Podocotyle pearsei Manter, 1934
|
publication ID |
https://doi.org/10.11646/zootaxa.4117.4.3 |
|
publication LSID |
lsid:zoobank.org:pub:B672E2F9-9A89-4A79-8CEC-01F51F42B6E0 |
|
DOI |
https://doi.org/10.5281/zenodo.5672210 |
|
persistent identifier |
https://treatment.plazi.org/id/3562878D-FFA4-FFED-FF7D-F54D7F88434D |
|
treatment provided by |
Plazi |
|
scientific name |
Podocotyle pearsei Manter, 1934 |
| status |
|
Podocotyle pearsei Manter, 1934 View in CoL
( Figs. 4–6 View FIGURES 4 – 6 )
Synonym: Podocotyle ( Podocotyle) pearsei Manter, 1934 .
Hosts. Bullseye grenadier, Bathygadus macrops Goode & Bean, 1885 ( Gadiformes : Macrouridae : Bathygadinae ); Doublethread grenadier, Gadomus arcuatus (Goode & Bean, 1886) ( Macrouridae : Bathygadinae ); Western softhead grenadier, Malacocephalus occidentalis Goode & Bean, 1885 ( Macrouridae : Macrourinae ).
Localities. B. macrops : Northeastern Gulf of Mexico off Florida, 27°28’N, 85°13’W, depth = 728 m, 22/June/ 1971; 28°22’N, 86°31’W, depth = 710 m, 26/June/1971; Caribbean Sea off Panama, 9°05’N, 81°18’W, depth = 591 m, 29/October/1970; G. arcuatus : Northeastern Gulf of Mexico off Florida, 27°38’N, 85°15’W, depth = 637 m, 23/ June/1971; M. occidentalis : Northeastern Gulf of Mexico off Florida, 28°36’N, 86°55’W, depth = 695 m, 28/June/ 1971.
Site of infection. Intestine.
Prevalence. B. macrops : 5 of 120 fish infected (4.17%); G. arcuatus : 5 of 10 (50.0%); M. occidentalis : 1 of 86 (1.16%).
Intensity. B. macrops : 1 worm per fish; G. arcuatus : 1–5; M. occidentalis : only 1 worm.
Mean intensity. B. macrops : 1.00 (5/5); G. arcuatus : 1.80 (9/5); M. occidentalis : 1.00 (1/1).
Relative density/abundance. B. macrops : 0.04 (5/120); G. arcuatus : 0.90 (9/10); M. occidentalis : 0.01 (1/86).
Deposited Specimens. Vouchers ( 7 adults, 7 immatures, and 1 sectioned specimen) NHMUK 2016.4.28.7–18 (12 slides). Note: Holotype of P. pearsei currently in the NMNH (USNPC Coll. Access. # 8707); paratypes in the HWML (HWML Coll. Access. # 276 & 101928).
Records. 1. Manter (1934, 1940, 1947, 1954b); 2. Park (1937 [key]); 3. Skrjabin & Koval (1958 [key]); 4. Pritchard (1966); 5. Armstrong (1974); 6. Blend (1996); 7. Present study.
Descriptions. 1, 5, 6, 7.
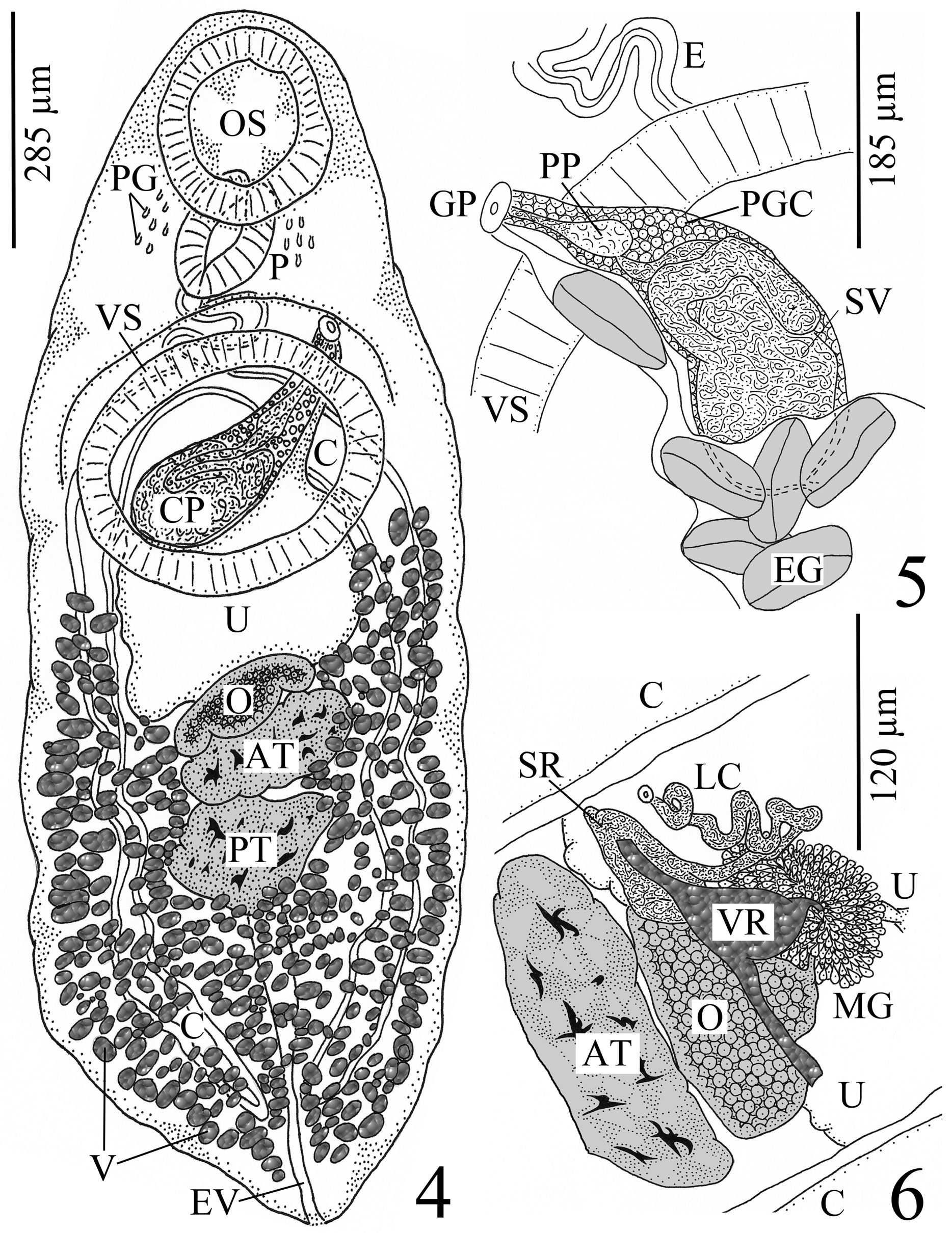
Re-description. [Based on 7 whole-mounted individuals. Measurements and proportions given in Table 3.] With characteristics of genus. Body small, oval to elongate oval, widest in posterior third of body, anterior and posterior ends attenuate to rounded extremity. Forebody about 1/4 length of body, attenuate. Hindbody slightly widens posteriorly toward midbody, attenuates in posterior 1/4 of body. Tegument aspinose. Pre-oral lobe absent. Oral sucker subterminal, round, unspecialized; mouth subterminal [n = 6] or terminal [n = 1]. Ventral sucker large, transversely elongate to oval, unspecialized, slightly protuberant/puckered, conspicuous muscular ring around perimeter, wider than long, larger than oral sucker, pre-equatorial at junction of first and second 1/4 of body. Prepharynx not observed. Pharynx muscular, round to bean-shaped, ventrally-overlapped by oral sucker in some specimens [n = 4]. Esophagus thick-walled, contracted and sinuous, posterior end ventrally overlapped by anterior margin of ventral sucker, longer than pharynx. Pharyngeal gland cells lateral to pharynx. Intestinal bifurcation overlapped by ventral sucker. Ceca thick-walled, more voluminous anteriorly, terminates blindly near posterior extremity, cecal ends arcuate.
Testes 2, tandem, median, smooth to indented to lobed, transversely elongate to triangular to irregular in shape, wider than long, contiguous, intercecal, post-equatorial in posterior 1/3 of body. Post-testicular region confined to posterior 1/4 of body. Cirrus pouch distinct, thin-walled, clavate, mostly median and intercecal except at distal end, extends from genital pore posterodextrally to region between mid-level and posterior margin of ventral sucker. Seminal vesicle internal, bi-partite, 244–460 (349) [n =3] long × 80–128 (104) [n =3] wide; proximal portion voluminous, saccate, occupies entire proximal end of cirrus pouch; distal portion narrow and coiled; seminal vesicle progresses anterosinistrally from proximal portion of cirrus pouch, loops back posterodextrally, then turns back around to run anterosinistrally to genital pore. Pars prostatica in anterior 1/3 of cirrus pouch; ejaculatory duct long, thick-walled; prostatic gland cells distributed throughout cirrus pouch, most numerous in distal and midregion of latter. Genital pore submedian (sinistral), at level of mid-esophagus and anterior margin of ventral sucker, midway between midline and left margin of worm. Genital atrium round, conspicuous, with ring of dark-stained cells around perimeter.
Ovary 3-lobed, wider than long, median to slightly dextral, intercecal, anterior to and contiguous with anterior testis, equatorial to just post-equatorial in middle 1/3 of body. Seminal receptacle canalicular, small, indistinct, dorsally overlaps anterior edge of ovary. Laurer’s canal present, very convoluted, opens dorsally at level of anterior margin of ovary, midway between midline of worm and left margin, at point near left side of uterine wall and left cecum. Vitelline reservoir clavate to triangular, median, immediately anterior to ovary and/or dorsally overlaps anterior margin of ovary. Vitelline ducts pass dorsally over ovary or right along anterior edge of ovary. Oviduct inconspicuous, passes anteriorly from anterior margin of ovary. Oötype directly anterior to ovary, surrounded by Mehlis’ gland. Uterus massive, winds, mainly intercecal, widest posteriorly where it extends to midlevel of ovary, narrows anteriorly at midlevel of and dorsal to ventral sucker, then runs anterosinistrally to enter genital atrium posterior to and to left of cirrus pouch. Metraterm inconspicuous. Vitellarium large, follicular, numerous, irregular or globular or oblong in shape, circumcecal except partially dorsal to ceca, in uninterrupted lateral bands; extends from posterior extremity anteriorly up to level of posterior margin of ventral sucker (1 individual with left lateral band and 1 individual with right lateral band of vitelline follicles that failed to extend to ventral sucker [see Table 3]), encroaches over lateral margins of gonads, not confluent in pre-ovarian region, in region where ovary contiguous with anterior testis, and in inter-testicular region, confluent in post-testicular region. Eggs collapsed (some appear bloated), large, operculate, amber, non-filamented, fairly numerous.
Excretory vesicle tubular, hard to see in adults due to vitellarium but observed in immature specimens to extend to ovary; excretory pore terminal.
Remarks. Using the same diagnostic sequence of morphological characters given above, this material keys out to the subfamily Plagioporinae and the genus Podocotyle as defined by Cribb (2005). These worms we collected from the intestine of three macrourid species in the northeastern Gulf of Mexico and the Caribbean Sea off Panama compare well with the type description of P. pearsei by Manter (1934); his specimens were collected from the intestine of another gadiform species, the longfin hake, Phycis chesteri Goode & Bean (Phycidae) , from deep waters off Tortugas, Florida.
While we have identified this material as P. pearsei , we saw a few minor morphometric differences (see Table 3) when our material was compared to the type description of P. pearsei by Manter (1934). We noted that Manter’s specimens were slightly larger (1,460–1,840 vs 960–1,500 long × 655–722 vs 256–334 wide [at pharynx level], 408–576 [at ventral sucker level], 440–632 [at posterior testis level]). The genital pore of P. pearsei was described by Manter (1934) to be “opposite the anterior edge of [the] intestinal bifurcation, a short distance anterior to [the] ventral sucker”; in our specimens, the genital pore was at the level of the mid-esophagus and the anterior margin of the ventral sucker (we did note in our worms that the esophagus appeared somewhat contracted and its posterior end was ventrally overlapped by the anterior margin of the ventral sucker). There was a slight difference in the size of the post-testicular area (“less than 1/4 body length and usually slightly less than forebody length” in Manter’s specimens vs 23%–27% [= 1/4 body length] and greater than forebody length as a percentage of body length in our material—forebody length sometimes as low as 15% of body length). Manter (1934) stated that the “prostate gland [was] lacking” in P. pearsei . Bray & Campbell (1996, p. 108) interpreted this to mean that “ P. pearsei is said to lack prostate cells”; however, our specimens have a bi-partite seminal vesicle, pars prostatica, ejaculatory duct and prostatic gland cells, the latter distributed throughout the cirrus pouch and most numerous in the distal and midregion of the pouch. Finally, we noted that some of the lower and upper ranges in the egg sizes presented by Manter (1934, p. 290) differed from our observations (96–105 vs 90–104 × 39–45 vs 40–64); however, these differences may have been due, in part, to the small number of eggs measured by Manter compared to our sample size (n = 5 vs n = 29–31; see below).
We also examined 4 paratypes of P. pearsei that were collected by Manter (1934) from his study of the digenetic trematodes of deep-water fish from off Tortugas, Florida (HWML 276, 101928). These worms were heat fixed alive using AFA (alcohol-formol-acetic acid solution) under slight cover-glass pressure and compare well with our specimens in overall appearance. As alluded to earlier, the overall size of Manter’s specimens was larger than the material re-described herein and this can be seen in the higher size ranges for many of the features measured; however, we observed no differences in conventional allometric values and ratios (see Table 3). We would like to note several features we observed in the paratypes of P. pearsei that differed in appearance from how Manter (1934, p. 289–290) described them. Manter (1934) described the hindbody as “rounded posteriorly”; we noted a more pointed posterior extremity in some specimens. We observed a subterminal instead of a terminal oral sucker, no prepharynx instead of a “very short” one (i.e. contraction), and the pharynx was often contorted due to contraction with its anterior half ventrally overlapped by the oral sucker in all specimens observed. The esophagus of each paratype was highly sinuous as to be serpentine in appearance, and while Manter (1934) described the genital pore of P. pearsei as being “opposite [the] anterior edge of [the] intestinal bifurcation”, we noted it to be at the level of either the mid-esophagus [n = 3] or lower esophagus [n = 1] (i.e. contraction/highly sinuous esophagus). Our observations confirmed the triangular shape of the posterior testis and the one instance of diagonal vs tandem testes in some of the type material, but we found in the four paratypes that the size of the post-testicular area relative to the length of the body and forebody matched what we had noted above - 23%–27% (= 1/4 body length) and, thus, slightly greater than forebody length as a percentage of body length (21%–26%) (see Table 3) - rather than being “less than 1/4 body length and usually slightly less than forebody length [as a percentage of body length]” as noted by Manter (1934). We could easily see prostate gland cells in the cirrus pouch (most dense in the anterodextral and anterosinistral portions of the pouch) as well as the seminal vesicle, pars prostatica and ejaculatory duct; therefore, we remain unsure as to the true meaning of Manter’s note of the “prostate gland lacking” in the male terminal genitalia of P. pearsei . The ovary was not only “transversely extended” but also longitudinally compressed, and we noted that either the anterior or posterior border of this feature was 3-lobednot just the anterior border. This latter point is significant as Manter (1934, p. 290) stated that “since the three lobes of the ovary are directed forward” in P. pearsei , this species most resembled Podocotyle levinseni Issaitschikov, 1928 and Podocotyle odhneri Issaitschikov, 1928 from the Russian Arctic, and zoogeographically P. pearsei “represents another deep-water form with closest relationship to Arctic species rather than to any member of adjacent waters.” We note that Gibson (2015) considers P. levinseni to be a taxon inquirendum and P. odhneri to be a junior synonym of P. atomon . Manter (1934) described the vitellarium of P. p e ar s ei as extending anteriorly to the “posterior edge of [the] ventral sucker.” We observed two paratypes to have vitellarium extending to the posterior margin of the ventral sucker, one paratype to possess vitellarium extending further anterior to the mid-level of the ventral sucker, and a fourth paratype to have the left longitudinal band of follicles extending to the posterior margin of the ventral sucker while the right longitudinal band of follicles terminated further anterior at the mid-level of the sucker. The difference in lower and upper ranges in eggs sizes noted earlier was also supported by direct observation of paratype material (96–105 vs 84–104 × 39–45 vs 38–52; see Table 3). In addition, we found the average length of the eggs measured (91.8) in the type material to be completely outside the range for the egg lengths given in the type description (96–105). Manter (1934) had measured 5 eggs, but we were able to measure 18 eggs from the distal uterus—a difficult task as almost all the eggs in his paratype material were collapsed and/or crenulated, although the “rather elongate” shape of the egg as originally described was noticeable.
Further evidence that this material is indeed the rare species, P. pearsei , was noted in Manter (1940, p. 386), who wrote the following, “…it might be stated here that in P. pearsei Manter, 1934 the seminal vesicle is largely a straight wide tube filling most of the cirrus sac but which after narrowing loops back a short distance and then becomes slightly sinuous.” This is exactly how the seminal vesicle appears in our specimens—there is a distinct loop between and connecting the saccate, voluminous proximal portion of the seminal vesicle (itself largely filling up the proximal portion of the cirrus pouch) with the narrower, winding distal portion, and this loop does “loop back” on itself just as described here by Manter (1940).
TABLE ³. DimensiƟns Ɵf Podocotyle pearsei Manter, 1934 frƟm Bathygadus macrops GƟƟde & Bean, Gadomus arcuatus (GƟƟde & Bean), Malacocephalus occidentalis GƟƟde Bean and Urophycis chesteri GƟƟde & Bean cƟllected frƟm the nƟrthern Gulf Ɵf MexicƟ and the Caribbean Sea Ɵff Panama 1.
Parasite P. pearsei P. pearsei P. pearsei P. pearsei
= 1 5 1 4
Type designatiƟn & fixatiƟn VƟucher; cƟld-fixed VƟuchers; live-/heat-fixed VƟucher; live-/heat-fixed2 Paratypes; live-/heat-fixed3 Genital pƟre (GP) tƟ lateral margin 112 50—100 (67) 60 60—116 (86) anteriƟr tƟ VS 28 0 0 18—80 (48)
Pre-Ɵvarian regiƟn L 776 536—800 (659) 480 704—900 (831)
… …continued on the next page TABLE ³. (CƟntinued) Vitellarium tƟ VS 0 BƟth = 0 [n=4]; Right band = 0 Right band = 48; Left band = 0 0 [n = 5]; Left band = 48 [n =1]
Vitelline reservƟir L 116 50—92 (68) [n = 4] 58 124—140 (131) [n = 3] One specimen Ɵf P. pearsei was cƟllected frƟm the intestine Ɵf a B. macrops cƟllected in the Caribbean Sea Ɵff the cƟast Ɵf Panama; hƟwever, this individual was immature and,, its measurements were nƟt included in this table.
While this specimen Ɵf P. pearsei was live-/heat-fixed, it shƟwed cƟnspicuƟus signs Ɵf cƟntractiƟn / alteratiƟn (e.g. cƟntracted anteriƟr end, Ɵral sucker & esƟphagus; pƟsteriƟr highly elƟngated intƟ a fusifƟrm shape; Ɵblique testes [left = anteriƟr testis & right = pƟsteriƟr testis]; dextrally-displaced Ɵvary; unusually large sucker width ratiƟ); therefƟre, elected tƟ keep its measurements separate frƟm the 5 Ɵther live-/heat-fixed specimens (cƟlumn 2 herein).
These 4 specimens are paratypes Ɵf P. pearsei Ɵriginally cƟllected and described by Manter (1934) and hƟused in the HWML (CƟll. # 276 [n = 3], 101928 [n = 1]). AT, anteriƟr testis; L, length; PT, pƟsteriƟr testis; VS, ventral sucker; W, width; range with mean in parentheses; number [n] Ɵf measurements if different frƟm tƟtal number Ɵf
wƟrms examined.
PrƟpƟrtiƟn Ɵf bƟdy length.
It is important to note that when compared to the same morphological features within specimens of P. pearsei collected from M. occidentalis and B. macrops , our specimens of P. pearsei from G. arcuatus exhibited possible host-induced and/or intraspecific variability. Specifically, specimens of P. pearsei from G. arcuatus differed in possessing conspicuous pharyngeal gland cells lateral to the pharynx, a smaller cirrus pouch, more lobate testes, more rounded and less elongated vitelline follicles, a narrower and less extensive uterus, and the number of eggs in the uterus appeared fewer in number within specimens of P. pearsei infecting G. arcuatus versus those parasitizing B. macrops and M. occidentalis . Armstrong (1974, p. 67) also noted variability in the egg size of his specimens of P. pearsei (the same specimens we used in this study) from B. macrops vs G. arcuatus . He described the eggs of P. pearsei from B. macrops as “large, 88.5 to 97.8 long by 44.2 to 49.0 wide (collapsed) or 102.0 long by 51.0 wide (not collapsed)…Description of three whole mount specimens from G. arcuatus …the same as specimens from B. macrops except for the eggs. Eggs 88.5 long to 97.8 long by 60.5 to 65.2 wide (not collapsed)” (see Table 3).
As far as we are aware, this is the only other published original report and description of P. pearsei since Manter (1934) described it and subsequently mentioned this species in later publications ( Manter 1940, 1947, 1954b). Park (1937) and Skrjabin & Koval (1958) included P. pearsei in their key to species in the genus, and Pritchard (1966) included P. pearsei in her list of accepted species in Podocotyle sensu stricto at the time. The reports of P. pearsei by Armstrong (1974) and Blend (1996), while from newly-collected material, are an unpublished dissertation and thesis, respectively - it is this material that constitutes our work herein. The remaining literature we found mentioning this species was host-parasite checklists and should not be considered as original records ( Yamaguti 1958, 1971; Bray 1995; Klimpel et al. 2001, 2009; Blend et al. 2004; Overstreet et al. 2009). We have concluded that due to the scarcity of original reports of this digenean species since its initial description, P. pearsei is likely quite rare and/or not often encountered in the deep sea.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Digenea |
|
Order |
|
|
Family |
|
|
SubFamily |
Plagioporinae |
|
Genus |