Echinolittorina pascua (Rosewater, 1970)
|
publication ID |
https://doi.org/ 10.11646/zootaxa.1420.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:B2E8D420-9177-44DB-9807-12472877F48F |
|
persistent identifier |
https://treatment.plazi.org/id/3B108794-591D-FFF8-BAF1-58ADEDDC4A7B |
|
treatment provided by |
Felipe |
|
scientific name |
Echinolittorina pascua (Rosewater, 1970) |
| status |
|
Echinolittorina pascua (Rosewater, 1970) View in CoL
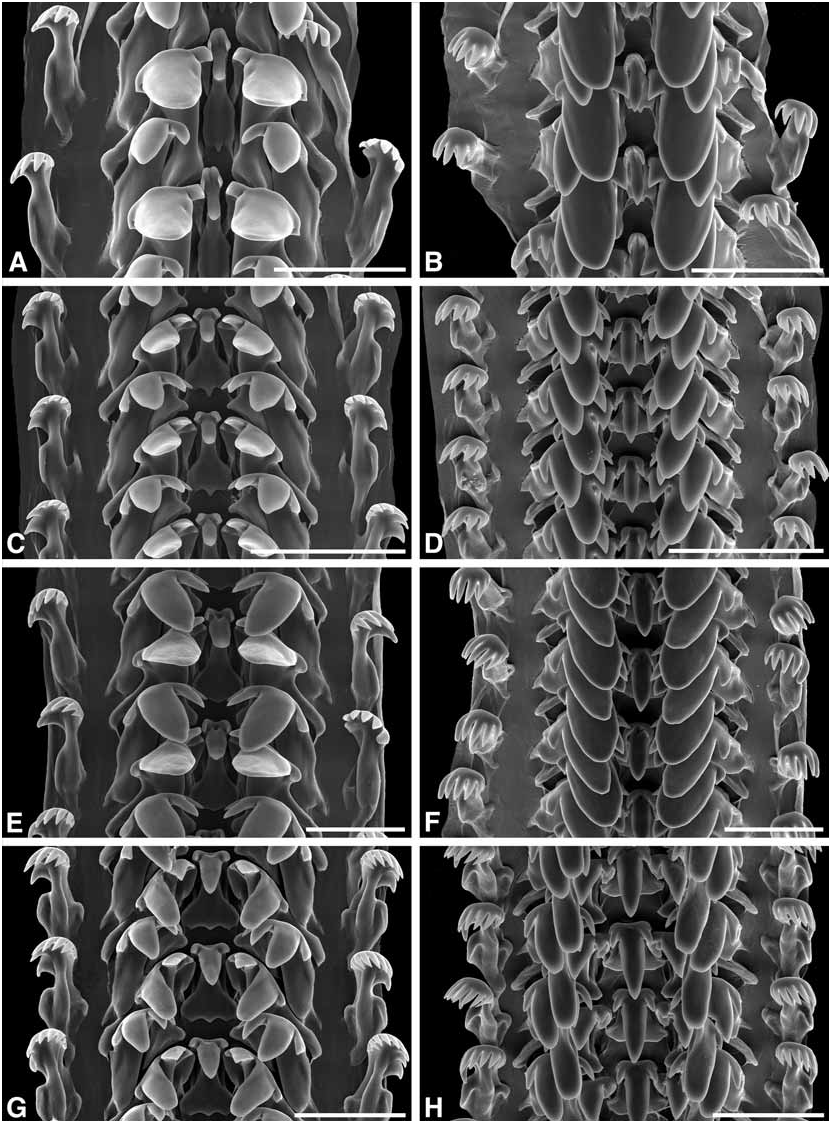
( Figures 15G, H View FIGURE 15 , 21–23 View FIGURE 21 View FIGURE 22 View FIGURE 23 )
? Trochus nodulosus Gmelin, 1791: 3582 View in CoL (Oceano australi [southern Ocean]; lectotype (Clench & Abbott 1942) Chemnitz, 1781: pl. 163, figs 1545, 1546; in part, includes E. tuberculata (Menke, 1828) ; perhaps = E. malaccana View in CoL group; not Trochus nodulosus Solander View in CoL in Brander, 1766).
Tectarius nodulosus View in CoL — Odhner, 1922: 248.
? Turbo trochiformis Dillwyn, 1817: 826 (new name for Trochus nodulosus Gmelin, 1791 View in CoL , not Turbo nodulosus Gmelin, 1791 ; not Turbo trochiformis Brocchi, 1814 ; type locality restricted to Southern Ocean [Pacific]).
? Littorina trochoides Gray, 1839: 140–141 View in CoL (no locality; lectotype (Rosewater 1970) BMNH 1887.4.26.1, Fig. 21H View FIGURE 21 ; nomen dubium). E.A. Smith, 1913: 410.
? Litorina trochoides — Philippi, 1847a: vol. 2: 159, Litorina pl. 3, fig. 3.
? Tectarius trochoides — H. Adams & A. Adams, 1854: 315.
Tectarius pyramidalis — Dall, 1908: 437 (not L. pyramidalis Quoy & Gaimard, 1833 = N. pyramidalis ).
Tectarium pyramidale — Lamy, 1938: 138–139 (not Quoy & Gaimard, 1833).
Nodilittorina (Nodilittorina) pyramidalis pyramidalis — Rosewater, 1970: 481–484, pl. 370, fig. 5 (in part, includes N. pyramidalis , E. malaccana View in CoL , E. austrotrochoides View in CoL , E. cecillei View in CoL , E. marquesensis View in CoL , E. wallaceana View in CoL , E. cinerea View in CoL ; not Quoy & Gaimard, 1833).
Nodilittorina (Nodilittorina) pyramidalis pascua Rosewater, 1970: 484–485 , pl. 370, figs 10–13, pl. 372 (map) ( Easter Island ; holotype USNM 679290 ( Fig. 21I View FIGURE 21 ), 24 paratypes USNM 679291, seen; 7 paratypes ANSP 315563, 23 alcohol paratypes ANSP 15238, not seen).
Nodilittorina pyramidalis var. pascua — Salvat & Rives, 1975: 263, fig. 40.
Nodilittorina pyramidalis pascua — Rehder, 1980: 25–26, pl. 5, fig. 1. Tsuchida & Shimura, 1986: 83, pl. 1, fig. 2.
Nodilittorina (Nodilittorina) pascua — Reid, 1989a: 100.
Nodilittorina pascua — Reid, 2002a: 259–281.
Echinolittorina pascua View in CoL — Williams et al., 2003: 83. Williams & Reid, 2004: 2227–2251.
Taxonomic history: Confusion surrounding Trochus nodulosus Gmelin, 1791 is discussed under the Taxonomic History of the E. malaccana group.
The identity of L. trochoides Gray, 1839 , has been a matter of uncertainty (Philippi 1847a; von Martens 1897), because Gray’s original description in The Zoology of Captain Beechey’s Voyage was brief; he gave no figure or type locality, stating only ‘my collection’. The first figure of the species was by Philippi (1847a), of a single specimen received from Cuming (which therefore may have been compared with, or even part of, Gray’s original material). Gray’s collection was incorporated in the BMNH and in 1887 a lot of eight shells was registered as the type collection of this species. One of these was subsequently designated lectotype (Rosewater 1970). No original label by Gray survives, but the museum register records the locality ‘Low Island’. Following its description, the name trochoides was used only rarely (Philippi 1847a; Reeve 1858; Nevill 1885; E.A. Smith 1913), and then fell into the synonymy of the nodulose IWP species complex under the name T. (or N.) nodulosus (e.g. Tryon 1887; Fischer 1969) or N. pyramidalis (Abbott 1954; Rosewater 1970). It was reestablished as the oldest available name for a tropical IWP species by Reid (1989a) and has become widely used (see Taxonomic History of E. malaccana group, and of E. malaccana ). However, now that this concept of N. trochoides is recognized to be a species complex, it is important to discover to which species the name belongs. Only now has thorough comparison been undertaken using much new material, and including nodulose shells from throughout the IWP region. This has revealed extraordinary similarity and convergence among the small, nodulose shells of the E. malaccana and E. natalensis groups.
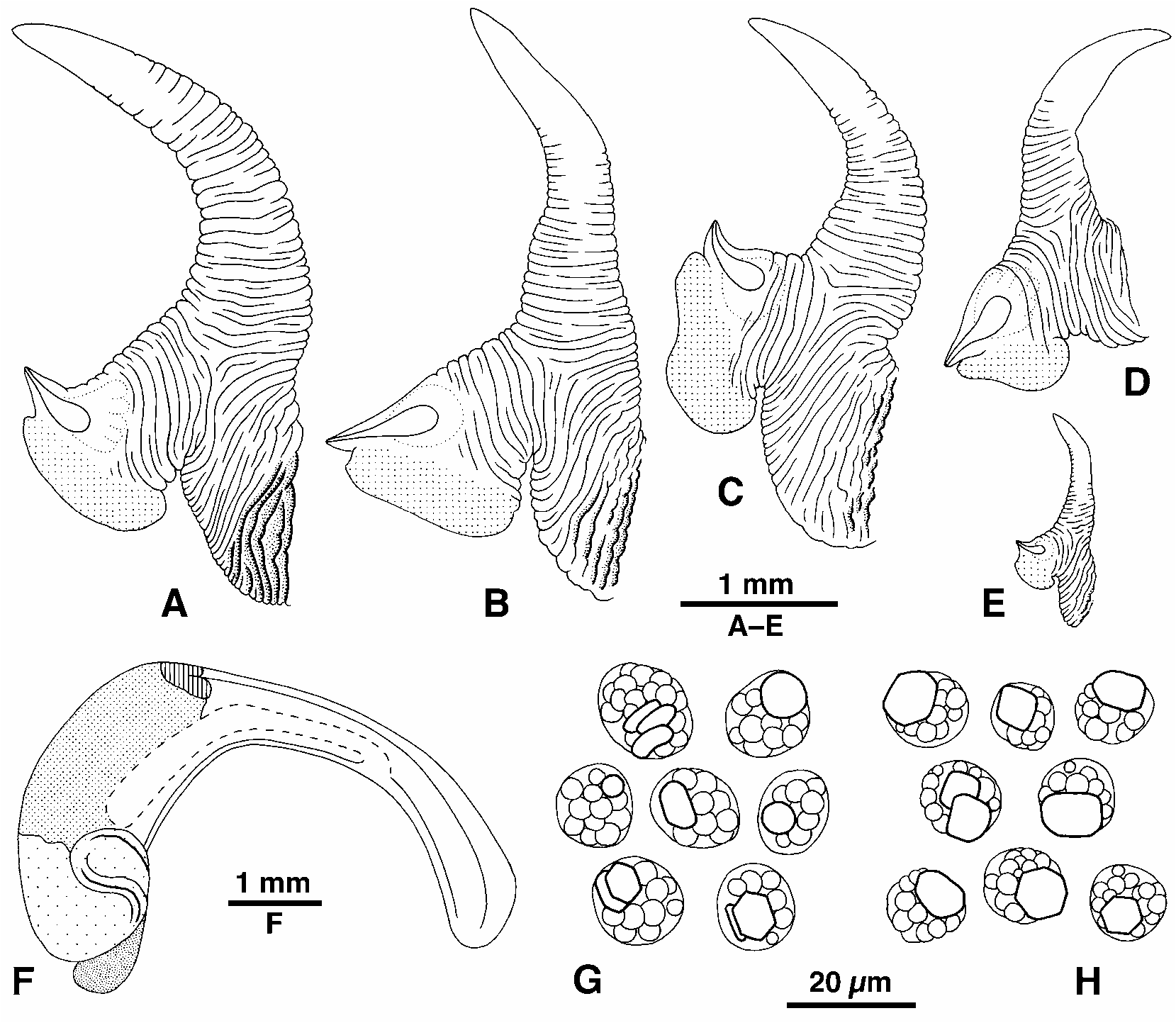
Consequently, it is now clear that the lectotype and paralectotypes of L. trochoides designated by Rosewater (1970) are small specimens of E. pascua (compare Fig. 21F and H View FIGURE 21 ). The short, recurved columellar base, partly fused nodules on the last whorl (forming axial flanges), concave profile above the shoulder leading to nodulose suture, dark colour and absence of conspicuously white nodules, strong regular spiral threads, and presence of a single row of small nodules of the base, are all characteristic of E. pascua . Some of these features can be found in occasional specimens of the E. malaccana group (Figs 27E, H, 32J), but together they unequivocally indicate E. pascua . In contrast, shells of the E. malaccana group have a sharp inner lip, two rows of white nodules on the last whorl, more rounded whorls, finer and more irregular spiral threads, and 0– 4 rows of small nodules on the base. It remains to be determined if the designated specimens are indeed types. Gray (1839) diagnosed the shell as ‘black’, but added the note ‘shell bluish, and the nodules white in some specimens’. The sculpture was described as ‘nodulose near the suture, with a series of compressed nodules on the upper and two on the last whorl, with a series of close-set, roundish granules round the edge in front of the last one’. This could describe the sculpture of the last whorl of the nodulose form of E. pascua , the ‘series of … granules … in front’ indicating the single row of small nodules on the base, but is not sufficient to exclude the E. malaccana group. The size quoted was 7 by 4 lines (14.8 x 8.4 mm; Gray was usually accurate in his measurements), which is large for the E. malaccana group, but common for E. pascua ; it is also considerably larger than the largest (11.2 mm) of the putative types. In the preface to his work, Gray (1839) admitted that not all the material described came from Beechey’s expedition, but stated that specimens from the voyage were deposited in the museums of the Zoological Society and of the Naval Hospital. He did not mention whether any of the material was in his own possession. The route of the voyage is therefore equivocal evidence. Nevertheless, it is interesting that the Blossom called at all the recorded localities of E. pascua (Easter I. and three islands of the Pitcairn group), as well as at Macao, Ryukyu Islands and Bonin Islands (where members of the E. malaccana group could have been obtained) (route described by Beechey 1831). Finally, although ‘Low Island’ is a common geographical appellation, ‘Low Archipelago’ was the current name for the Tuamotu Archipelago in the early nineteenth century, in which Easter Island was at that time also included (e.g. Darwin 1842, although Beechey 1831 did not use the name). The shell figured by Philippi (1847a) is large (18 mm, i.e. larger than any specimen of the E. malaccana group) and entirely black, but for a pale basal band, and is therefore probably also a specimen of E. pascua . (The specimen figured by Reeve 1858, as L. trochoides exists in BMNH, and is a member of the E. malaccana group that does not correspond with Gray’s diagnosis.)
In summary, while the putative types of L. trochoides are E. pascua , and there is circumstantial evidence that Gray could have obtained specimens of that species, the discrepancy in size and provenance between the type specimens and Gray’s inconclusive description, and the lack of clear type locality (or of clear connection with Beechey’s voyage), all combine to raise doubts. It would be undesirable to replace the now familiar E. pascua with an older name that has not been used in this sense since E.A. Smith (1913), and then only in a list. Furthermore, the name trochoides has become familiar (since Reid 1989a) in another sense, for the E. malaccana group, to which E. pascua does not belong. The epithet pascua has not been used sufficiently frequently to justify reversal of precedence (ICZN 1999: Art. 23.9) in order to conserve its current usage. Therefore, stability is best served by considering L. trochoides Gray, 1839 a nomen dubium. (See also Taxonomic History of E. malaccana group for further discussion.)
Rosewater (1970) introduced pascua as a subspecies of N. pyramidalis , the name then used for most of the nodulose western Pacific littorinids (i.e. N. pyramidalis s.s. and E. malaccana group). The type collection consisted of the distinctively large, broad, weakly nodulose forms found on Easter Island ( Fig. 21I View FIGURE 21 ). Neither he nor Reid (1989a, 1992, 2001a) noticed that the smaller, nodulose shells from the limestone rocks of Henderson Island ( Fig. 21F, G View FIGURE 21 ) were identical with the types of L. trochoides ( Fig. 21H View FIGURE 21 ). Earlier authors had identified E. pascua with either N. pyramidalis or the E. malaccana group, probably on the basis of geographical proximity ( E. marquesensis of the E. malaccana group occurs as far east as the Marquesas Is).
Diagnosis: Shell high conical, 1–2 rows of axially elongate nodules on last whorl; 15–23 spiral threads on last whorl (including base); columella turned out at base to make inner lip rounded and forming a thickened boss; brown to black, nodules same colour or sometimes brown to grey. Easter Island and Pitcairn Group. COI: GenBank AJ623025 View Materials , AJ623026 View Materials .
Material examined: 18 lots (including 10 penes, 4 sperm samples; 5 pallial oviducts, 2 radulae).
Shell ( Fig. 21 View FIGURE 21 ): Mature shell height 3.8–15.3 mm (to 17.9 mm, Rehder 1980). Shape conical to high conical (H/B = 1.33–1.68; SH = 1.77–2.33); spire whorls flat or concave between suture and peripheral nodules, suture indistinct; spire profile straight to slightly concave; periphery of last whorl weakly angled. Columella short, concave, turned out at base to make inner lip of aperture rounded (i.e. there is no projecting anterior lip at base of columella, and edge of inner lip is not sharp); inner lip forms a smooth boss continuous with eroded pseudumbilical and parietal area. Sculpture of last whorl: single series of axially elongate nodules from midwhorl to below periphery, 10–17 at periphery; in strongly sculptured shells nodules are divided to form a paired series and suture is irregularly nodulose ( Fig. 21F–H View FIGURE 21 ); entire surface with strong, regular, spiral threads, 11–17 at and above periphery, continuous across nodules; microstriae over entire surface; base with 4–7 threads and single row of small nodules (occasionally divided by a spiral groove). Protoconch not well preserved, approx. 0.28 mm diameter, 2.5 whorls. Colour: purple-brown to black, fading to grey, sometimes pale sutural band and basal band, peripheral nodules usually brown to black, sometimes pale grey, basal nodules occupy pale basal band; aperture black-brown, with pale band at base; columella purple-brown.
Animal ( Fig. 22 View FIGURE 22 ): Head black, no unpigmented stripe across snout, tentacle dark grey to black (owing to fusion of two longitudinal stripes, as seen in palest animals only), pale around eye and rarely across base, pale at tips; sides of foot black. Opercular ratio 0.48–0.55. Penis ( Fig. 22A–E View FIGURE 22 ): filament gradually tapering to pointed tip, with fine annular wrinkles for most of its length, filament 0.5–0.6 total length of penis, sperm groove extends to tip; mamilliform gland about half size of glandular disc, borne together on projection of base; penis unpigmented or slightly pigmented at base. Euspermatozoa 114–128 µm; paraspermatozoa ( Fig. 22G, H View FIGURE 22 ) spherical or slightly oval, 12–17 µm diameter, filled with large round granules, containing 1–2 short irregularly oval to rectangular rod-pieces, hexagonal in section and not projecting from cell. Pallial oviduct ( Fig. 22F View FIGURE 22 ): bursa opening at one third to one half length of straight section (from anterior) and extending back almost or fully to albumen gland. Development predicted to be planktotrophic.
Radula ( Fig. 15G, H View FIGURE 15 ): Relative radula length 4.65–6.64. Rachidian: length/width 1.33–1.50; tip of major cusp pointed. Lateral and inner marginal: major cusp on each of similar size, tips rounded. Outer marginal: 8– 10 cusps.
Range ( Fig. 23 View FIGURE 23 ): Southeastern Polynesia. Range limits: Oeno I. (USNM 789558); Bounty Bay, Pitcairn I. (CUMZ; USNM 793954); North Beach, Henderson I. (CUMZ; BMNH 20040233); Hanga Tee, Easter I. (BMNH 20040232; USNM 756082); Ovahe, Easter I. (BMNH 20040231; USNM 756048).
Habitat: On high volcanic islands this species is abundant on basalt rocks in the littoral fringe, on both strongly exposed shores and in moderately sheltered inlets. On raised coral islands it occurs on limestone.
Remarks: The range of this species is restricted to a few small and isolated islands in the extreme southeast of Polynesia: those of the Pitcairn Group ( Pitcairn, Oeno and Henderson Islands, within 200 km of each other) and Easter Island about 1900 km to the east (Rosewater 1970; Rehder 1980; Paulay 1989). On all these islands it is the sole species of Echinolittorina . The distance to Easter Island is close to the maximum dispersal distance of 2100 km estimated from extralimital records of Echinolittorina species (see Remarks on E. cinerea and Discussion), so that conspecificity of the Pitcairn and Easter populations could be questioned. Nevertheless, molecular data from single examples from Henderson and Easter Islands are closely similar, within the expected range for conspecifics (Williams & Reid 2004). If there is gene flow between the populations, it is most likely to occur from east to west in the South Equatorial Current; prevailing surface currents in the Pitcairn Group are from the north east (Paulay & Spencer 1988). There are no records of the species from Ducie Island, an atoll 1570 km west of Easter Island, but during low sea-level stands this may have provided a stepping stone for dispersal. This species is undoubtedly not present on Polynesian islands to the west; the littorinid faunas of the Tuamotu Islands and of Rapa are well known and do not include it. Several authors have noted the existence of a small number of endemic molluscs shared between the Pitcairn and Easter Groups (Rehder 1980; Paulay & Spencer 1988; Preece 1995). The degree of endemism among the impoverished molluscan fauna of Easter Island has been estimated at 36%, attributable to its extreme geographical isolation (Boyko 2003). Only a single, poorly preserved, protoconch was found in available samples, but this did not differ significantly from those of other Echinolittorina species , suggesting that there are no peculiarities in the larval development of this species despite the isolation of the islands on which it occurs.
The island localities of E. pascua fall into two quite different groups. All are situated in oceanic waters of low productivity, but Easter and Pitcairn Islands are volcanic high islands with basalt shores strongly exposed to waves, whereas Henderson is a raised coral island and Oeno an atoll, in both cases sheltered by coral reefs and with only limestone substrates. This supports the apparent indifference of Echinolittorina species to the nature of their rock substrate. Specimens from the limestone islands are, however, narrower and more strongly nodulose ( Fig. 21F, G View FIGURE 21 ) than those from basalt shores, as has been observed in other species (see E. natalensis , E. malaccana , E. wallaceana , E. hawaiiensis ).
Mitochondrial gene sequences (COI, 12S rRNA, but not nuclear 28S rRNA) suggest that E. pascua is sister to the other three species of the E. natalensis clade, E. natalensis , E. subnodosa and E. omanensis , all of which are distributed in the western Indian Ocean, about 18000 km (or 180° longitude) distant from E. pascua . This is far too great a distance to be explained by dispersal, past or present. Instead, it has been suggested that this clade of Echinolittorina was formerly more widespread and has become restricted to the periphery of the IWP region by extinction (Williams & Reid 2004). Interestingly, the five members of the E. malaccana clade occupy the intervening area, from western India to the Marquesas Islands, and these two clades are nowhere sympatric. Their habitats and shells are closely similar, and competitive replacement of the E. natalensis clade by the E. malaccana clade is a possibility. The time of separation of E. pascua from the rest of the E. natalensis group is estimated at 10–20 Ma (Williams & Reid 2004) and the origins of Oeno and Henderson Islands fall in this range (16 and 13 Ma respectively), whereas Pitcairn and Easter Islands are much younger (less than 1 Ma and 2.5 Ma, respectively) (Preece 1995; Boyko 2003).
No other Echinolittorina species are sympatric with E. pascua , so no misidentification of correctly localized specimens should occur. Shells of other members of the E. natalensis clade ( E. natalensis , E. subnodosa and E. omanensis ) are similar, but all usually have a third row of nodules at the shoulder, which is absent in E. pascua ; anatomically, the four species do not differ significantly. Large specimens from Easter and Pitcairn Islands are larger and broader than members of the E. malaccana group, have a distinctively rounded inner lip, and usually have a sculpture of axial flanges at the periphery rather than two rows of discrete nodules. However, the narrower and more nodulose shells from limestone islands ( Fig. 21F, G View FIGURE 21 ) are more similar to those of the E. malaccana group (see comparison in Taxonomic History above), although they differ anatomically.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Echinolittorina pascua (Rosewater, 1970)
| Reid, David G. 2007 |
E. austrotrochoides
| Published 2007 |
E. marquesensis
| Published 2007 |
E. wallaceana
| Published 2007 |
Nodilittorina (Nodilittorina) pyramidalis pascua
| Rosewater 1970: 484 - 485 |
Nodilittorina pyramidalis var. pascua
| Rosewater 1970 |
Nodilittorina pyramidalis pascua
| Rosewater 1970 |
Littorina trochoides
| Gray 1839: 140 - 141 |
Tectarius trochoides
| Gray 1839 |
Tectarius pyramidalis
| Quoy & Gaimard 1833 |
L. pyramidalis
| Quoy & Gaimard 1833 |
N. pyramidalis
| Quoy & Gaimard 1833 |
Litorina
| Menke 1828 |
Turbo trochiformis
| Dillwyn 1817: 826 |
Turbo trochiformis
| Brocchi 1814 |
Trochus nodulosus
| Gmelin 1791: 3582 |
Trochus nodulosus
| Gmelin 1791 |
Turbo nodulosus
| Gmelin 1791 |
Trochus nodulosus
| Solander 1766 |