Gazella leptoceros (F. Cuvier, 1842 )
|
publication ID |
https://doi.org/10.1093/mspecies/sead007 |
|
publication LSID |
lsid:zoobank.org:pub:7ED7B9F8-DF5A-4416-9C6B-1731764BDA52 |
|
persistent identifier |
https://treatment.plazi.org/id/35047F0B-F035-247C-FF29-FBE1FEE791F7 |
|
treatment provided by |
Plazi |
|
scientific name |
Gazella leptoceros (F. Cuvier, 1842 ) |
| status |
|
Gazella leptoceros (F. Cuvier, 1842) View in CoL
Slender-horned Gazelle
Antilope leptoceros F. Cuvier, 1842 in É. Geoffroy Saint-Hilaire and F. Cuvier, 1842:2, plates 73 and 374. Type locality “rapportés du Sennaar [= Sennar, Sudan] par M. Burton;” corrected to “probably desert between Giza and Wadi Natron, lower Egypt ” by Flower (1932:438).
( Gazella) leptoceros : Temminck, 1853:193. First use of current name combination.
Leptoceros Abu Harab Fitzinger, 1869:160 . Type locality “Afr. Libysche Wüste” (= Libyan desert, Africa).
Leptoceros Cuvieri Fitzinger, 1869:160 . Type locality “Afr. Süd-Nubien, Bajuda Wüste, Berber, Setit, Taka, Senaar, Kordofän, Bahr-el-abiad” (= Bajuda desert along the Nile, in what is now the region of northern Sudan and southern Egypt).
Gazella Loderi Thomas, 1894a:452 . Type locality “ Sand-dunes of Le Souf , about 100 miles south from Biskra,” Algeria.
Gazella leptoceros loderi View in CoL : Sclater and Thomas, 1898:148. Name combination.
Gazella leptoceros typica Sclater and Thomas, 1898:149 . Usage equivalent to Gazelle leptoceros leptoceros View in CoL and not intended as a new name.
CONTEXT AND CONTENT. Order Artiodactyla, suborder Ruminantia, infraorder Pecora, family Bovidae, subfamily Antilopinae, tribe Antilopini ( Burgin et al. 2020). No subspecies are currently recognized ( Groves and Grubb 2011; Burgin et al. 2020).
NOMENCLATURAL NOTES. Taxonomic affiliations of Gazella leptoceros have been revised multiple times since the 1960s and are yet to be fully resolved. Two subspecies of G. leptoceros have been named but are not currently recognized: eastern G. l. leptoceros from Egypt and Libya and western G. l. loderi from Algeria and Tunisia ( Groves and Grubb 2011; Mallon et al. 2020). The named forms were never clearly distinguished (Lydekker and Blaine 1914; Lavauden 1926), and their allopatry was inferred but never defined; their separation may be a recent result of human pressures causing range fragmentation (Convention on International Trade in Endangered Species of Wild Flora and Fauna [CITES] 2007). Sparse genetic sampling from eastern populations ( n = 2 from Egypt, none from Libya) and overall low degree of genetic difference between eastern and western populations did not provide sufficient evidence for Mallon et al. ( 2020) to change the monotypic treatment of G. leptoceros.
Ellerman and Morrison-Scott (1966) included Gazella marica (sand gazelle) as a subspecies of G. leptoceros because the taxa share pale coloration and indistinct markings, elongated hooves, and some skull characters. This was supported by discriminant function analysis of four Egyptian G. leptoceros skulls by Groves (1969), which demonstrated that this eastern population (given as G. l. leptoceros ) clustered closer to Arabian G. marica than to Algerian G. leptoceros ( loderi ). Groves (1969) suggested that G. leptoceros might belong to the same subgenus as G. ( Trachelocele) subgutturosa (including G. marica ). Lange (1972) proposed G. leptoceros as a subspecies of G. subgutturosa (Persian gazelle— Kingswood and Blank 1996) along with G. marica , and Effron et al. (1976) noted similar karyotypes between G. subgutturosa (sensu lato) and G. leptoceros , particularly in the large size of the Y2 chromosome.
Karyotypic similarities led Kumamoto and Bogart (1984) to suggest that G. leptoceros (western population) and G. cuvieri (Cuvier’s gazelle) might be conspecific; Vassart et al. (1995) also identified identical karyotypes between the two species. Genetic sequencing of nuclear genes and mitochondrial cytochrome b confirmed close kinship between G. leptoceros and G. cuvieri ( Rebholz and Harley 1999; Lerp et al. 2016). Similarities in mitochondrial DNA led Hassanin et al. (2012) to suggest that both G. leptoceros and G. marica be considered as subspecies of G. cuvieri . Deeper genetic analysis (using both nuclear and mitochondrial genes) showed Algerian G. leptoceros and G. cuvieri to form a monophyletic group (53 G. leptoceros and 255 G. cuvieri samples, 12 haplotypes, exhibiting 0.3% divergence) to the exclusion of Egyptian G. leptoceros (two samples, one haplotype identified, 1.4% genetic divergence from the other samples— Silva et al. 2017). These authors proposed that Algerian G. leptoceros and G. cuvieri be considered as allopatric ecotypes (lowland and mountain) of one species ( G. cuvieri — Silva et al. 2017).
Molecular (e.g., Wacher et al. 2010; Hassanin et al. 2012), cytogenetic (e.g., Vassart et al. 1995; Cernohorska et al. 2015), and phylogenetic (e.g., Lerp et al. 2016) analyses support a clade comprised of G. leptoceros , G. cuvieri , and G. marica . However, given the physical and ecological differences between G. leptoceros , G. cuvieri , and G. marica , these three taxa continue to be treated separately (as distinct evolutionary significant units) for conservation planning ( Lerp et al. 2016; Silva et al. 2017; Mallon et al. 2020). Further study is needed to elucidate the relationships between the two populations of G. leptoceros , their relatedness to G. cuvieri and G. marica , and the resolution of species within this clade ( Mallon et al. 2020). The present account follows the arrangement of Burgin et al. (2020), covering G. leptoceros (both western and eastern populations) to the exclusion of G. cuvieri and G. marica .
The genus Gazella and the common name “gazelle” are derived from the Arabic name “ghazal” ( Kingswood and Blank 1996). The specific epithet comes from the Greek leptós meaning thin or narrow and ceros meaning horn—a description reflected in the common English name slender-horned gazelle ( Gotch 1995). Besides slender-horned gazelle, other common English names include Loder’s gazelle (cf. Thomas, 1894a; Sclater and Thomas 1898) and sand gazelle (sometimes African sand gazelle or Algerian sand gazelle to distinguish from G. marica , the Arabian sand gazelle— Devillers et al. 2006). The Arabic name rim (variously spelled rhim, rhime, reem, rheem) has been adopted (as rhim) in English and French to refer specifically to G. leptoceros ( Mallon et al. 2020) . However, in Arabic this name is also used for other gazelle species (e.g., in Arabia, it refers to G. marica — Lydekker and Blaine 1914). Other Arabic names for G. leptoceros include ghazal abiad, meaning white gazelle, and abu el haráb (alternately abu el harabat— Sclater and Thomas 1898); an early preference was noted for the use of rim in Algeria and ghazal abiad in Tunisia and Egypt ( Sclater and Thomas 1898; Joleaud 1929; Ellerman and Morrison-Scott 1966). The species is known as adam (alternately edemi) or hankut in the Tamacheq language ( Devillers et al. 2006; Mallon et al. 2020) and ezim at the Sioua [Siwa] Oasis in Libya ( Joleaud 1929). In French, it is gazelle des dunes or gazelle des sables (both meaning “sand gazelle”), gazelle leptocère or gazelle à cornes fines (“slender-horned gazelle”), gazelle blanche (“white gazelle”), or rhim; in German, Dünengazelle (“dune gazelle”); and in Spanish, gacela de las dunas (“dune gazelle”) or gacela de astas delgadas (“slender-horned gazelle”— Mallon et al. 2020).
DIAGNOSIS
Gazelles are a widespread group of antelope divided generally by size into three genera: the smaller desert-dwelling species of Gazella , the intermediate-sized savannah-dwelling species of Eudorcas , and the larger species of Nanger ( Groves and Leslie 2011) . In the most recent taxonomy, Gazella contained 21 species distributed from the Sahara Desert in Africa to the Gobi Desert in Asia ( Burgin et al. 2020), characterized by ridged horns, distinctive dark stripes on the face, and a lateral band across the flanks. Among the five African species of Gazella ( G. cuvieri , G. dorcas [dorcas gazelle— Yom-Tom et al. 1995], G. leptoceros , G. pelzelni [Pelzeln’s gazelle, formerly a subspecies of G. dorcas ], and G. spekei [Speke’s gazelle]— Burgin et al. 2020), G. leptoceros has the palest body coloration, varying from pale buff to nearly white ( Fig. 1 View Fig ; Thomas 1894b; Osborn and Helmy 1980). Diagnostically, the typical gazelle markings on the face and body of G. leptoceros are pale and poorly defined ( Sclater and Thomas 1898; Lydekker and Blaine 1914; Osborn and Helmy 1980). The lambdoid (or occipitoparietal) suture of the skull is rounded in both Algerian and Egyptian G. leptoceros populations; in all other members of Gazella the suture forms angles at both ends of its upper aspect ( Groves and Harrison 1967; Groves 1969).
Among species of Gazella , G. leptoceros is sympatric only with G. dorcas . Gazella leptoceros is always pale in color, whereas G. dorcas is typically a richer, darker brown and has a distinct dark band on the flanks, although paler individuals are known ( Mallon et al. 2020). The elongated, upright horns of male G. leptoceros are straight in frontal view, diverging to varying extents at the tips, while the horns are distinctly lyrate in male G. dorcas ( Ellerman and Morrison-Scott 1966; Osborn and Helmy 1980). Females (especially young individuals) of the two species can be more challenging to distinguish in the field; facial markings of G. leptoceros are consistently paler and lack rufous tones seen on G. dorcas ( Sclater and Thomas 1898; Osborn and Helmy 1980). In addition to the lambdoid suture, key skull features that distinguish G. leptoceros include a deep infraorbital fossa with elongated fenestra (the fossa is shallow with no fenestration in G. dorcas ), two foramina in the supraorbital pit (a single foramen present in G. dorcas ), and rounded (rather than triangular) posterior margins of the nasals ( Osborn and Helmy 1980).
Gazella leptoceros and G. marica are frequently confused because of their similar coloration, elongated hooves, and habitat preference (they also share the same Arabic name, rim); some authors have considered them synonymous (e.g., Ellerman and Morrison-Scott 1966). Gazella leptoceros is overall larger and males lack the throat swelling of G. marica ( Ellerman and Morrison-Scott 1966) . Horns of G. leptoceros differ from those of G. marica in they lack significant curvature, have parallel horn bases, and rise nearly vertically (rather than at an angle) from the skull ( Groves and Harrison 1967).
GENERAL CHARACTERS
Gazella leptoceros is a medium-sized gazelle ( Mallon et al. 2020). Fully grown male specimens from Egypt weighed 16.5–17.5 kg ( Flower 1932), and an additional individual (sex unreported) from Egypt weighed 15.0 kg ( Osborn and Helmy 1980). One adult male from Algeria with entrails removed weighed 15 kg ( Loder 1894), and a captive female at the San Diego Zoo (from Tunisian stock) weighed 12.5 kg ( Benirschke 2002). Lavauden (1926) reported unusually large sizes for G. leptoceros in Algeria ( 60 kg for large males), disagreeing with contemporary authors but providing no explanation for this discrepancy. General body weights (without specimen references) are reported to be 20–27 kg for adult males and 14–18 kg for adult females ( Devillers et al. 2006; Mallon et al. 2020). Head–body length of four Egyptian G. leptoceros males was 885–999 mm (mean 937 mm), shoulder height 696–752 mm (mean 718 mm), and tail length 155–166 mm (mean 162 mm — Osborn and Helmy 1980). Measurements from a single Egyptian female were head– body length 955 mm, shoulder height 697 mm, and tail length 125 mm ( Osborn and Helmy 1980). Length of hind foot was 300–335 mm (mean 316 mm) from five Egyptian specimens ( Osborn and Helmy 1980). Sizes of individuals from Tunisia and Algeria, inferred from skull measurements, were slightly larger than those from Egypt ( Sclater and Thomas 1898; Osborn and Helmy 1980).
The short-haired pelage of G. leptoceros is an overall pale sandy-fawn color ( Sclater and Thomas 1898; Flower 1932; Groves 1969). A faintly darker stripe (“pale sandy with a tinge of brownish”— Sclater and Thomas 1898:137) with indistinct borders runs along the flanks between the front and rear legs; a band dorsal to this stripe is only slightly paler than the dorsum ( Sclater and Thomas 1898; Anderson and de Winton 1902; Lydekker and Blaine 1914). Typical facial markings of the genus Gazella are very pale in G. leptoceros : the central facial blaze and darker streaks from eyes to muzzle are sandy in color, with no rufous or brown hairs ( Osborn and Helmy 1980). Areas around eyes, the muzzle, and sides of face are white ( Cuvier 1842). Ears are very pale externally and are long, narrow, and pointed; ear length is 13–15 cm ( Cuvier 1842; Sclater and Thomas 1898; Osborn and Helmy 1980; Devillers et al. 2006). Throat, chest, and belly are white ( Cuvier 1842; Osborn and Helmy 1980). Fronts of forelegs are the same sandy buff as the body with white inner surfaces; hind legs are paler and may be fully white ( Thomas 1894b). Rump is white, and the indistinct pygal borders are only slightly darker than the sandy-colored back (Lydekker and Blaine 1914; Osborn and Helmy 1980). Tail is brownish-black ( Thomas 1894b; Sclater and Thomas 1898; Lydekker and Blaine 1914). Coloration does not differ markedly between sexes or age classes ( Cuvier 1842). Individuals from Egypt are “somewhat smaller and darker” than those from Algeria and Tunisia ( Osborn and Helmy 1980: 493).
Inguinal pockets, containing inguinal glands, are present in males and females; interdigital and lacrimal glands are also present ( Cuvier 1842; Osborn and Helmy 1980). Short but distinct “knee brushes” of dark hair occur on carpal joints of front legs ( Sclater and Thomas 1898; Osborn and Helmy 1980). Stiff hairs between dew claws and hooves are brown to blackish ( Fitzinger 1869; Osborn and Helmy 1980). There are two mammae ( Cuvier 1842).
Both sexes have the characteristic slender horns that give G. leptoceros its scientific and common names. In males, horns are generally straight with a slight backward bend; in mature individuals, horns possess 24–30 encircling ridges that become less pronounced approaching the smooth tips ( Fig. 2 View Fig ; Pease 1896; Sclater and Thomas 1898; Lydekker and Blaine 1914; Osborn and Helmy 1980). Horn bases are close together ( 14 mm apart in the type specimen of G. l. loderi ) and nearly circular; basal circumference is 89–114 mm ( Thomas 1894b; Lydekker and Blaine 1914; Groves 1969). Horns form an evenly tapered profile with a variable degree of divergence; tip-to-tip distance can measure 89–260 mm (Lydekker and Blaine 1914). In general, horns of mature males are roughly twice the length of the skull (Lydekker and Blaine 1914). Six adult male specimens from Algeria and Tunisia (labeled as G. l. loderi ) had an average horn length of 297.5 mm ± 15.8 SD; six comparable specimens from Egypt (labeled as G. l. leptoceros ) had overall longer horns, with an average length of 337.3 ± 28.3 mm ( Groves 1969). Flower (1932:439) reported that “good horns of male leptoceros in Egypt are from 360 to 395 mm,” and horns from three skulls from Egypt examined by Osborn and Helmy (1980) averaged 326.6 mm (range 294–366 mm). Maximum recorded horn length is 412 mm (from Egypt — Beudels and Devillers 2013); the longest horn from Tunisia measured 403 mm (Lydekker and Blaine 1914; Devillers et al. 2006). Horns of females are significantly thinner than those of males, although they approach similar lengths (78–83.5% of male horn length in Egypt and Algeria, respectively— Sclater and Thomas 1898; Groves 1969). Horn profile in females tends to be particularly straight, with the surface annulations being much shallower and less distinct ( Anderson and de Winton 1902; Osborn and Helmy 1980). The thin horns of females are prone to deformities, both in the wild ( Meliane et al. 2023b) and under human care. As with males, a location-based trend is seen in female horn length, with western populations generally having shorter horns: mean 248.4 ± 19.0 mm (from five skulls) compared to 273.0 mm (mean of two skulls) from eastern populations ( Groves 1969).
DISTRIBUTION
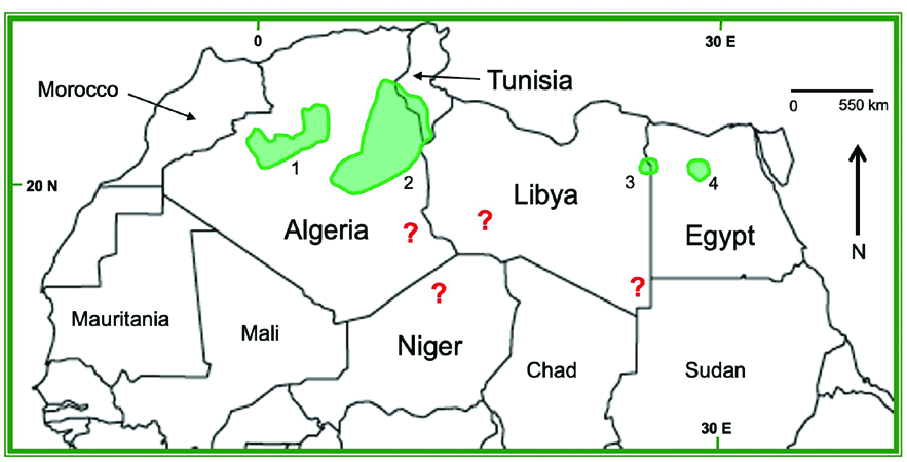
Gazella leptoceros is sparsely distributed throughout the Sahara Desert in northern Africa from longitude W 4°20ʹ east to the Nile River ( Flower 1932; Mallon et al. 2020). Confirmed records of occurrence, collected from 2000 to 2020, are limited to Algeria, Tunisia, Libya, and Egypt ( Fig. 3 View Fig ; Mallon et al.
2020), with the current extent of known occurrence covering an estimated 182,005 km 2 ( Durant et al. 2014). In Algeria and Tunisia, distribution of G. leptoceros closely matches the large ergs (sand seas) south of the Atlas Mountains, particularly the Grand Erg Occidental (Great Western Sand Sea) of northwestern Algeria and the Grand Erg Oriental (Great Eastern Sand Sea) that straddles the border of Algeria and Tunisia ( Devillers et al. 2006; Mallon et al. 2020). Gazella leptoceros was once present in the Erg Iguidi in western Algeria but is now extirpated ( De Smet and Smith 2001). It was historically found in the Erg d’Admer (southeastern Algeria) where it was noted as rare by Lavauden (1926); there have been no confirmed observations in recent decades (CITES 2007). In the Tunisian Grand Erg Oriental, a ground survey in October 2020 directly observed and recorded signs of G. leptoceros on three occasions between Jbil and Senghar-Jabbes national parks, but an aerial survey in October 2019 did not find G. leptoceros south of Senghar-Jabbes National Park (although unspecified ungulate spoor was observed— Meliane et al. 2023a). Distribution in Egypt and Libya has contracted markedly over the past century: G. leptoceros was formerly found throughout the western desert of Egypt ( Saleh 2001) and in numerous localities in Fezzan of southwestern Libya (e.g., Erg Edeyen— Lavauden 1926) into the 1950s ( Setzer 1957; Khattabi and Mallon 2001). Most recent records from Egypt come from scattered localities in the Qattara Depression and areas west of the Siwa Oasis adjacent to the Libyan border ( El Alqamy and El Din 2006). There are no confirmed data on current areas of occurrence in Libya ( Khattabi and Mallon 2001; Mallon et al. 2020).
Limits of distribution of G. leptoceros are unclear. A single record of a male G. leptoceros shot in northeastern Morocco in 1954 has since been attributed as either misidentification or an accidental occurrence ( Devillers et al. 2006; Mallon et al. 2020). There are no records of G. leptoceros from southwestern Algeria or Mauritania ( Devillers et al. 2006; Mallon et al. 2020). Although Devillers et al. (2006) suggested that the distribution might extend into Mali based on proximity to appropriate habitat in Algeria, there are no records to confirm this. In the central Sahara, G. leptoceros was present in northern Niger (Ténéré) and northern Chad (near Tibesti) as recently as the 1970s; modern records in these areas are scant and unconfirmed and might represent transitive movements from the north ( Devillers et al. 2006; Mallon et al. 2020). Further historical records suggest past presence in southeastern Libya and northeastern Chad ( Devillers et al. 2006; Mallon et al. 2020). There are no confirmed records from northwestern Sudan ( Devillers et al. 2006; Mallon et al. 2020)—the type locality originally given from southeastern Sudan by F. Cuvier (1842) was corrected by Flower (1932) to lower Egypt.
FOSSIL RECORD
Distinct antelope-like species were abundant in Eurasia during the middle Miocene 14–15 million years ago (mya; e.g., Korotkevich 1968; Leslie et al. 2010; Zang and Yang 2016). Pilgrim (1939) considered Central Asia the evolutionary center of gazelle-like forms, but recent phylogenetic analyses suggested that Gazella originated in the Middle East ( Lerp et al. 2016). Gazella has been the most species-rich and widespread genus in the tribe Antilopini , occurring throughout Africa and Eurasia ( Bibi et al. 2009; Zang andYang 2016). Earliest fossils of Gazella date to the mid-Miocene of Fort Ternan, Kenya, about 14 mya ( Gentry 1970, 2010; Shipman et al. 1981). Gazella praegaudryi was named from fossils (from ca. 10 mya) found near Bou Hanifia, Algeria ( Arambourg 1959; Zang and Yang 2016), just north of the current distribution of G. leptoceros ( Fig. 3 View Fig ).
Gazella leptoceros is absent from the Quaternary fossil assemblage in Algeria (along with other desert specialists such as addax [ Addax nasomaculatus] and fennec fox [ Vulpes zerda ]), although there are some similarities to the extinct G. subkevella known from the region ( Lavauden 1926; Joleaud 1929). The fossil species G. triquetricornis , known from Algeria, has affinities to G. bennettii (chinkara or Indian gazelle) and might represent an intermediate form between Asian gazelles (with horns only in males) and African species (horns in both sexes— Joleaud 1929). The absence of G. leptoceros fossils is supported by phylogenetic work that suggests that it arose relatively recently in the upper Pleistocene ( Joleaud 1929; Lerp et al. 2016).
FORM AND FUNCTION
The skull of Gazella leptoceros is “of normal proportions” for the genus Gazella ( Fig. 2 View Fig ; Sclater and Thomas 1898:138). Thomas (1894b:470) compared the skull of the type specimen of G. leptoceros with that of G. bennettii and found it similar in size and proportion, “but rather lighter and more delicate.” Series of skull measurements are presented in Groves (1969), Osborn and Helmy (1980), and Thomas (1894b). Horn cores are conspicuously pitted and have longitudinal grooves running from base to tip ( Osborn and Helmy 1980). Premaxillary bones articulate broadly with the nasals ( Sclater and Thomas 1898).
Dental formula of G. leptoceros is typical of the family Bovidae ( Groves and Leslie 2011) : i 0/3, c 0/1, p 3/3, m 3/3, total 32. Dental mesowear patterns from five wild-caught museum specimens (localities unspecified) showed unequal wear of upper molars depending on their position ( Louys et al. 2011). For M1, 60% of the teeth examined had blunt cusps, 20% had rounded cusps, and 20% had sharp cusps. A similar proportion (58.3%) of M2 had blunt cusps, and the remainder (41.7%) had sharp cusps. No samples were available for M3 or lower molars. This tooth wear pattern resembles that of a browser despite G. leptoceros generally being considered as a mixed feeder ( Louys et al. 2011).
Hooves of G. leptoceros are longer and more splayed than in the similar G. cuvieri and G. dorcas ( Lavauden 1926; Schomber and Kock 1960; Osborn and Helmy 1980; Newby 1984), but these differences are generally attributed to environmental effects (wear pattern caused by walking on soft sand) rather than direct adaptations to desert existence ( Osborn and Helmy 1980). In Egypt, length of hind foot hoof from G. leptoceros specimens taken in hard desert was notably shorter ( 45–55 mm, mean 50.5 mm, from four males) than from specimens from sandy desert ( 60 mm, one male — Osborn and Helmy 1980); a male from sand dunes in Algeria had a hind hoof length of 56 mm ( Thomas 1894b). Forehoof measurements showed greater variability than hind hooves ( Osborn and Helmy 1980). Hooves of G. leptoceros are very narrow ( Thomas 1894b; Lavauden 1926), unlike the sympatric but heavier addax, whose widened hooves provide increased surface area and are believed to prevent the addax from sinking in sand ( Newby 1984; Krausman and Casey 2007). The lateral hoof on each foot of G. leptoceros is broader than the medial hoof, and the angle of wear suggests the hooves are habitually splayed ( Thomas 1894b; Newby 1984; Beudels and Devillers 2013). The footprints of G. leptoceros are considerably larger and more pointed than those of the sympatric G. dorcas ( Loder 1894; Newby 1984; Mallon et al. 2020).
Captive G. leptoceros in Riyadh, Saudi Arabia (of unreported provenance, and potentially attributable to G. marica ) tolerated average daily temperatures in summer of over 35°C and were used to study key physiological adaptations to thermal stress ( Babor et al. 2014). Average rectal temperature in a combined sample of 24 G. leptoceros and G. gazella (mountain gazelle) fluctuated between seasons, with an increase of 1.20°C ± 0.20 SE from winter to summer; average skin temperature was 10.80 ± 0.60°C warmer in summer compared to winter ( Babor et al. 2014). These changes resulted in significantly lower thermal gradients (rectal-to-skin, skin-to-ambient, and rectal-to-ambient) in summer compared with winter ( Babor et al. 2014). Lack of physiological disruption caused by these thermal changes suggested that heterothermy was actively used to reduce evaporative water loss ( Babor et al. 2014).
Hematological changes between winter and summer conditions demonstrated significantly higher serum albumin, serum glucose, and serum haptoglobin levels in summer and concurrent declines in red blood cell count, hemoglobin concentrations, and total protein concentrations ( Babor et al. 2014). Lower erythrocyte indices may be a function of reduced oxygen demand and energy requirements during hot conditions; the observed increased albumin levels may help maintain blood volume and may also have antioxidant properties, providing protection from free radicals produced from heat stress ( Babor et al. 2014). Although some authors have claimed that the pale sandcolored pelage serves to reflect heat, this is generally discounted; the principal benefit of the color pattern appears to be its cryptic function ( Newby 1984).
ONTOGENY AND REPRODUCTION
Ontogeny.— Neonatal Gazella leptoceros resemble adults in their markings but tend to be even paler ( Fig. 4 View Fig ; Cuvier 1842). Birth weight of one male at Hannover Zoo, Germany, was 1.835 kg; typical initial weight gain is 60–80 g /day, with birth weight doubling in the first month ( Dittrich 1969). During the first 2 weeks of life, neonates are cached in hiding spots while their mothers forage ( Dittrich 1968). A mother returns to her cached offspring 3–4 times daily to nurse, groom, and stimulate urination and defecation; any urine or feces that are produced are consumed ( Dittrich 1968). Infants begin to test solid foods at 4 weeks of age and weaning occurs at 4–6 months ( Dittrich 1968).
Growth and maturation are rapid. One female born at the Hannover Zoo gave birth to her first offspring at 10.6 months, indicating conception, and thus sexual maturity, was achieved at 5.1 months of age ( Dittrich 1972). Among males, signs of maturity and lack of tolerance by mature males occur at 8–10 months of age ( Abáigar et al. 2009). Mean generation time of G. leptoceros in the North American population monitored by the Association of Zoos and Aquariums (AZA) was 3.8 years ( Randle 2021). Females have reproduced up to age 11; males do not appear to experience reproductive senescence and have sired offspring to maximum longevity ( Randle 2021).
Reproduction.— Reproduction in the wild is largely seasonal. Births of wild Gazella leptoceros occur primarily in winter and early spring ( Saleh 2001). Among 17 captive births between 1899 and 1924 in the Giza Zoological Gardens, Egypt, 13 occurred in February–April, three in May–June, and one in October ( Flower 1932). Early reports from the western desert of Egypt stated that fawns were most common in November–December, when they were hunted with dogs ( Sclater and Thomas 1898). Mallon et al (2020) provided one report (location unspecified) that 33% of births occurred in October–November. Births at Sidi Toui, Tunisia, occurred in February–April ( Mallon et al. 2020); elsewhere in Tunisia, the peak birthing season was in January–February ( Smith et al. 2001). At the Brezina Breeding Station, Algeria, 60% of 10 births in 2000–2008 occurred in February, with two births each in March and April ( Abáigar et al. 2009). Based on typical gestation lengths, the peak of rut (breeding season) is believed to occur in August–September in Tunisia ( Smith et al. 2001) and September–November in Brezina, Algeria ( Abáigar et al. 2009). In captivity outside of the natural geographical distribution, reproductive seasonality diminishes ( Dittrich 1968).
Gestation lengths of G. leptoceros from two documented pregnancies at the Hannover Zoo, Germany, were 156 days (female offspring produced) and 169 days (male offspring), or about 5.5 months ( Dittrich 1972). Observations at the Giza Zoo, Egypt, similarly suggested that gestation lasted “not more than 5½ months and may be less, i.e., under 167 days” ( Flower 1932:439). One offspring per litter is common in G. leptoceros from Egypt: multiple offspring were not observed among 17 births in the Giza Zoo ( Flower 1932). Few clear data exist from western populations ( Algeria and Tunisia), but early reports suggested that twinning was common ( Sclater and Thomas 1898). This trend of twinning has also been noted in recent publications (e.g., Devillers et al. 2006; Lerp et al. 2016; Mallon et al. 2020) and both twins and singletons have occurred in a captive group at the Brezina Breeding Station, Algeria ( Abáigar et al. 2009). Median litter size in captive G. leptoceros in North America (descended from Tunisian founders) is one, but twins have occurred ( Fig. 4 View Fig ; Benirschke 2002; Randle 2021).
Labor is generally quick (1.5 h or less), although Dittrich (1968) observed one parous female in labor for 10 h between the first contractions and delivery of the offspring. The placenta is passed within an hour of birth and is typically consumed by the dam ( Dittrich 1968). Based on one full-term placenta and one uterine necropsy from captive G. leptoceros at the San Diego Zoo, California, the placenta is epitheliochorial, villous, and polycotyledonary ( Benirschke 2002). Forty to 57 ellipsoid cotyledons, each measuring 0.5–2.5 cm, develop in four rows. Implantation is apparently more common in the left uterine horn, and there is no invasion of the endometrium by the trophoblast. One placenta from a term gestation weighed 70 g; its greatest dimensions were 17 by 4 cm ( Benirschke 2002). At Hannover Zoo, Germany, females did not experience an immediate postpartum estrus and began cycling about 26 days after giving birth, but typically did not conceive on this first cycle ( Dittrich 1968).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Gazella leptoceros (F. Cuvier, 1842 )
| Huffman, Brent A., Leslie, David M. & Jr. 2023 |
Gazella leptoceros loderi
| Sclater and Thomas 1898: 148 |
Gazella leptoceros typica
| Sclater P. L. & Thomas O. 1898: 149 |
Gazella Loderi
| Thomas 1894: 452 |
Leptoceros Abu Harab Fitzinger, 1869:160
| Fitzinger 1869: 160 |
Leptoceros Cuvieri Fitzinger, 1869:160
| Fitzinger 1869: 160 |
( Gazella ) leptoceros
| Temminck 1853: 193 |