the group are likely to be discovered, and as the species in the key may prove to be more variable with regard to number of podous rings and body diameter, any identification should be checked against the detailed gonopod description and illustrations, and the size (no. of podous rings vs. body diameter) should be compared with the diagrams, Figs. 2–3 View Fig View Fig . The limbus ( Fig. 4 View Fig ) offers additional diagnostic characters.
The key is based on adult males.
1. Body diameter> 2.5 mm …………………………………………………………………………2
– Body diameter < 2.5 mm …………………………………………………………………………5
2. Gonopod coxa with two lateral processes ………………………………………… basiliscus View in CoL sp. nov.
– Gonopod coxa without lateral processes ……………………………………………………………3
3. Anterior lamella of telomere divided into a horizontal lobe with deeply laciniate edges and a long, straight, distal spine-like part ( Fig. 19C View Fig ). Setation of walking leg tarsi normal (as Fig.5A View Fig ) … krai View in CoL sp. nov.
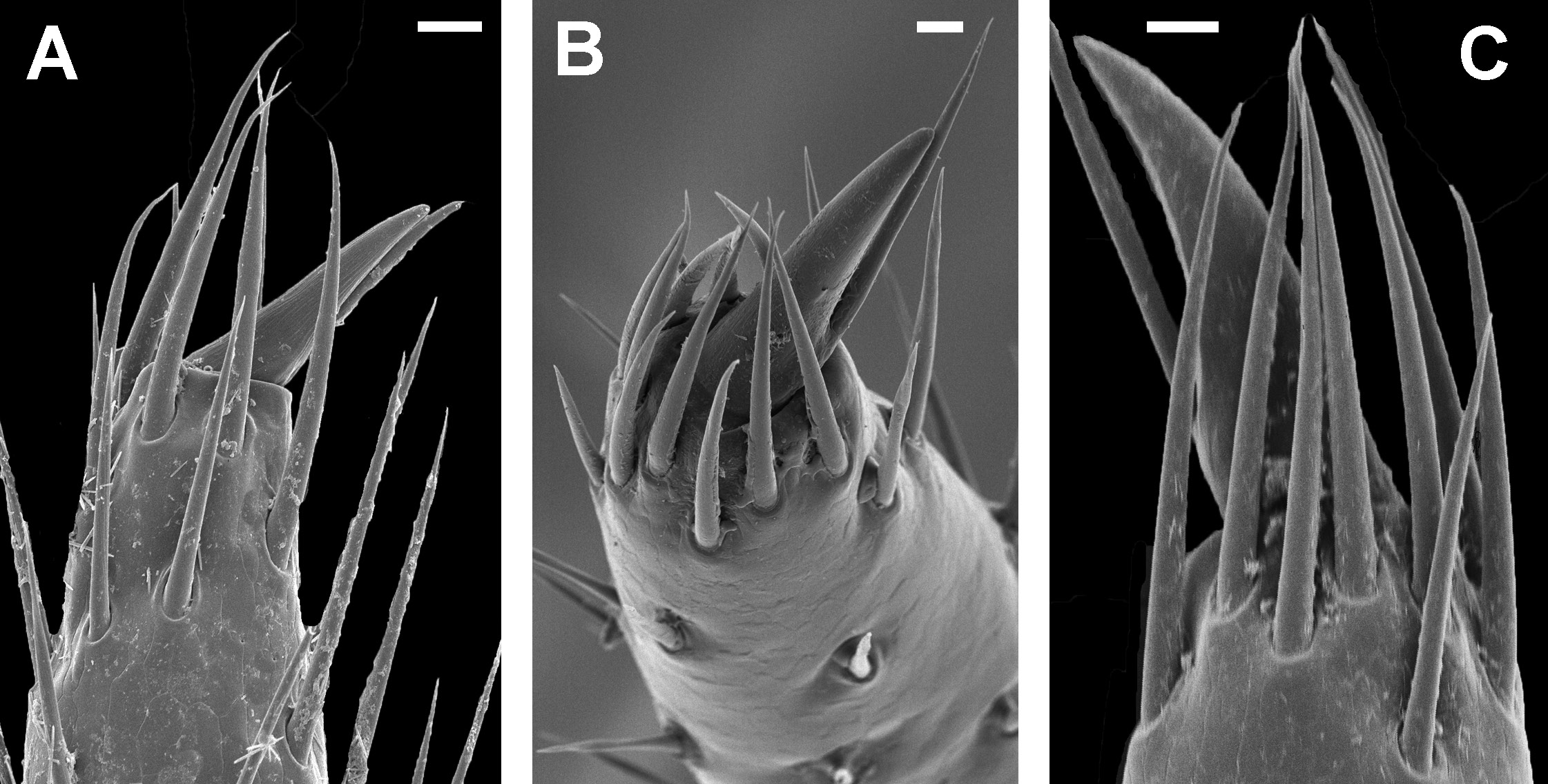
– Anterior lamella of telomere different. Setation of walking legs unusual: claws surrounded by dense ‘palisade’ of setae ( Fig. 5 View Fig B–C) ………………………………………………………………4
4. Posterior lamella of telomere ending in two long spines. Anterior lamella of telomere with small accessory lamella, otherwise with smooth edges ( Fig. 21B View Fig ) ……… circumvallatus View in CoL sp. nov.
– Posterior lamella of telomere ending in two short processes. Anterior lamella of telomere with a coarsely dentate basal part ( Fig. 22C View Fig ) …………………………………………………… ibis View in CoL sp. nov.
5 45–49 podous rings. Body diameter 1.5–1.8 mm ( Fig. 2 View Fig ). Cucullus of gonopod coxa subrectangular ( Fig. 28 View Fig ). Limbus with spatulate lobes ( Fig. 4F View Fig ) ………………………………… gracilior View in CoL sp. nov.
– <45 body rings and/or body diameter ≥ 1.9 mm. Cucullus and limbus different …………………6
6 Gonopod coxa with a lateral process ………………………………………………………………7
– Gonopod coxa without a lateral process …………………………………………………………14
7 <40 body rings. Body diameter 1.7–1.8 mm. No dorsal spine on anal valves. Lateral process of gonopod coxa a strongly bent hook ( Fig. 14 View Fig ) ……………………………… malleolus View in CoL sp. nov.
–> 40 body rings. Body diameter ≥ 1.8 mm. Each anal valve with a dorsal spine. Lateral process of gonopod coxa different …………………………………………………………………………8
8 Metaplical shelf of gonopod coxa in two levels between which metaplical shelf-spine fits. Lateral process of gonopod coxa small to inconspicuous ( Fig. 24 View Fig ) ……………………… vilici View in CoL sp. nov.
– Metaplical shelf in one level. Metaplical shelf-spine and lateral process different …………9
9 Lateral margin of gonopod coxa basal to lateral process pronouncedly ‘hunch-backed’ ( Figs 12–13 View Fig View Fig ) ……………………………………………………………………………………………………10
– Lateral margin of gonopod coxa basal to lateral process not pronouncedly ‘hunch-backed’ ………11
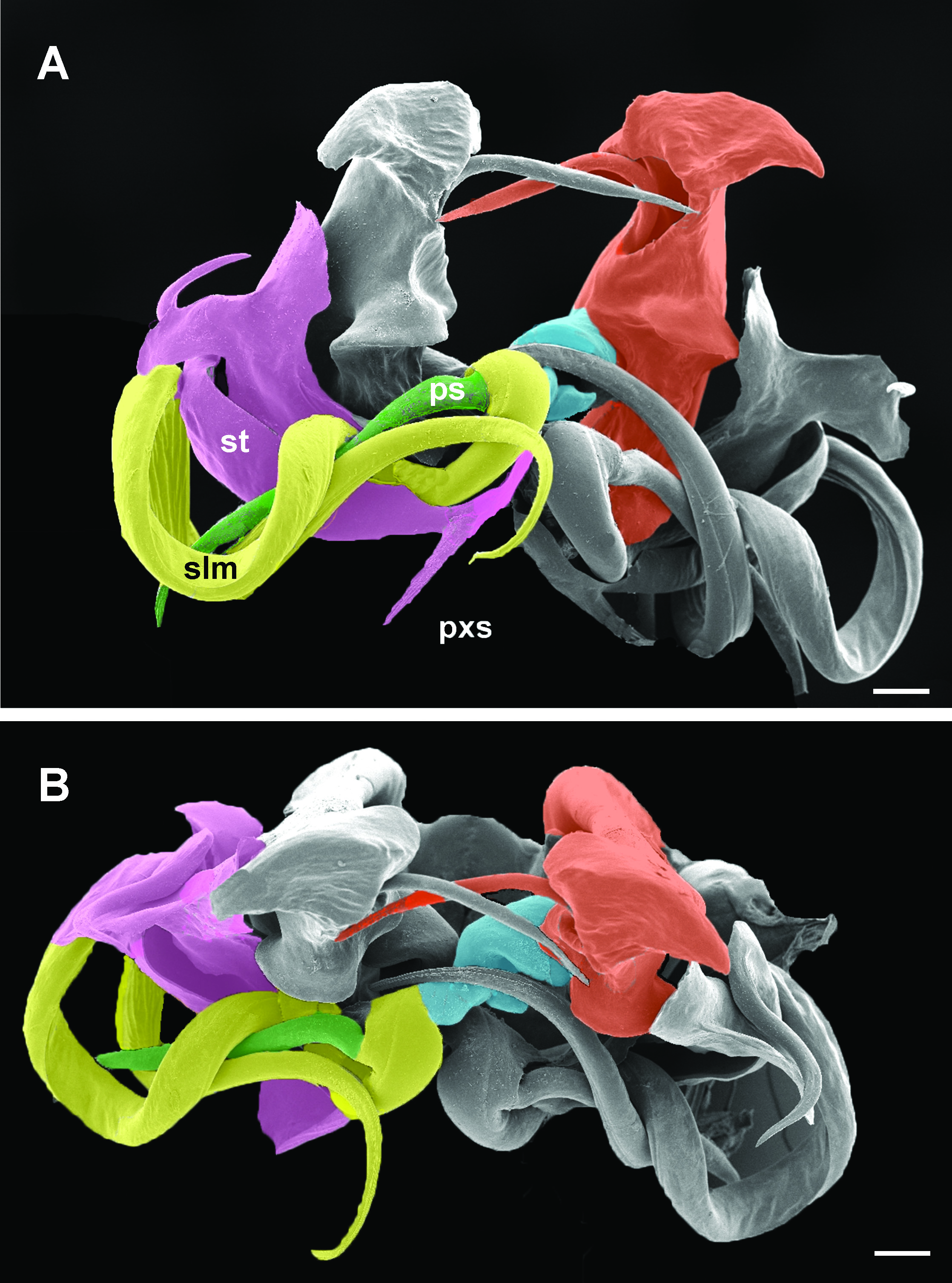
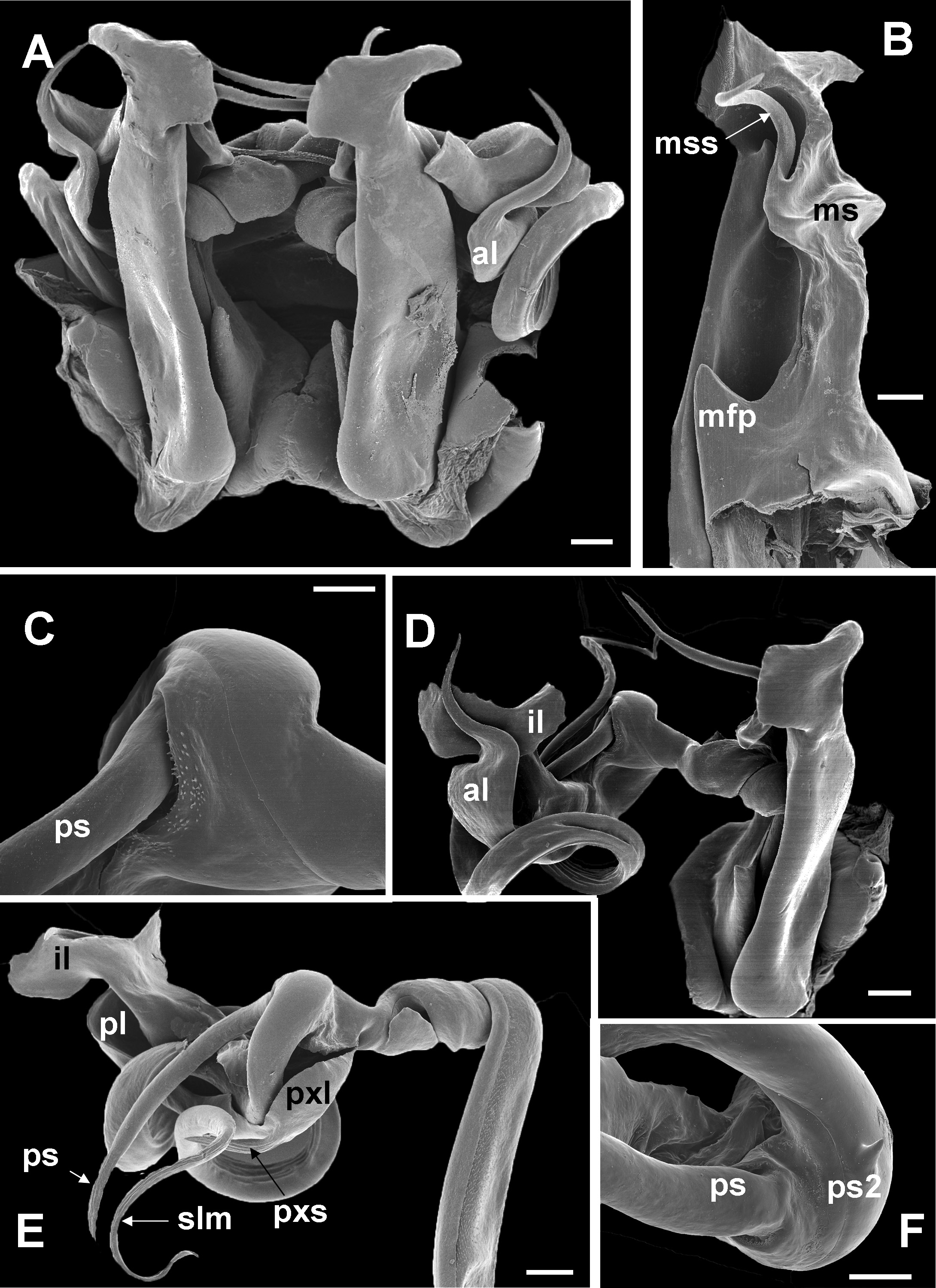
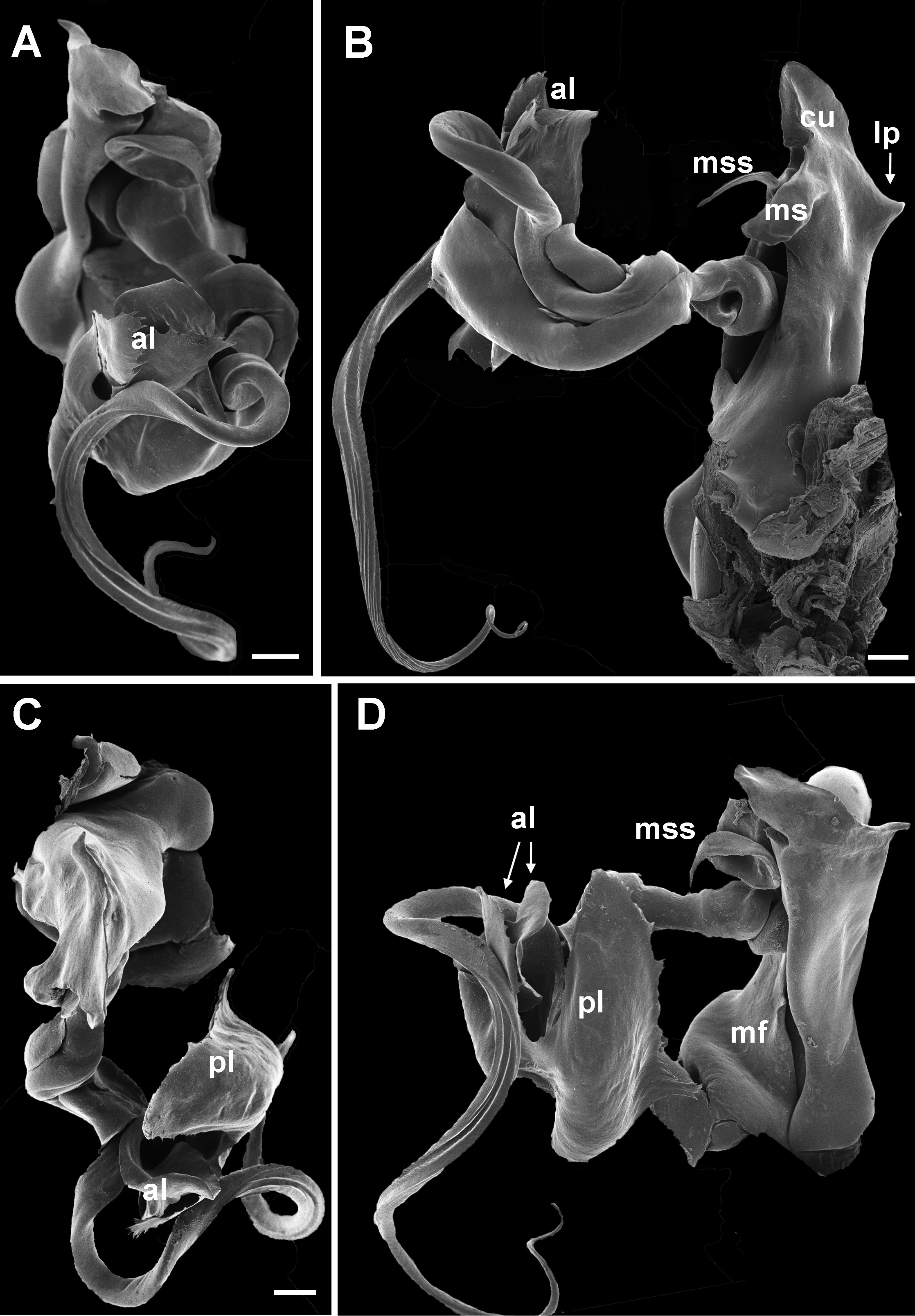
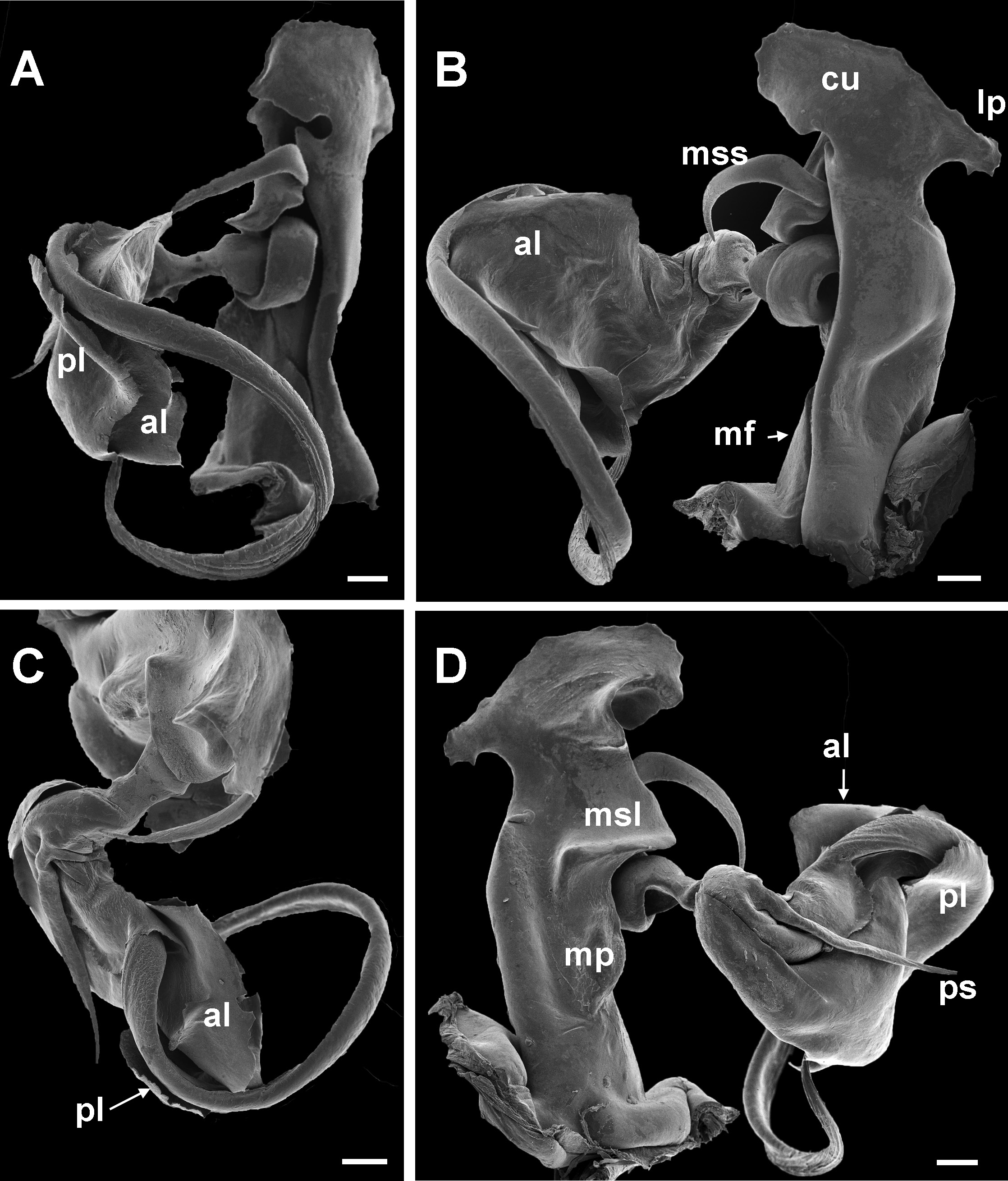
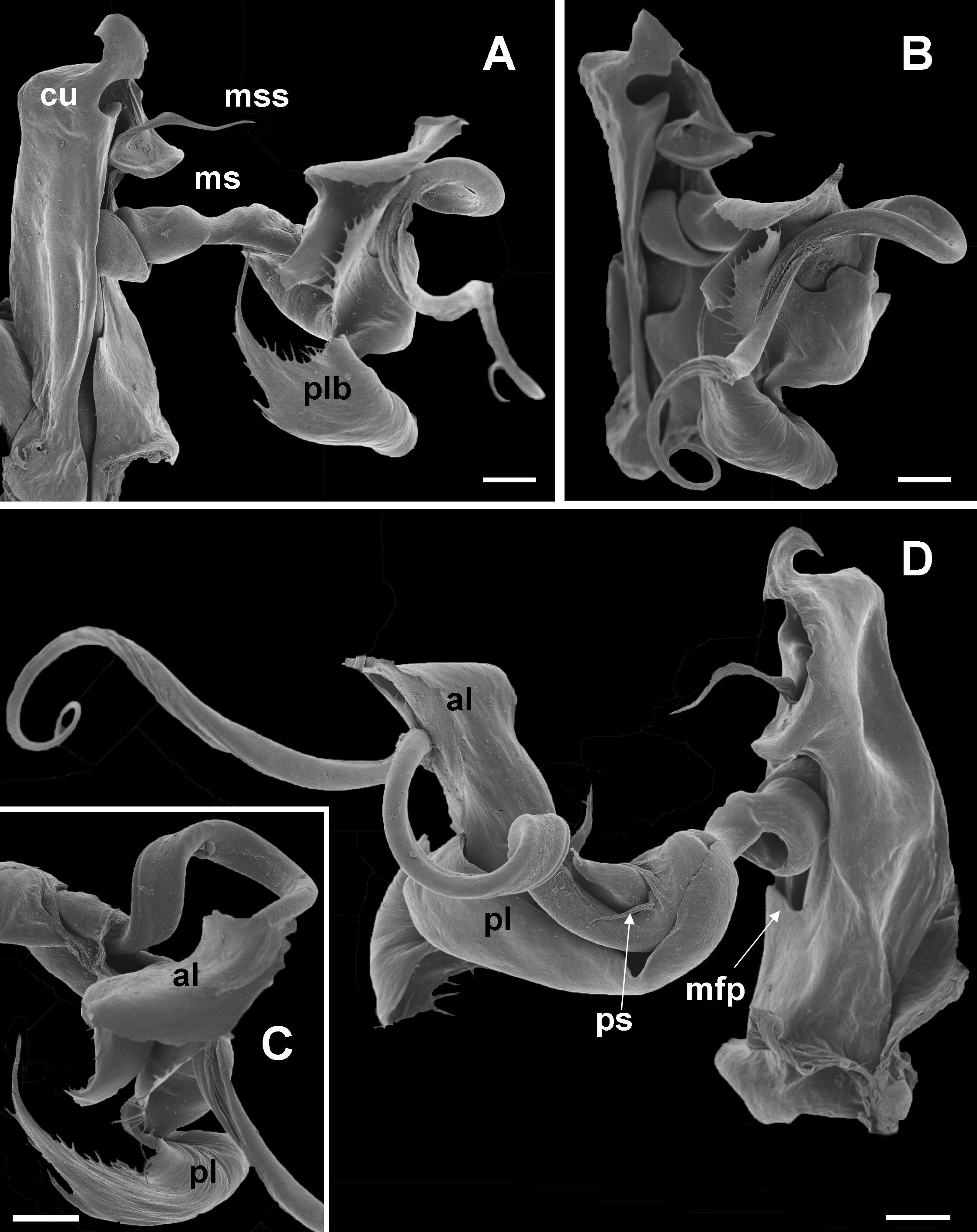
10 Main stem of telomere with thumblike process ( Fig. 12D View Fig ) ………………………… dabagaensis View in CoL – Main stem of telomere without thumblike process ( Fig. 13 View Fig ) …………………… quasimodo View in CoL sp. nov.
11 Proximal lobe of telomere with long, fluted spine ( Figs 10A View Fig , 11E View Fig ) …………………… netus View in CoL sp. nov. – Proximal lobe of telomere without a spine ………………………………………………………12
12 Lateral process of gonopod coxa small. Cucullus without a large mesal extension ( Fig. 17 View Fig ) ……………………………………………………………………………… mwanihanensis View in CoL sp. nov.
– Lateral process of gonopod coxa larger. Cucullus with a large mesal extension ( Figs 15–16 View Fig View Fig ) …13
13 Lateral process of gonopod coxa pointed ( Figs. 15A View Fig ) ……………………… scopus View in CoL sp. nov.
– Lateral process of gonopod coxa broadly rounded, irregularly dentate ( Fig. 16B View Fig ) ……………………………………………………………………………… nikolajscharffi View in CoL sp. nov.
14 Metaplical shelf-spine of gonopod coxa long, projecting ± mesad ( Figs 20 View Fig A–B, 23A, D) ………15 – Metaplical shelf-spine not long, projecting ± mesad ……………………………………………16
15 Posterior lamella of telomere divided into a horizontal lobe with spinose edges and a long, straight, distal spine-like part ( Fig. 20C, E View Fig ) …………………………………… nectarinia View in CoL sp. nov.
– Posterior lamella of telomere with strongly laciniate edges but without a long, straight, spine-like part ………………………………………………………………………… vandenspiegeli View in CoL sp. nov.
16 Metaplical shelf of gonopod coxa in two levels between which metaplical shelf-spine fits. Gonopod coxa with a small to inconspicuous lateral process ( Fig. 24 View Fig ) ………… vilici View in CoL sp. nov.
– Metaplical shelf in one level. Metaplical shelf-spine different …………………………………17
17 Gonopod coxa with distal hook-like extension ( Fig. 31A View Fig ). 41 podous rings, body diameter 1.6 mm ………………………………………………………………………………………… tintin View in CoL sp. nov.
– Gonopod coxa without distal hook-like extension. Mostly>41 podous rings and/or body diameter> 1.6 mm ………………………………………………………………………………18
18 Anal valves without dorsal spines ( Fig. 6D View Fig ) ……………………………………… teres View in CoL sp. nov.
– Each anal valve with a dorsal spine ……………………………………………………………19
19 Cucullus of gonopod coxa subrectangular in outline ( Fig. 26 View Fig B–C) ………… hamerae View in CoL sp. nov.
– Outline of cucullus more rounded ……………………………………………………………20
20 Anterior lamella of gonopod telomere with long, smooth spine ( Fig. 30 View Fig C–D) …… howelli View in CoL sp. nov.
– No such spine ……………………………………………………………………………………21
21 Anterior lamella of gonopod telomere divided into two triangular lobes with serrate margins ( Fig. 27B, E View Fig ). Posterior lamella simple …………………………………………… termini View in CoL sp. nov.
– Anterior lamella of gonopod telomere simple. Posterior lamella with a slender, spinose branch with a long, thin, bifurcate side branch ( Fig. 29E View Fig ) ………………………………… mwabvui View in CoL sp. nov.
Distribution and habitat
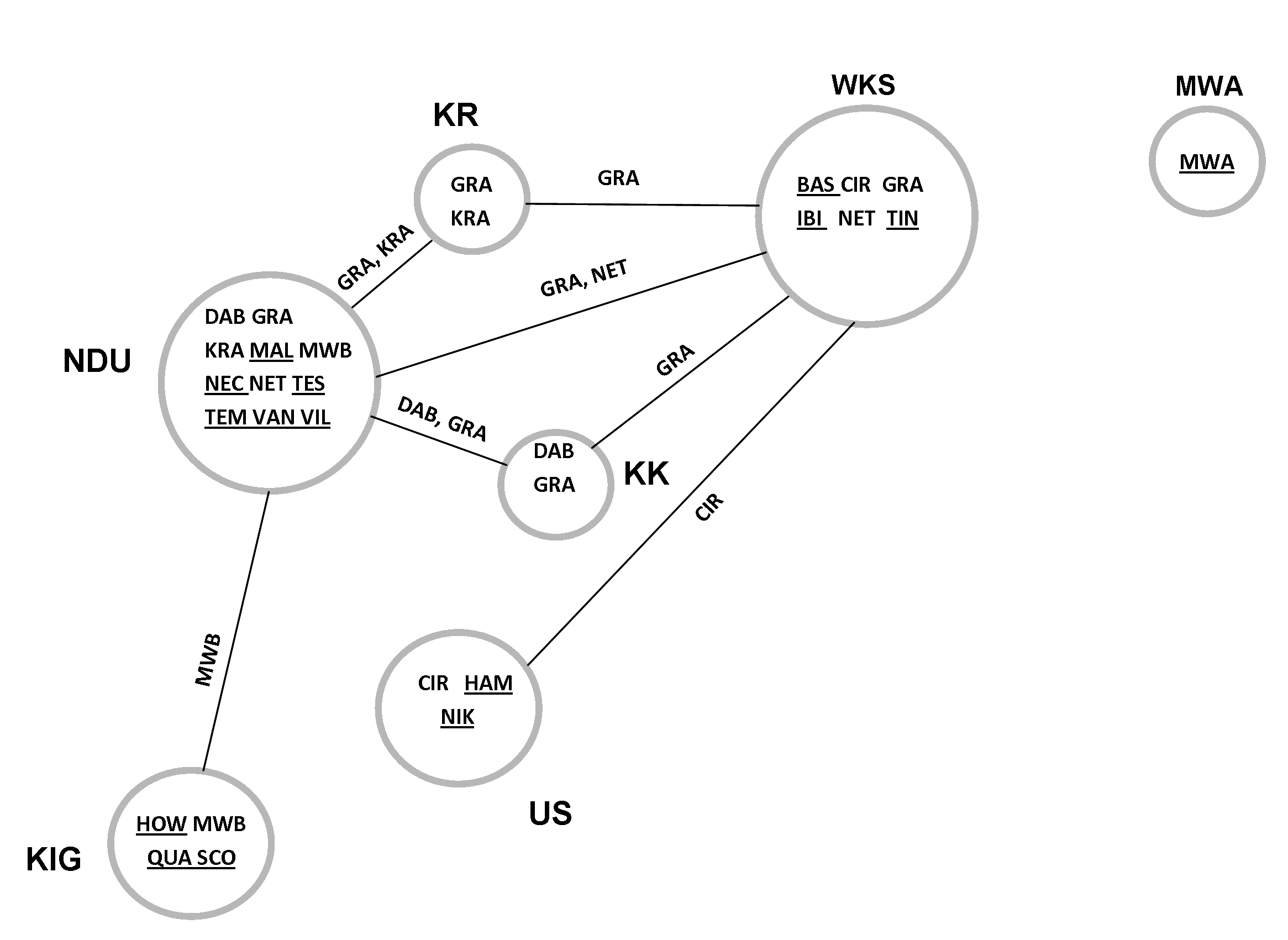
Fig. 32 View Fig shows the distribution of the 21 species of the Chaleponcus dabagaensis group plotted on a semi-diagrammatic map of the Forest Reserves (FR) from which species of the group have been collected. Fifteen of the species have been collected in only one FR, with New Dabaga/Ulangambi FR being the richest (6 exclusive species, 11 species in all).
Five species are shared between two FR, and C. gracilior sp. nov. even occurs in four FR, being clearly the most widespread of the species. The easternmost FR, Mwanihana, does not share any species with any other FR.
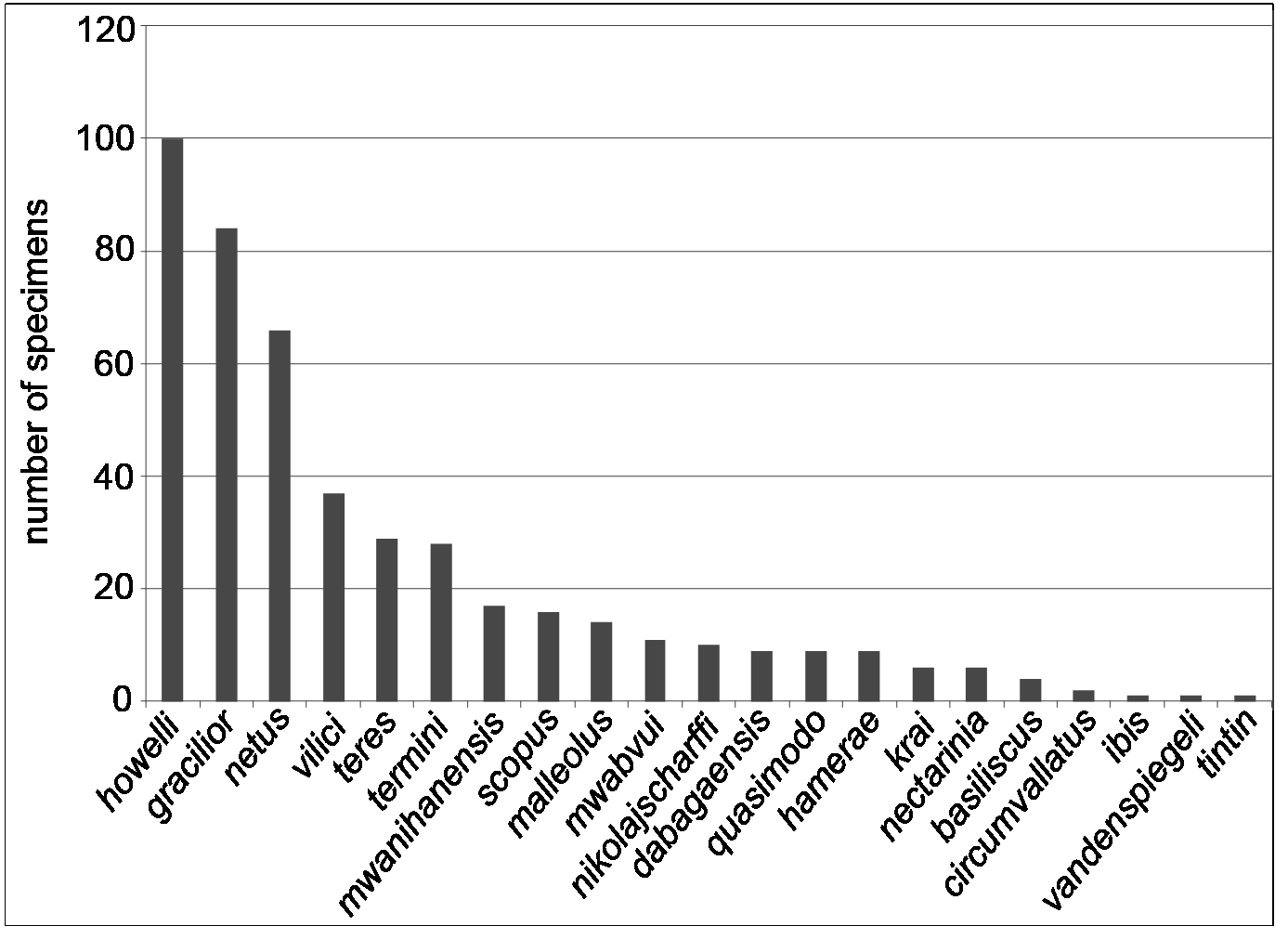
As Fig. 1 View Fig clearly shows, there are several forested areas in the Udzungwa Mts where the C. dabagaensis group has not been collected. This is probably due to a collecting bias, since the very comprehensive millipede material from the Udzungwa Mts kept in ZMUC largely derives from the FRs where the C. dabagaensis group is now known to occur. Additional collection effort, especially in the large southerncentral Matundu forest, is likely to reveal several additional species of the group. Even renewed collecting in the forest reserves already sampled is likely to reveal new species, since three ( ibis sp. nov., vandenspiegeli sp. nov., tintin sp. nov.) out of the 21 species are singletons and one ( circumvallatus sp. nov.) is a doubleton ( Fig. 33 View Fig ).
The Chaleponcus dabagaensis group consists of high-altitude species, all material having been collected at 1390–2100 m asl, and with the highest species diversity above 1700 m ( Table 2 View Table 2 ). The vast majority of specimens was collected in montane forest, but some species were found in disturbed habitats as well, e.g., the relatively widespread C. gracilior sp. nov. Very often several (up to 5) species were represented in a sample, but whether such coexisting species occupy different microhabitats is not known.
Two notes on general morphology
Torsion of the gonopod telopodite
Torsion of the gonopod telopodite is characteristic of the majority of species of Odontopygidae and the related family Spirostreptidae ( Kraus 1966, Hoffman 2008) and is important for the function of the gonopod (Barnett & Telford 1996). In all odontopygids examined by me the torsion always begins with an anteriad bend, i.e., the torsion is clockwise when followed from coxa towards the gonopod tip and viewed from a mesal point of view on the RIGHT gonopod. Published drawings by, e.g., Kraus (1966) and Frederiksen (2013a,b) confirm the generality of this pattern.
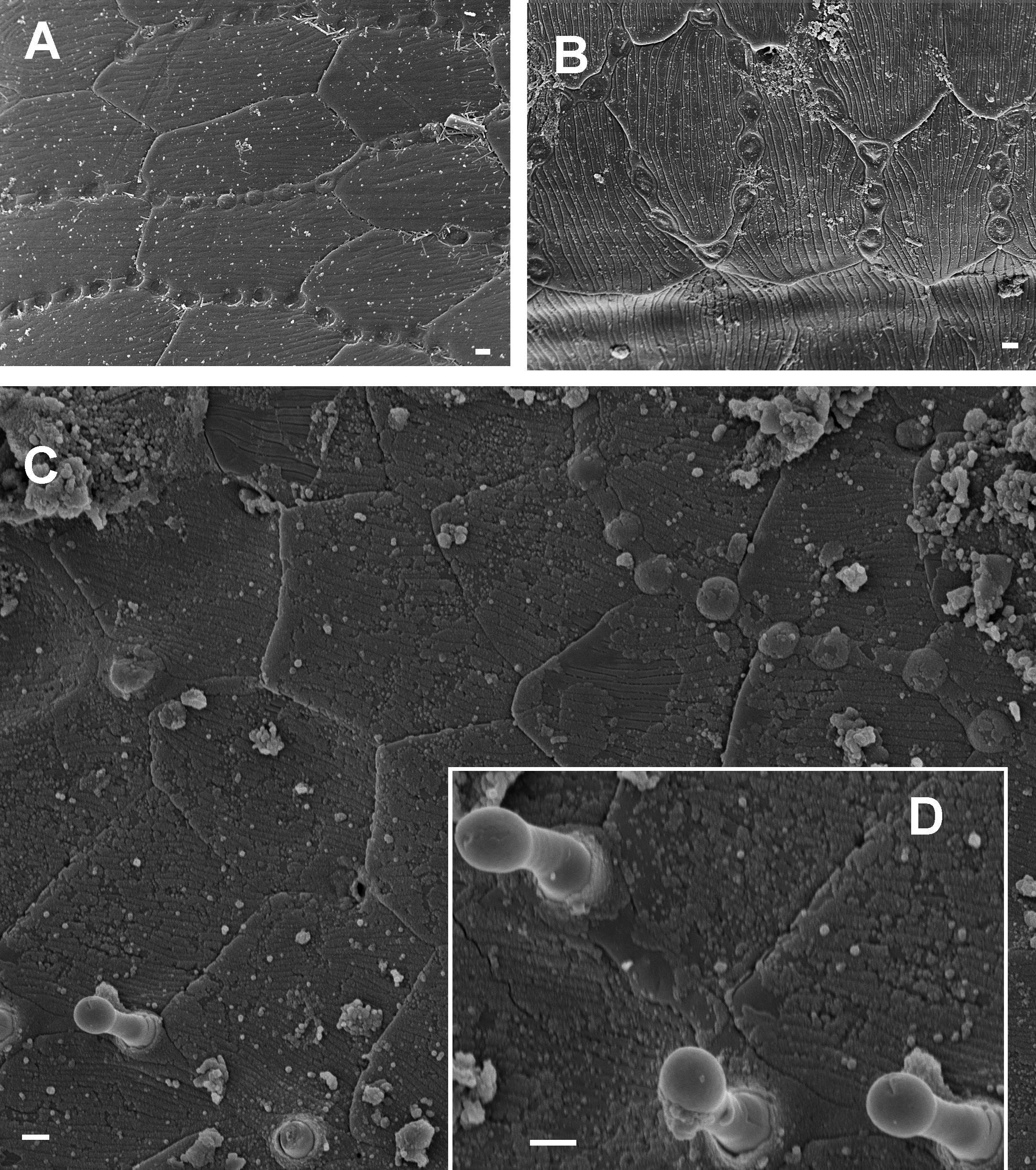
Intercalary cuticular microscutes
Very small ( ca. 1 µm) circular microstructures were frequently observed between cuticular microscutes on body rings ( Fig. 34 View Fig ). Similar structures were reported by Akkari & Enghoff (2011) from several families of the order Polydesmida and have also been observed on Lusitanipus alternans (Verhoeff, 1893) ( Callipodida : Dorypetalidae ) (HE and S. Reboleira unpublished), but have not been recorded from other millipede orders. Such intercalary cuticular microscutes are, however, discernible on fig. 8 of Frederiksen (2013a) ( Chaleponcus parensis Frederiksen, 2013 ), as well as on fig. 1 of Frederiksen (2013b) ( Lamelloramus rhombiformis Frederiksen, 2013b ). They have also been observed on a species of the genus Aquattuor Frederiksen, 2013 , and may be widespread in Odontopygidae . The nature of the microscutes is unknown. In a preparation of C. nikolajscharffi sp. nov. some of the microscutes give rise to projecting, club-shaped outgrowths of a few µm length ( Fig. 34D View Fig ) which may represent parasitic fungi of a kind?
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Archepyginae |
|
Tribe |
Prionopetalini |
|
Genus |