Cladomorphini
|
publication ID |
https://doi.org/10.11646/zootaxa.4128.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:B4D2CD84-8994-4CEF-B647-3539C16B6502 |
|
DOI |
https://doi.org/10.5281/zenodo.6084902 |
|
persistent identifier |
https://treatment.plazi.org/id/387F3068-D32F-FF94-FF27-E95220081874 |
|
treatment provided by |
Plazi |
|
scientific name |
Cladomorphini |
| status |
|
4.2.2. Cladomorphini View in CoL Bradley & Galil, 1977
( Figs. 6–12 View FIGURES 6 – 12 , 50 View FIGURES 45 – 51 )
Type-genus: Cladomorphus Gray, 1835: 15 View in CoL .
Cladomorphini View in CoL Bradley & Galil, 1977: 189.
Otte & Brock, 2005: 32.
Hennemann & Conle, 2010: 101.
Cladoxerinae Karny, 1923: 237 (in part).
Cranidiini View in CoL , Zompro, 2004: 134 (in part).
Otte & Brock, 2005: 32 (in part).
Phibalosomatini (Sectio V: Phibalosomata) Redtenbacher, 1908: 399 (in part). Phibalosomini Günther, 1953: 557 View in CoL .
Comments: Günther (1953: 557) established Phibalosomini as a tribe of Phibalosominae (= Cladomorphinae Bradley & Galil, 1977) and distinguished it from the other three tribes in that subfamily i.e. Cladoxerini , Haplopodini and Cranidiini , either by the long antennae, ♀♀ lacking tegmina and alae or distinct medioventral carina of the profemora, which is conspicuously displaced towards the anteroventral carina. As the type-genus Phibalosoma Gray, 1835 is a synonym of Cladomorphus Gray, 1835 , Bradley & Galil (1977: 188, 189) renamed Günther's subfamily and tribe Cladomorphinae and Cladomorphini . Günther contained exclusively Neotropical genera except for the Madagascan Parabactridium Redtenbacher, 1908 , but since the type specimen(s) of the typespecies Parabactridium mirum Redtenbacher, 1908 are lost a confirmed assignment to an existing subfamily or tribe is currently impossible. Certainly however, Parabactridium is rather unlikely to be a member of Cladomorphini and presently attributed to Cladoxerini (→ 4.2.1). The genus Aplopocranidium Zompro, 2004 was misplaced in Cranidiini and transferred to Cladomorphini by Hennemann & Conle (2010: 104).
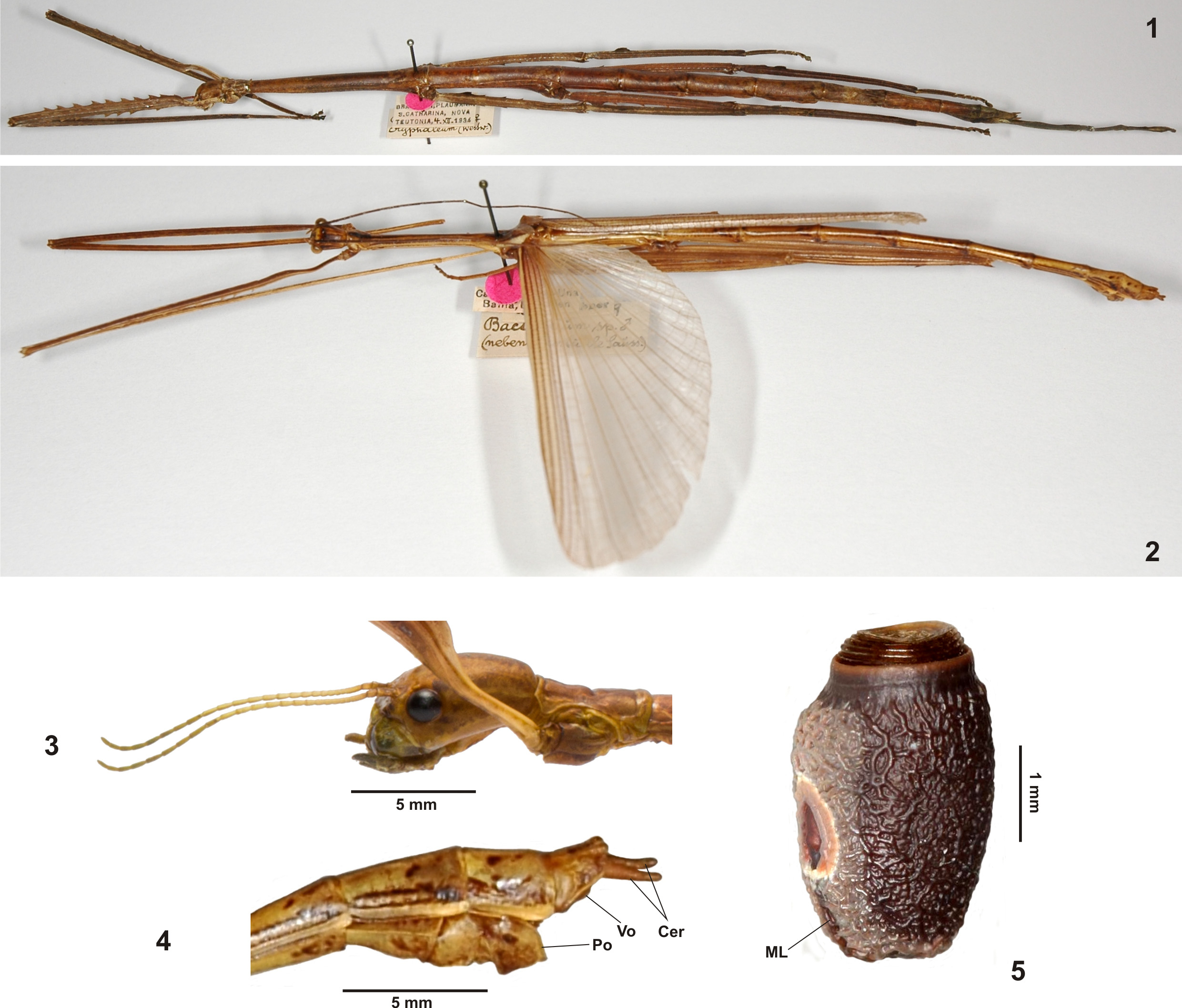
The strongly elongated and filiform gonapophyses VIII, which considerably project over the apex of the anal segment ( Figs. 8–9 View FIGURES 6 – 12 ), and distinct praeopercular organ ( Fig. 8 View FIGURES 6 – 12 ) are shared with ♀♀ of Cranidiini . The developed gonoplacs and presence of a gula are in common with Cranidiini and Cladoxerini . A sister-group relationship with Cladoxerini ( Fig. 409 View FIGURES 409 ) is supported by the morphology of the profemora, fairly slender, stick-like body of ♀♀ and morphology of the eggs.
As currently recognized Cladomorphini appears to be paraphyletic. In particular, genera that were placed in the “ Phanocles -group” of Diapheromeridae : Diapheromerinae : Diapheromerini by Zompro (2001: 195) share numerous morphological characters that support close relation. These include the elongated and filiform gonapophyses VIII, well developed gonoplacs and distinct praeopercular organ of ♀♀, various specializations of the genitalia of ♂♂ as well as the prominent, lamellate and considerably displaced medioventral carina of the profemora and presence of a gula in both sexes. Also the egg-morphology of members of the “ Phanocles -group” supports close relationship with Cladomorphini , the capitulum bearing a raised hollow structure and the internal micropylar plate being open with a distinct median line. A more detailed discussion of the tribe Cladomorphini and revision that also covers the genera attributed to the “ Phanocles -group” by Zompro (2001: 195) is subject of a forthcoming study by the two first authors (Hennemann & Conle, in preparation). Since this will clarify the here suggested relationships, no broader discussion shall be presented at this point.
The following brief and certainly preliminary diagnosis of the tribe is based on the eight genera that are currently attributed to Cladomorphini :
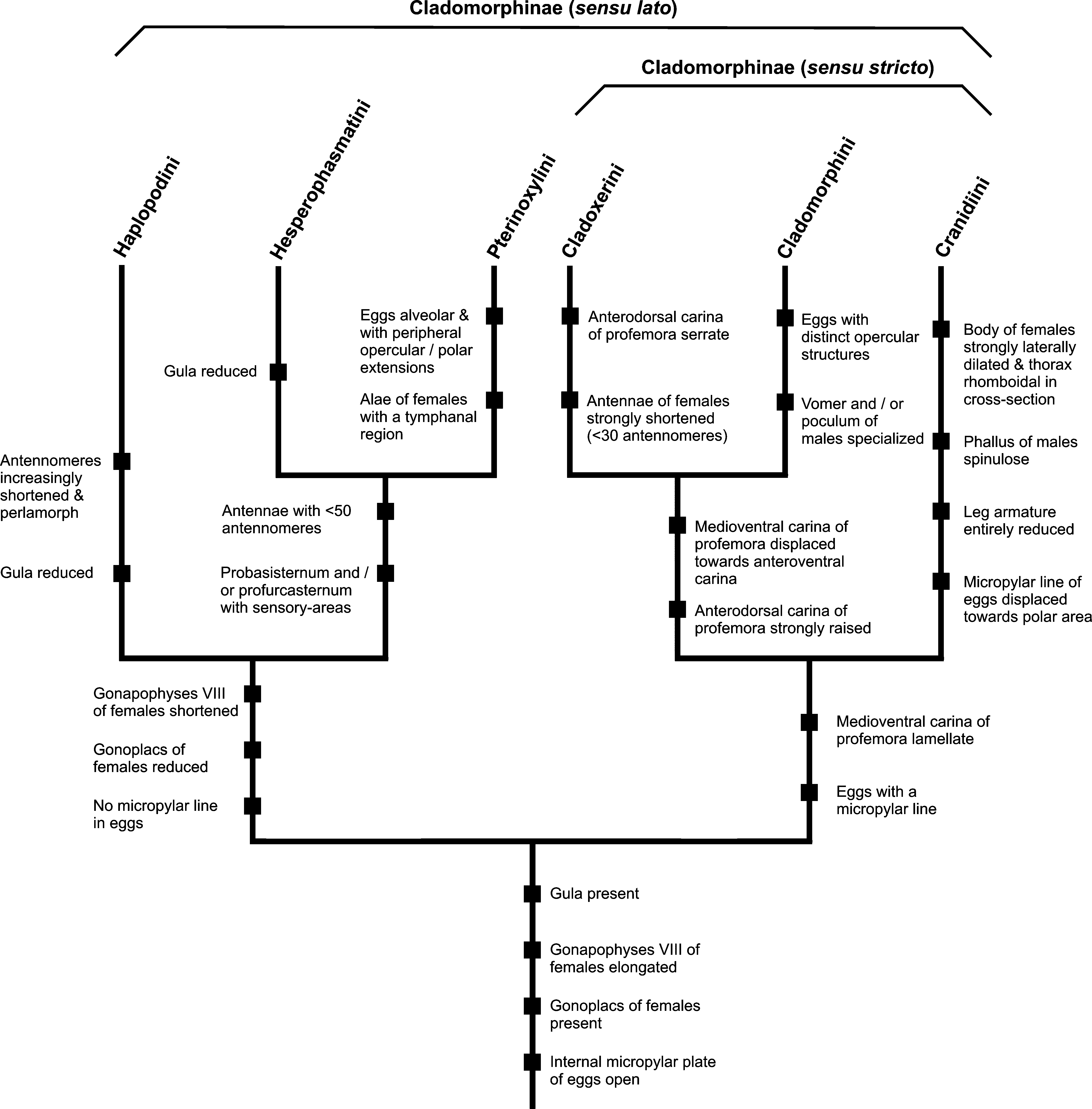
Large to very large (♀♀ up to 245.0 mm), moderately slender to fairly stocky and robust Cladomorphinae with strong sexual dimorphism. Females are apterous and considerably larger than ♂♂, which are slender, stick-like insects mostly with scale-like tegmina and well developed alae (exception are the apterous ♂♂ of Otocraniella Zompro, 2004 ). The anal region of the alae is transparent grey or brown. Ocelli are lacking in both sexes. The body surface is smooth (spines of mesonotum disregarded) and slightly shiny ( Jeremia Redtenbacher, 1908 or Aplopocranidium Zompro, 2004 ) to densely granulose ( Jeremiodes Hennemann & Conle, 2006 or Cladomorphus Gray, 1835 ) or rugose ( Xylodus Saussure, 1859 ). The head is either elongate or oval with the vertex almost flat and unarmed ( Jeremiodes ), has the vertex ± convex and either unarmed (e.g. Jeremia & Hirtuleius Stål, 1875 ), tuberculate ( Cladomorphus ), or bears two prominent horns or foliaceous appendages ( Otocrania Redtenbacher, 1908 and Xylodus ). A gula is present. The antennae are filiform, longer than the mesothorax and consist of>50 antennomeres. The scapus is dorsoventrally flattened, the remaining antennomeres cylindrical. The mesothorax is elongate, at least 2x longer than the head and pronotum combined and often armed with enlarged tubercles, spines or irregular swellings dorsally. The median segment is longer than the metanotum. Abdominal tergum V often bears crest-like posterior lobes and on sternum VII of ♀♀ there is a ± distinct praeopercular organ ( Fig. 8 View FIGURES 6 – 12 ). Occasionally, also tergites VI and VII of ♀♀ may bear posterolateral lobes. Females have the gonapophyes VIII strongly elongated, filiform and projecting considerably over the apex of the abdomen ( Figs. 8–9 View FIGURES 6 – 12 ). Gonoplacs are well developed. The subgenital plate is elongated, either lanceolate or irregularly spatulate, and extends considerably over the apex of the abdomen ( Figs. 8–9 View FIGURES 6 – 12 ). Males show conspicuous specializations of the genitalia, either having the cerci strongly enlarged and hook-like ( Aplopocranidium , Jeremia and Jeremiodes ), the poculum apically elongated into a tube-like or spatulate appendage ( Cladomorphus , Otocraniella , Otocrania and Xylodus , Fig. 10 View FIGURES 6 – 12 ) or the posterior margin of the poculum with two terminal teeth ( Jeremiodes and Jeremia ). The vomer is well developed and sclerotized, being a rather elongate hook-like organ with one (e.g. Jeremia and Jeremiodes ) or two terminal points ( Cladomorphus , Otocraniella and Xylodus ). The profemora are triangular in cross-section, having the anterodorsal carina raised and ± lamellate and the posterodorsal carina considerably reduced. The medioventral carina is distinct, mostly lamellate and ± conspicuously displaced towards the anteroventral carina. The meso- and metafemora and tibiae are trapezoidal in cross-section and often bear single enlarged teeth, spines or lobes. The basitarsi are carinate dorsally with the the two dorsal carinae melted with another (exceptions are Aplopocranidium and Jeremiodes ). The eggs are ± laterally compressed, have a hollow, net-like capitulum and a ± elongate, roughly parallel-sided micropylar plate, which covers at least half of the dorsal capsule surface ( Figs. 11–12 View FIGURES 6 – 12 ). Internally the plate is open with a narrow posteromedian gap and a distinct but clearly separated median line ( Fig. 50 View FIGURES 45 – 51 ).
A well supported autapomorphy of Cladomorphini is represented by the conspicuous specializations of the ♂♂ genitalia, some of which are unique within the entire Phasmatodea (i.e. the strongly elongated and tube-like or spatulate poculum seen in Cladomorphus , Xylodus and Otocraniella , which projects considerably over the apex of the abdomen and resembles an elongated ♀ subgenital plate, Fig. 10 View FIGURES 6 – 12 ).
Distribution: Northern half of South America.
Genera included:
1. Aplopocranidium Zompro, 2004: 134 . Type-species: Bacteria waehneri Günther, 1940: 456 , 495, by original designation of Zompro, 2004: 134.
2. Cladomorphus Gray, 1835: 15 . Type-species: Cladomorphus phyllinus Gray, 1835: 15 , by subsequent designation of Rehn, 1904: 61. ( Figs. 3–8 View FIGURES 1 – 5 View FIGURES 6 – 12 , 45 View FIGURES 45 – 51 )
= Phibalosoma Gray, 1835: 42 . Type-species: Phibalosoma lepelletieri Gray, 1835: 42 , by monotypy. [Synonymised by Kirby, 1904a: 356]
3. Hirtuleius Stål, 1875: 29 . Type-species: Hirtuleius laeviceps Stål, 1875: 81 , by monotypy.
4. Jeremia Redtenbacher, 1908: 425 . Type-species: Jeremia grossedentata Redtenbacher, 1908: 425 , by monotypy.
5. Jeremiodes Hennemann & Conle, 2007: 2 . Type-species: Jeremiodes guianensis Hennemann & Conle, 2007: 6 , by original designation of Hennemann & Conle, 2007: 2.
6. Otocrania Redtenbacher, 1908: 423 . Type-species: Bacteria aurita Burmeister, 1838: 565 , by subsequent designation of Brock, 1998: 26.
7. Otocraniella Zompro, 2004: 137 . Type-species: Otocraniella flagelloantennata Zompro, 2004: 137 , by original designation of Zompro, 2004: 137.
8. Xylodus Saussure, 1859: 62 . Type-species: Xylodus adumbratus Saussure, 1859: 62 , by monotypy.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Cladomorphini
| Frank H. Hennemann, Oskar V. Conle & Daniel E. Perez-Gelabert 2016 |
Phibalosomini Günther, 1953 : 557
| Gunther 1953: 557 |
Cladoxerinae
| Karny 1923: 237 |