Physopelta ( Neophysopelta ) parviceps Blöte, 1931
|
publication ID |
https://doi.org/10.11646/zootaxa.4951.3.3 |
|
publication LSID |
lsid:zoobank.org:pub:ADA22E04-9548-4ECC-AF84-18E51955852B |
|
DOI |
https://doi.org/10.5281/zenodo.4684752 |
|
persistent identifier |
https://treatment.plazi.org/id/351AAE4D-0255-FFF7-3295-BA1AFCA7FE30 |
|
treatment provided by |
Plazi |
|
scientific name |
Physopelta ( Neophysopelta ) parviceps Blöte, 1931 |
| status |
|
Physopelta ( Neophysopelta) parviceps Blöte, 1931 View in CoL
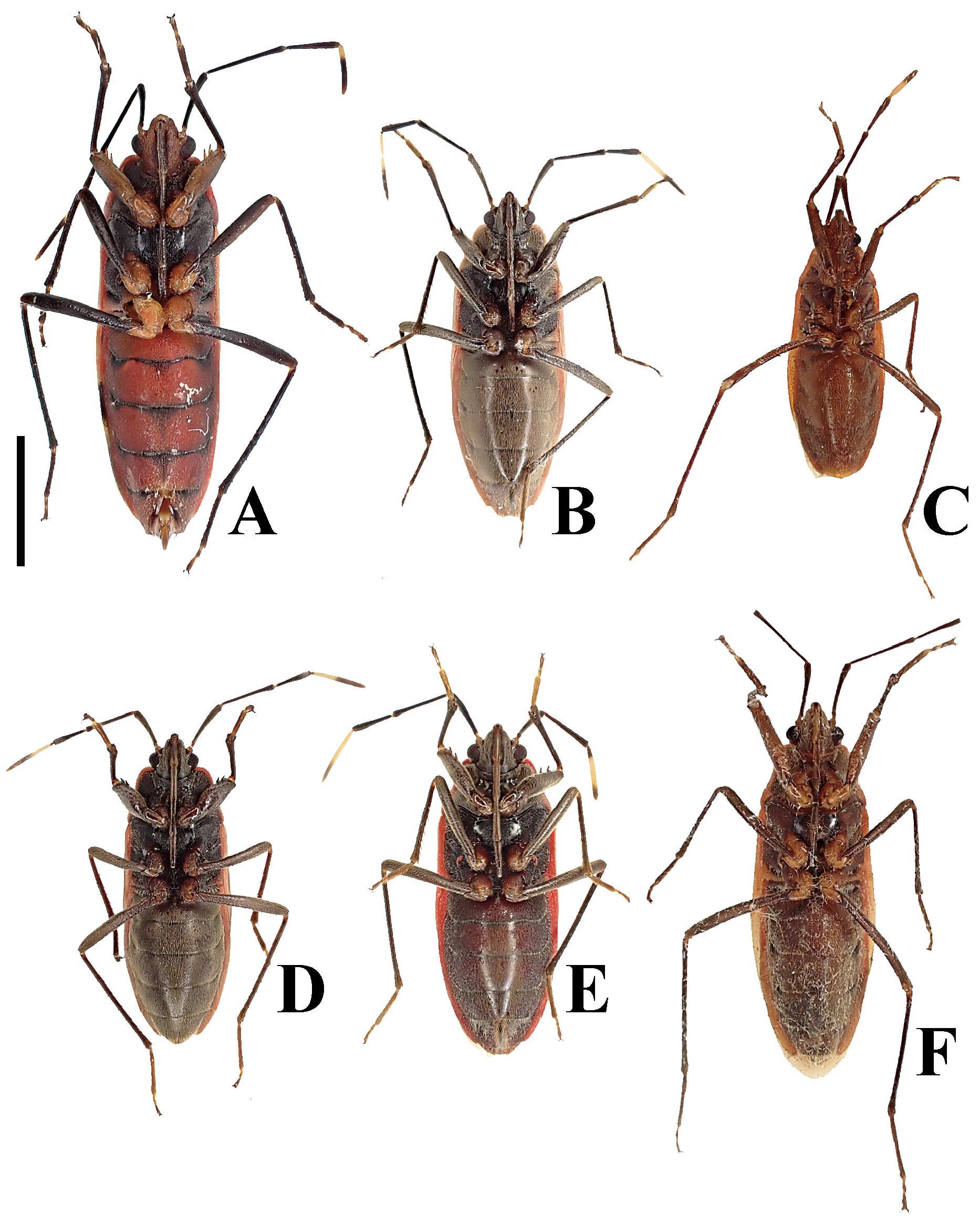
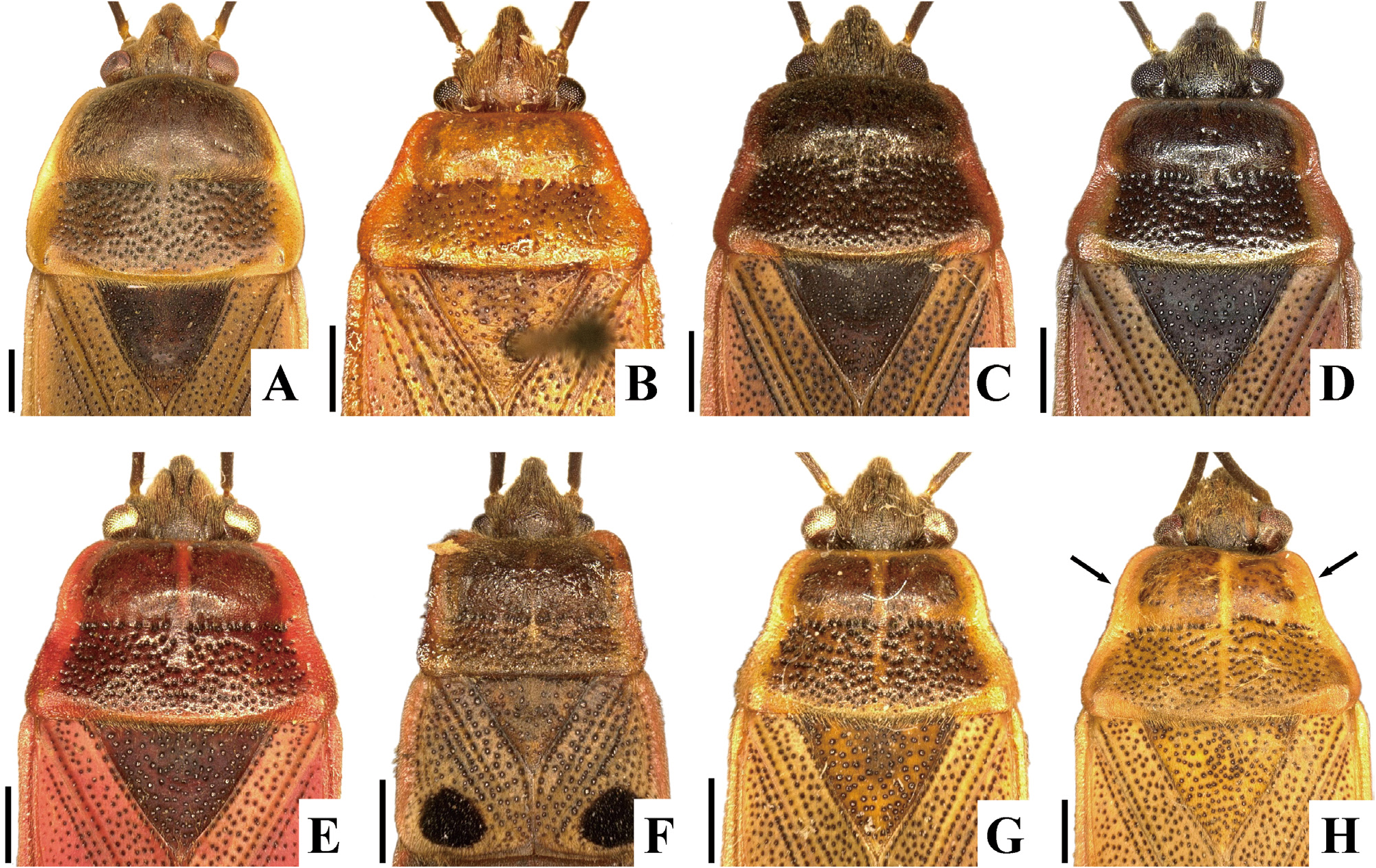
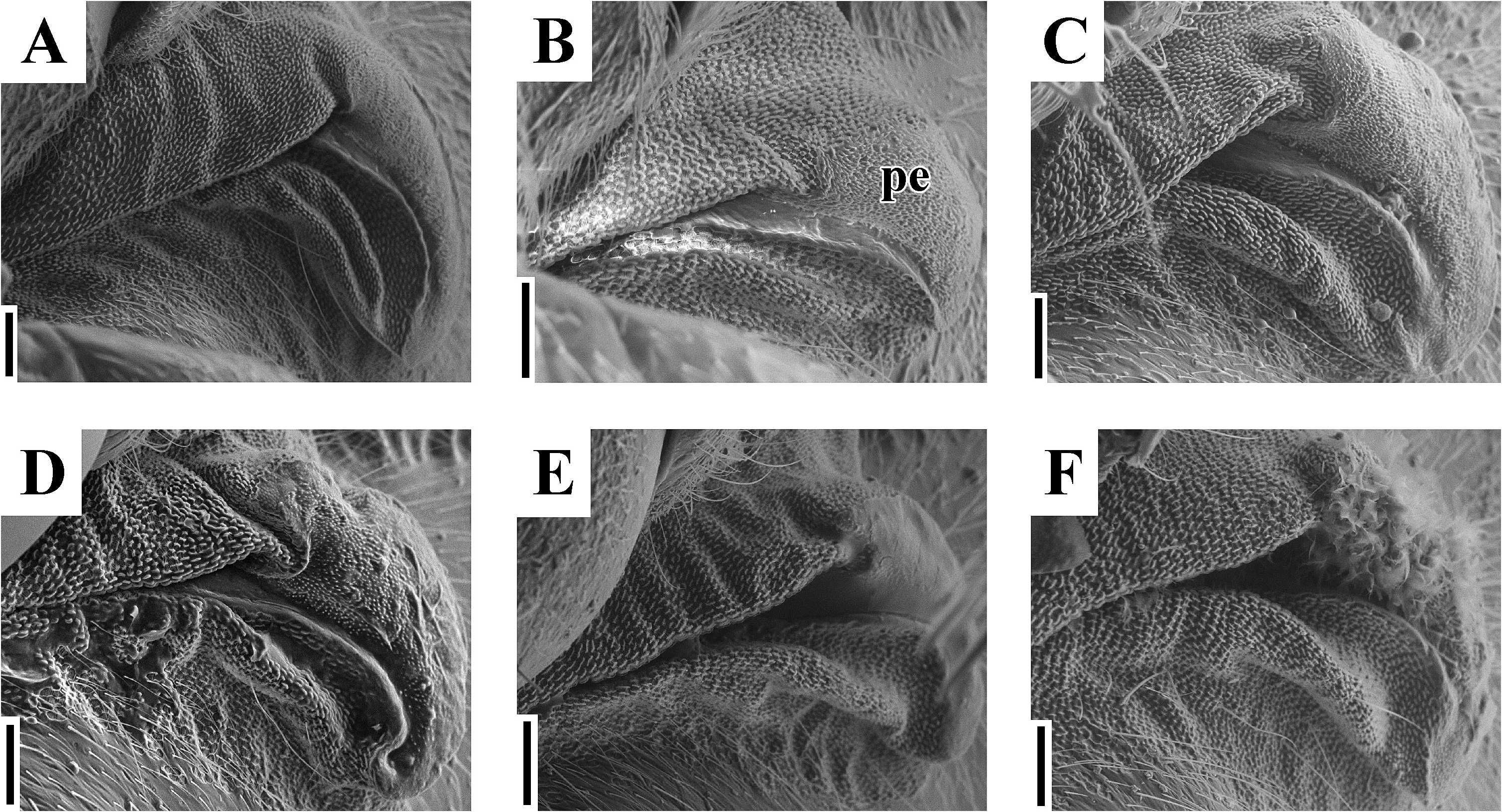
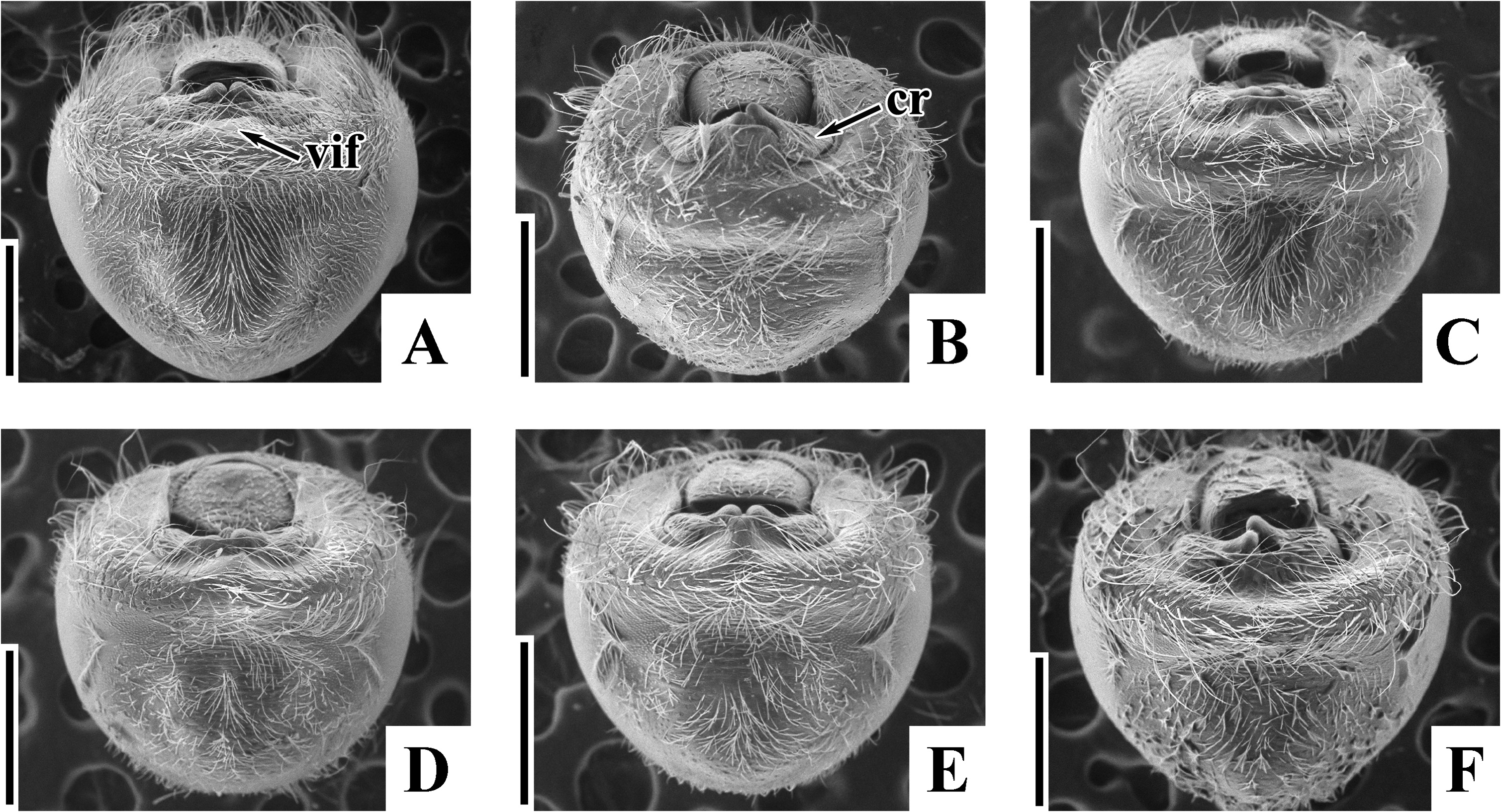
( Figs. 2A–H View FIGURE 2 , 3D, E View FIGURE 3 , 4D, E View FIGURE 4 , 5C–F View FIGURE 5 , 6C, D View FIGURE 6 , 7C, D View FIGURE 7 , 8C, D View FIGURE 8 , 9C, D View FIGURE 9 , 10C, D View FIGURE 10 , 11C, D View FIGURE 11 , 12C, D View FIGURE 12 , 13B, C View FIGURE 13 , 14B, C View FIGURE 14 )
Physopelta parviceps Blöte, 1931: 100 View in CoL . Holotype: macropterous ♂, Japan; NCB ( Stehlík 2013: 536 View Cited Treatment ).
Physopelta albofasciata DeGeer, 1773 View in CoL : Matsumura (1905: 26) (distribution). Misidentification ( Stehlík 2013: 536 View Cited Treatment ).
Physopelta slanbuschii Fabricius, 1787 View in CoL : Matsumura (1905: 27) (distribution, as Physopelta Schlanbuschii View in CoL ); Kohno et al. (2012: 400) (monograph); Aukema et al. (2013: 401) (checklist: Palaearctic); Hayashi et al. (2016: 478) (checklist: Japan); Stehlík (2013: 543) View Cited Treatment (monograph). Misidentifications.
Physopelta cincticollis Stål, 1863 View in CoL : Scott (1874: 291) (distribution); Scott (1880: 306) (distribution); Kato (1940: 704) (distribution); Esaki (1952: 230) (monograph); Takara (1957: 55) (distribution); Miyamoto (1965a: 89) (monograph); Sawada & Watanabe (1969: 7) (distribution); Miyamoto (1970: 265) (distribution); Lee (1971: 528) (monograph); Watanabe & Sohma (1972: 8) (distribution); Yamaguchi et al. (1973: 90) (distribution); Ishihara et al. (1974: 72) (distribution); Tomokuni (1981: 108) (distribution); Urata (1981a: 429) (distribution); Urata (1981b: 738) (distribution); Manna et al. (1985: 621) (distribution); Tomokuni (1985: 156) (distribution); Higuchi & Sato (1988: 83) (distribution); Miyamoto & Yasunaga (1989: 179) (checklist: Japan); Tomokuni (1989: 190) (distribution); Fukuda (1991: 23) (distribution); Hatada (1991: 28) (distribution); Lee & Kwon (1991: 50) (distribution); Yasunaga et al. (1993: 197) (monograph); Ehira (1996: 53) (distribution); Tomokuni et al. (2000: 43) (distribution); Hiromori (2001: 49) (distribution); Kerzhner (2001: 246) (checklist: Palaearctic); Kwon et al. (2001: 300) (checklist: Korea); Hayashi (2002: 144) (distribution); Kohno et al. (2002: 393) (predator); Tomokuni & Ishikawa (2002: 175) (distribution); Tomokuni & Hayashi (2006: 298) (distribution); Yano et al. (2012: 92) (distribution); Kanai et al. (2013: 17) (distribution); Stehlík (2013: 523) View Cited Treatment (monograph); Kanai (2017: 16) (distribution); Kanai (2018: 10) (distribution). Misidentifications.
References. Stehlík & Kerzhner (1999: 122) (distribution); Kerzhner (2001: 246) (checklist: Palaearctic); Japanese Society of Applied Entomology and Zoology (2006: 59) (pest); Miyamoto (2008: 155) (monograph); Yazaki (2009: 32) (distribution); Kohno et al. (2012: 399) (monograph); Ito (2013: 42) (distribution); Stehlík (2013: 536) (monograph); Tomokuni (2014: 365) (distribution); Nozaki et al. (2015: 30) (distribution); Ishikawa (2016: 477) (checklist: Japan); Komatsu (2016: 37) (distribution); Nozaki et al. (2016: 89) (distribution); Ahn et al. (2018: 202) (distribution); Hayashi & Kadowaki (2018: 313) (distribution); Ito et al. (2020: 116) (distribution); Okuda (2020: 47) (distribution).
Material examined. Non-types, a total of 4,198 specimens (spec.) of macropterous morph deposited in ELKU, KUM, NMPC and TUA from the following localities: JAPAN: Honshu: Yamagata Pref. ( 5 spec.); Tochigi Pref. ( 7 spec.); Gunma Pref. ( 2 spec.); Ibaraki Pref. ( 7 spec.); Saitama Pref. ( 137 spec.); Chiba Pref. ( 14 spec.); Tokyo Metropolis ( 35 spec.); Kanagawa Pref. ( 191 spec.); Niigata Pref. ( 1 spec.); Yamanashi Pref. ( 258 spec.); Nagano Pref. ( 2 spec.); Shizuoka Pref. ( 26 spec.); Aichi Pref. ( 1 spec.); Mie Pref. ( 34 spec.); Shiga Pref. ( 1 spec.); Osaka Pref. ( 1 spec.); Nara Pref. ( 1 spec.); Wakayama ( 2 spec.); Tottori pref. ( 1 spec.); Hiroshima Pref. ( 7 spec.); Izu Islands: Izu- Oshima Is. ( 91 spec.); Toshima Is. ( 2 spec.); Shikine Is. ( 1 spec.); Miyake Is. ( 80 spec.); Mikura Is. ( 46 spec.); Kozu Is. ( 20 spec.); Hachijo Is. ( 50 spec.); Hachijokojima Is. ( 11 spec.); Aogashima Is. ( 5 spec.); Shikoku: Kagawa Pref. ( 2 spec.); Ehime Pref. ( 9 spec.); Kochi Pref. ( 731 spec.); Kyushu: Fukuoka Pref. ( 18 spec.); Saga Pref. ( 11 spec.); Nagasaki Pref. ( 6 spec.); Oita Pref. ( 1 spec.); Kumamoto Pref. ( 6 spec.); Kagoshima Pref. ( 19 spec.); Nokonoshima Island: 2 spec.; Iki Island: 11 spec.; Tsushima Island: 112 spec.; Danjo Islands: Onna Is. ( 1 spec.); Koshiki Islands: Shimokoshiki Is. ( 6 spec.); Ryukyu Islands: Osumi Islands: Tanegashima Is. ( 4 spec.); Yaku Is. ( 14 spec.); Kuro Is. ( 24 spec.); Tokara Islands : Kuchinoshima Is. ( 7 spec.); Nakanoshima Is. ( 141 spec.); Akuseki Is. ( 6 spec.); Takara Is. ( 1 spec.); Amami Islands: Amami-Oshima Is. ( 412 spec.); Tokunoshima Is. ( 138 spec.); Okinawa Islands: Okinawa-Honto Is. ( 300 spec.); Iheya Is. ( 3 spec.); Zamami Is. ( 1 spec.); Kume Is. ( 10 spec.); Miyako Islands: Miyako Is. ( 2 spec.); Yaeyama Islands: Ishigaki Is. ( 117 spec.); Kohama Is. ( 33 spec.); Iriomote Is. ( 830 spec.); Hateruma Is. ( 1 spec.); Yonaguni Is. ( 155 spec.). KOREA: Korean Peninsula: Jeollanam-do, Yeosu-shi, Odondo, 6.viii.1965, leg. C. E. Lee ( 4 ♂♂ 6 ♀♀, KUM); Chejudo Island: Tonnaeko, 8.vi.1991, leg. T. Kishimoto ( 1 ♀, TUA); Kinneikutsu, 24.vii.1968, leg. T. Doi, S. Hidaka, M. Nakahara, S. Hayakawa, Y. Nishida & S. Omatsu ( 1 ♀, KUM); Kaewol bridge, 23.vii.1990, leg. S. Kamitani ( 1 ♀, KUM). TAIWAN: Lan-Yu Is., 3.iv.1987, leg. K. Baba ( 2 ♀♀, KUM). CHINA: Guangdong Province: Nanling National Nature Reserve Dadongshan (border of mixed forest; at light), alt. 690 m, 24°56.0′N 112°42.9′E, 18–21.v.2013, leg. J. Hájek & J. Ružička ( 1 ♀, NMPC); W of Qixing, Heishiding (Stream; pools) (forested stream valley; at light), alt. 190 m, 23°27.9′N 111°54.3′E, 1–3.v.2011, leg. M. Fikáček & J. Hájek ( 1 ♀, NMPC); Zhejiang Province: West Tianmu Shan (Mts) reserve from “Blind Alley” to “Immotal Peak” mountainous low forest, alt. 1,200 –1,500 m, 30°20.5–21.0′N 119°25.4–7′E, 27–28.vi.2017, leg. J. Hájek & J. Ružička ( 3 ♂♂, TUA; 3 ♀♀, NMPC); West Tianmu Shan (Mts) reserve Immotal Peak, mountainous low forest, esp. on flowering Castanea seguinii , alt. 1,500 m, 30°20.58.5′N 119°25.26.5′E, 5–6.vii.2017, leg. J. Hájek & J. Ružička ( 1 ♀, TUA; 1 ♀, NMPC). Brachypterous morph: JAPAN: Honshu: Tochigi Pref., Utsunomiyashi, Mt. Kogashi, 6.xi.1986, leg. H. Ohkawa ( 1 ♀, TPM); Tochigi Pref., Motegi, Mt. Kamakura, 6.v.2013, leg. S. Maehara ( 1 ♂, TUA). Ten specimens collected by C. E. Lee belong to a series which part was recorded as “ Physopelta cincticollis ” from Korea by Lee (1971). A single brachypterous female has been recorded as “brachypterous morph of Physopelta cincticollis ” from Japan by Higuchi & Sato (1988).
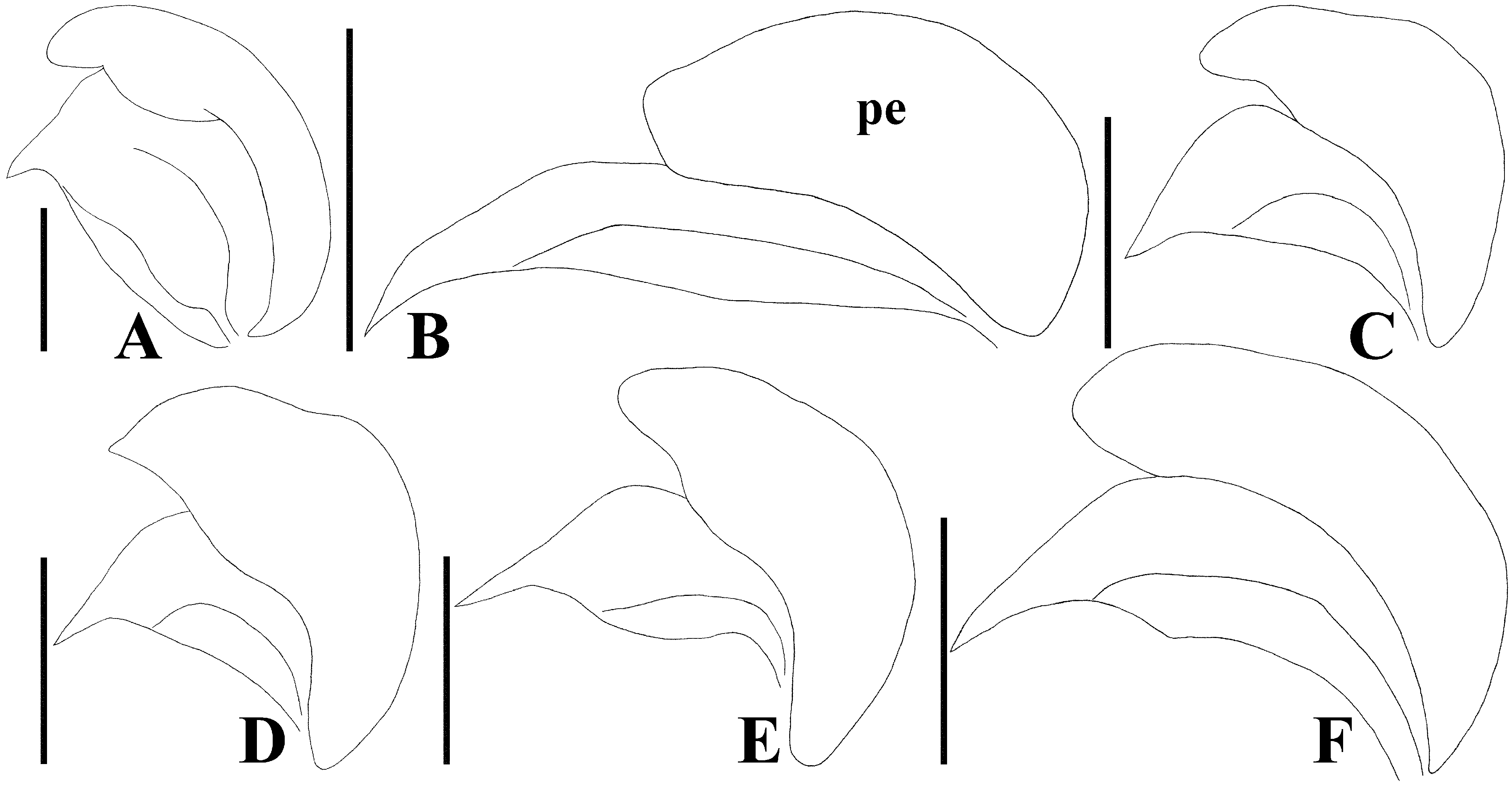
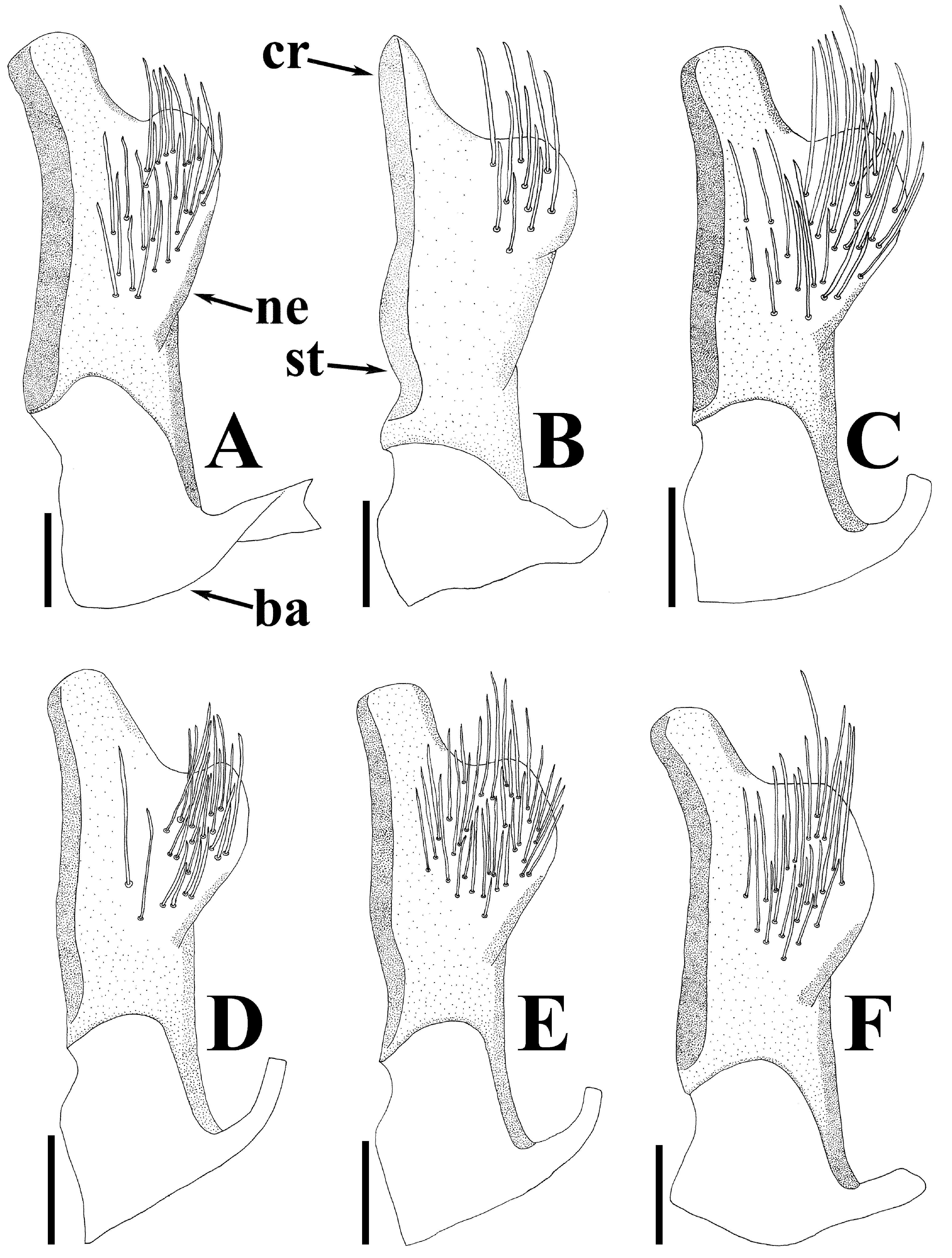
Diagnosis. Recognized among other species of Physopelta by a combination of the following characters: both macropterous and brachypterous morphs known; body 2.8 times as long as maximum width across fore wings ( Fig. 2A–F View FIGURE 2 ); calli and disc of pronotum dark brown ( Fig. 5C–E View FIGURE 5 ); scutellum dark brown in macropterous morph dark brown, without pale callosities; membrane of fore wing dark brown ( Fig. 6C, D View FIGURE 6 ); abdominal sternites dark brown without contrasting lunulae on sutures ( Figs. 3D, E View FIGURE 3 , 4D, E View FIGURE 4 ); compound eye in macropterous morph more than 0.3 times as wide as vertex in dorsal view; antennomere I shorter than antennomere II; antennomere II nearly clavate; calli in male convex; punctures of scutellum in density uniform throughout its surface, smaller than punctures of pronotum; anterior margins of fore wings not parallel to each other in rest; procoxal projection present, horn-shaped, straight, less than 1.5 times as long as its maximum width ( Fig. 7C, D View FIGURE 7 ); protrochanteral wrinkles present; male profemur more than 2 times as wide as mesofemur at widest part of each; male protibia lacking tooth at apex, with a single row of denticles throughout its length ventrally; peritreme of scent gland ostiole crescent-shaped, protruding posterolaterad ( Figs. 8C, D View FIGURE 8 , 10C, D View FIGURE 10 ); infolding of ventral rim of genital capsule less convex in middle part ( Fig. 9C, D View FIGURE 9 ); outer margin of endophallic reservoir outgrowth emarginate in apical part ( Fig. 11C, D View FIGURE 11 ); stem of paramere not emarginate in basal part ( Fig. 12C, D View FIGURE 12 ); crown of paramere at apex not convex in posterior view; and inner margins of ring sclerites nearly parallel to each other ( Fig. 13B, C View FIGURE 13 ).
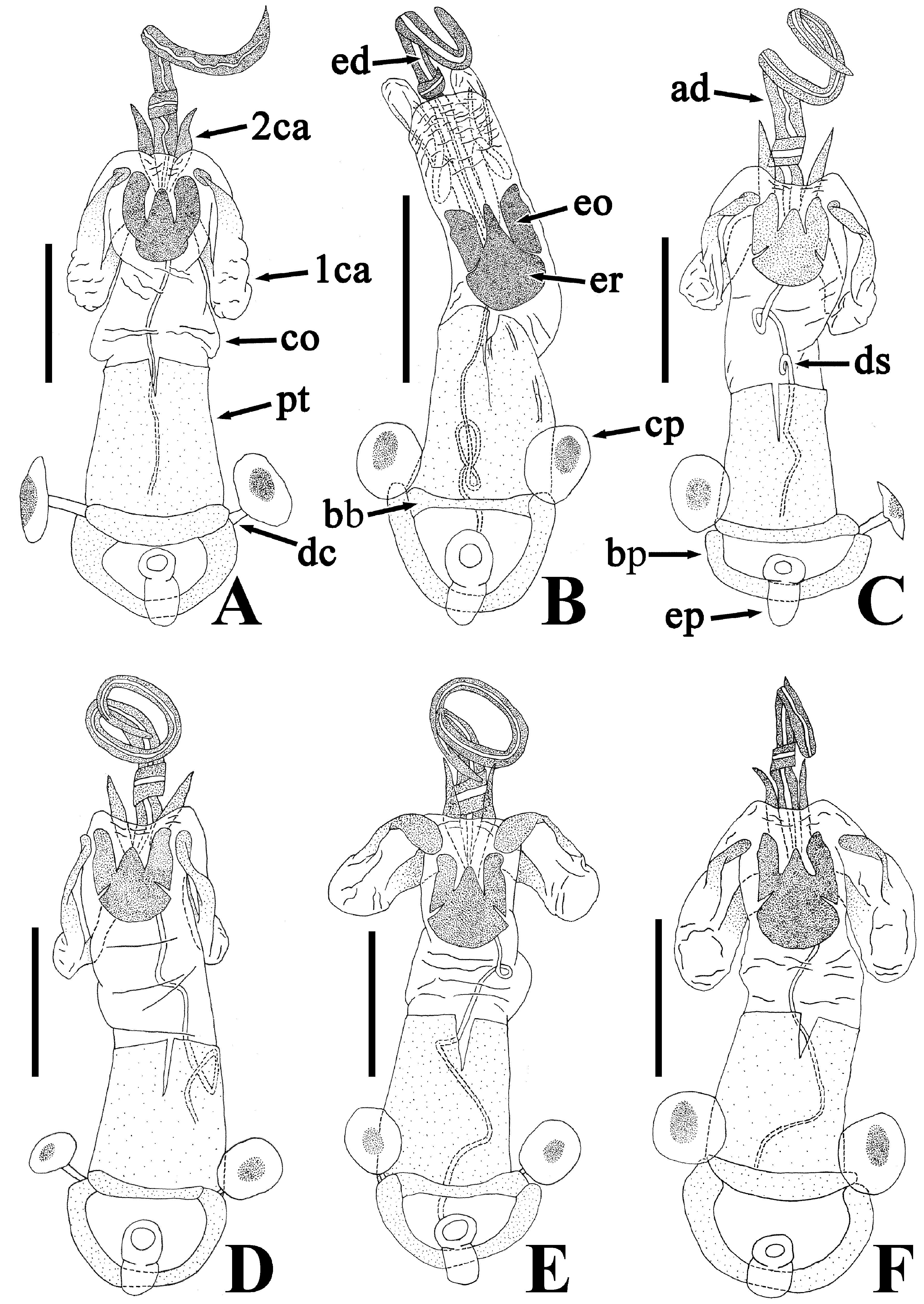
Description of genitalia. Genital capsule ( Fig. 9C, D View FIGURE 9 ) spherical, semicircular in ventral view, smooth on surface; infolding of ventral rim less convex in middle part. Phallus ( Fig. 11C, D View FIGURE 11 ) oblong; capitate process membranous; basal plate and phallotheca coriaceous; conjunctiva with two pairs of partly sclerotized conjunctival appendages; endophallic reservoir with a pair of outgrowths; outer margin of outgrowth emarginate in apical part. Paramere ( Fig. 12C, D View FIGURE 12 ) longer than its maximum width across crown; stem not emarginate in basal part; crown at apex not convex in posterior view.
Female terminalia ( Fig. 13B, C View FIGURE 13 ) triangular in anterior view; valvulae VIII and IX combining to form an ovipositor, fused with valvifers VIII and IX, respectively; ring sclerites coriaceous in marginal part, membranous in central part, protruding inward, with inner margins nearly parallel to each other. Spermatheca ( Fig. 14B, C View FIGURE 14 ) membranous; apical receptacle spherical; intermediate part in width uniform; spermathecal duct apically widened.
Brachypterous morph. General appearance is very similar to that of macropterous morph except for the following characters: scutellum brown, with numerous pale callosities ( Fig. 5F View FIGURE 5 ); compound eye less than 0.3 times as wide as vertex in dorsal view; calli longer than disc of pronotum, wider in male than in female; fore wing not concealing abdominal tergite IV ( Fig. 2G–H View FIGURE 2 ); membrane of fore wing indistinct; meso- and metafemur in both sexes with two rows of denticles; procoxal projection absent; peritreme of scent gland ostiole teardrop-shaped, protruding laterad.
Remarks. This species strongly resembles Physopelta ( Neophysopelta) cincticollis in general appearance; therefore, the distributional records of both species from Japan and Korea have been confused for a long time (cf. Stål 1863; Scott 1874; Blöte 1931; Lee 1971; Stehlík & Kerzhner 1999; Stehlík 2013). However, based on a comparison between thousands of specimens together with photographs of the male holotype and the female paratype (M. Hayashi, pers. comm. 2016) of Ph. ( N.) parviceps and photographs of the syntype ( Swedish Museum of Natural History 2007) together with a single Chinese and three Vietnamese specimens ( Figs. 15C, D View FIGURE 15 , 16B, E View FIGURE 16 ) of Ph. ( N.) cincticollis , four main characteristics were recognized to easily differentiate Ph. ( N.) cincticollis from Ph. ( N.) parviceps : both macropterous and brachypterous morphs known; compound eye more than 0.3 times as wide as vertex in dorsal view; antennomere II nearly clavate; and punctures of scutellum smaller than punctures of pronotum. In contrast, P. ( N.) cincticollis has the following features: only macropterous morph known; compound eye less than 0.3 times as wide as vertex in dorsal view; antennomere II nearly cylindrical; and punctures of scutellum as large as punctures of pronotum. It seems that “true” Ph. ( N.) cincticollis described from “ India orientalis” (southeastern Asia) is not distributed in Japan and Korea.
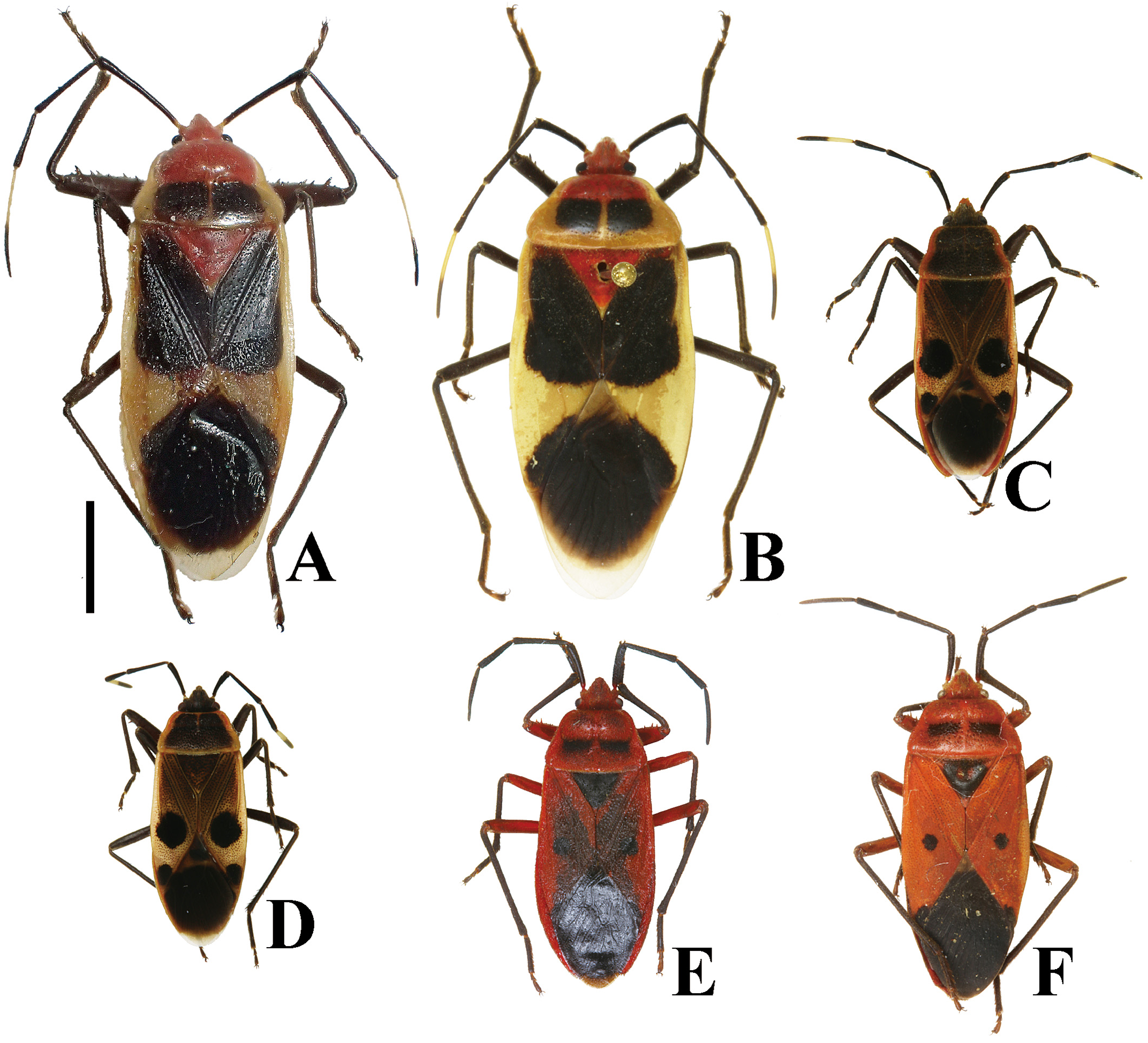
Yasunaga et al. (1993) pointed out that the dorsum color of Ph. ( N.) parviceps is frequently reddish in the southern part of Japan, whereas Ph. ( N.) parviceps with red dorsum has been identified as Ph. ( N.) slanbuschii ( Matsumura 1905; Kohno et al. 2012). However, based on a morphological examination of thousands of specimens of Ph. ( N.) parviceps ( Fig. 2A–C View FIGURE 2 ) and specimens identified as “ Ph. ( N.) slanbuschii ” ( Fig. 2D–F View FIGURE 2 ) from Japan, no morphological differences were observed between the two populations in structure of pronotum, scutellum, procoxa, peritreme of scent gland ostiole, genital capsule, phallus, paramere, female terminalia, and spermatheca ( Figs. 5C–E View FIGURE 5 , 6C, D View FIGURE 6 , 7C, D View FIGURE 7 , 8C, D View FIGURE 8 , 9C, D View FIGURE 9 , 10C, D View FIGURE 10 , 11C, D View FIGURE 11 , 12C, D View FIGURE 12 , 13B, C View FIGURE 13 , 14B, C View FIGURE 14 ). Therefore, the specimens identified as “ Ph. ( N.) slanbuschii ” from Japan corresponds to Ph. ( N.) parviceps without doubt. On the other hand, Ph. ( N.) parviceps can be easily distinguished from “true” Ph. ( N.) slanbuschii ( Figs. 15E, F View FIGURE 15 , 16C, F View FIGURE 16 ) described from China (probably southern part) by the following characteristics: head dark brown; basal part of antennomere IV white; calli dark brown; femur dark brown; compound eye more than 0.3 times as wide as vertex in dorsal view; and peritreme of scent gland ostiole protruding posterolaterad. In contrast, Ph. ( N.) slanbuschii has the following features: head red; basal part of antennomere IV dark brown; calli red; femur red; compound eye less than 0.3 times as wide as vertex in dorsal view; and peritreme of scent gland ostiole protruding anteroposteriad.
Physopelta ( Neophysopelta) parvicep s had been misidentified as Ph. ( Ph.) albofasciata DeGeer, 1773 ( Figs. 15A, B View FIGURE 15 , 16A, D View FIGURE 16 ) in the Ryukyu Islands, Japan (cf. Matsumura 1905; Stehlík 2013), but the former can be easily distinguished from the latter by the following characteristics: body length less than 15 mm; head dark brown; calli dark brown; lateral margin of pronotum brown; scutellum dark brown; apical part and lateral margin of fore wings of corium brown; antennomere I shorter than antennomere II; male protibia lacking tooth at apex; and peritreme of scent gland ostiole crescent-shaped, protruding posterolaterad. In contrast, Ph. ( Ph.) albofasciata has the following characteristics: body length more than 15 mm; head red; calli red; lateral margin of pronotum white; scutellum red; apical part and lateral margin of fore wings of corium white; antennomere I longer than antennomere II; male protibia with a single tooth in apical part; and peritreme of scent gland ostiole oblong, protruding laterad.
According to Stehlík (2013), only macropterous morph has been known in Physopelta species. However, “brachypterous morph of Physopelta cincticollis ” has been recorded from Japan proper ( Higuchi & Sato 1988). The brachypterous specimens matched well with the definition of Physopelta mentioned in the previous section, and appears to correspond to Ph. ( N.) parviceps described from Japan based on the following morphological characteristics: antennomere I shorter than antennomere II ( Fig. 2G–H View FIGURE 2 ); antennomere II nearly clavate; calli more convex in male than in female; punctures of scutellum in density uniform throughout its surface ( Fig. 5F View FIGURE 5 ); profemur wider in male than in female; protibia in male with a single row of denticles throughout its length ventrally; infolding of ventral rim of genital capsule less convex in middle part; crown of paramere at apex not convex in posterior view; and inner margins of ring sclerites nearly parallel to each other. Additionally, “brachypterous morph of Physopelta cincticollis ” and specimens of Ph. ( N.) parviceps were collected from Japan proper at the same locality ( Higuchi & Sato 1988; S. Maehara, pers. comm. 2019). Therefore, “brachypterous morph of Physopelta cincticollis ” belongs to Physopelta without doubt, and appears to correspond to Ph. ( N.) parviceps described from Japan based on macropterous morph. On the other hand, the morphological differences between macropterous and brachypterous morphs of Ph. ( N.) parviceps strongly resemble that of the monotypic genus strongly related to Physopelta , Jindraia Stehlík, 2006 (cf. Stehlík 2006). In the general similarity, Jindraia may be a junior synonym of the subgenus Neophysopelta , and Ph. ( N.) redeii Stehlík, 2013 may be a macropterous morph of Jindraia (P. Kment, pers. comm. 2021). Thus, the delimitation of both the genera would need a revision.
Distribution. Japan: Honshu, Izu Islands (Izu-Oshima Island, Toshima Island, Niijima Island, Shikine Island, Miyake Island, Mikura Island, Kozu Island, Hachijo Island, Hachijokojima Island, Aogashima Island), Sado Island, Oki Islands, Shikoku, Kyushu, Okinoshima Island, Iki Island, Tsushima Island, Goto Islands (Hira Island, Nakadori Island, Fukue Island), Danjo Islands (Otoko Island, Onna Island), Amakusa Islands (Shimo Island), Koshiki Islands (Kamikoshiki Island, Shimokoshiki Island), Ryukyu Islands (Tanegashima Island, Yaku Island, Kuchinoerabu Island, Kuro Island, Kuchinoshima Island, Nakanoshima Island, Taira Island, Akuseki Island, Takara Island, Amami-Oshima Island, Tokunoshima Island, Okinoerabu Island, Okinawa-Honto Island, Iheya Island, Aka Island, Zamami Island, Kume Island, Iotorishima Island, Miyako Island, Ishigaki Island, Kohama Island, Iriomote Island, Hateruma Island, Yonaguni Island) ( Scott 1874; Matsumura 1905; Yasunaga et al. 1993; Kohno et al. 2012; present study, etc.); Korea (southern part of Korean Peninsula, Chejudo Island) ( Lee 1971; Lee & Kwon 1991; Kwon et al. 2001; Ahn et al. 2018; present study); Taiwan (Lanyu Island) (present study); China ( Guangdong Province, Zhejiang Province) (present study).
This species is newly recorded from Lanyu Island and China ( Guangdong Province and Zhejiang Province) .
Thousands of specimens from Japan, Korea, and Taiwan including “ Physopelta cincticollis ” recorded by Lee (1971) were examined, and all of these were identified as Ph. ( Neophysopelta) parviceps . In addition, the illustrations and photographs of Ph. ( N.) cincticollis from Japan provided by the previous authors ( Esaki 1952; Miyamoto 1965a; Yasunaga et al. 1993) in fact represent Ph. ( N.) parviceps . At least some, possibly all of the previous dis- tributional records of Ph. ( N.) cincticollis from Japan and Korea ( Stehlík 2013, etc.) and Ph. ( N.) slanbuschii from Japan ( Kohno et al. 2012, etc.) correspond to Ph. ( N.) parviceps .
Host plant. In Japan, Mallotus japonicus (Euphorbiaceae) is a host plant for Physopelta ( Neophysopelta) parviceps because nymphs have been observed to feed on the seeds in the field, whereas adults feed on various flowers and fruits belonging to three plant families other than Euphorbiaceae , namely, Castanea crenata Siebold et Zucc. (Fagaceae) , Castanopsis spp. ( Fagaceae ), Citrus spp. ( Rutaceae ), and Morus spp. ( Moraceae ) (Miyamoto 1965; Japanese Society of Applied Entomology and Zoology 2006; Yasunaga et al. 1993; Miyamoto 2008; Kohno et al. 2012; Komatsu 2016; present study).
This species is found on various woody angiosperms belonging to Rosaceae and Rutaceae in Korea (cf. Kwon et al. 2001) and on flowers of Castanea seguinii Dode (Fagaceae) in China (present study).
Biology. Adults and nymphs are attracted to artificial light (Miyamoto 1965; Tomokuni 1993; Miyamoto 2008; Kohno et al. 2012; present study).
In Japan proper, adults are collected in almost all seasons ( Yasunaga et al. 1993; Kohno et al. 2012; Nozaki et al. 2016; Okuda 2020; present study, etc.), suggesting that the overwintering stage is adult.
Teneral adults were observed from August to September in Japan proper (present study). Nymphs were observed from August to October in Japan proper and in April in the Ryukyu Islands ( Kohno et al. 2012; present study). In Japan proper, host plant of Physopelta ( Neophysopelta) parviceps , Mallotus japonicus blooms from June to July, and mature seed is fallen to ground surface from September to November ( Tomikawa et al. 2013). Due to one season blooming of its host plant and emergence time of teneral adult and nymph, Ph. ( N.) parviceps could be univoltinism in Japan proper. Number of generations in other distribution areas is unknown.
In Japan, this species is abundant in lowlands ( Kohno et al. 2012). However, a number of individuals were collected using artificial light in the montane areas of Japan proper (present study), suggesting that Ph. ( N.) parviceps is abundant, at least in Japan proper, regardless of altitude.
| NMPC |
National Museum Prague |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
|
SubGenus |
Neophysopelta |
Physopelta ( Neophysopelta ) parviceps Blöte, 1931
| Souma, Jun & Ishikawa, Tadashi 2021 |
Physopelta slanbuschii
| Aukema, B. & Rieger, C. & Rabitsch, W. 2013: 401 |
| Stehlik, J. L. 2013: 543 |
| Kohno, K. & Hayashi, M. & Miyamoto, S. 2012: 400 |
| Matsumura, S. 1905: 27 |
Physopelta cincticollis Stål, 1863
| Kanai, K. 2018: 10 |
| Kanai, K. 2017: 16 |
| Kanai, K. & Moriyama, T. & Nakamura, K. 2013: 17 |
| Stehlik, J. L. 2013: 523 |
| Yano, S. & Kikuhara, Y. & Takechi, L. & Watanabe, K. 2012: 92 |
| Tomokuni, M. & Hayashi, M. 2006: 298 |
| Hayashi, M. 2002: 144 |
| Kohno, K. & Takahashi, T. & Sakakibara, M. 2002: 393 |
| Tomokuni, M. & Ishikawa, T. 2002: 175 |
| Hiromori, T. 2001: 49 |
| Kerzhner, I. M. 2001: 246 |
| Kwon, Y. J. & Suh, S. J. & Kim, J. A. 2001: 300 |
| Tomokuni, M. & Hayashi, M. & Usui, T. 2000: 43 |
| Ehira, K. 1996: 53 |
| Yasunaga, T. & Takai, M. & Yamashita, I. & Kawamura, M. & Kawasawa, T. 1993: 197 |
| Fukuda, H. 1991: 23 |
| Hatada, K. 1991: 28 |
| Lee, C. E. & Kwon, Y. J. 1991: 50 |
| Miyamoto, S. & Yasunaga, T. 1989: 179 |
| Tomokuni, M. 1989: 190 |
| Higuchi, H. & Sato, K. 1988: 83 |
| Manna, G. K. & Ueshima, N. & Dey, S. K. & Deb-Mallick, S. 1985: 621 |
| Tomokuni, M. 1985: 156 |
| Tomokuni, M. 1981: 108 |
| Urata, A. 1981: 429 |
| Urata, A. 1981: 738 |
| Ishihara, T. & Miyatake, M. & Tomokuni, M. & Tokihiro, G. 1974: 72 |
| Yamaguchi, T. & Miyagi, I. & Miyata, A. & Noda, M. & Ejima, M. & Yoshida, K. 1973: 90 |
| Watanabe, Y. & Sohma, K. 1972: 8 |
| Lee, C. E. 1971: 528 |
| Miyamoto, S. 1970: 265 |
| Sawada, G. & Watanabe, Y. 1969: 7 |
| Miyamoto, S. 1965: 89 |
| Takara, T. 1957: 55 |
| Esaki, T. 1952: 230 |
| Kato, M. 1940: 704 |
| Scott, J. 1880: 306 |
| Scott, J. 1874: 291 |