Naupactus xanthographus (Germar)
|
publication ID |
https://doi.org/ 10.1080/00222933.2017.1346715 |
|
publication LSID |
lsid:zoobank.org:pub:051587DD-37C2-4216-AA61-0E563BB44D64 |
|
persistent identifier |
https://treatment.plazi.org/id/3C7887C4-7572-CE16-53EF-FA3AAE37B77D |
|
treatment provided by |
Felipe |
|
scientific name |
Naupactus xanthographus (Germar) |
| status |
|
Naupactus xanthographus (Germar) View in CoL
( Figures 1 View Figure 1 (a – c), 3(a, c, e), 4(a, b), 5a)
Leptocerus xanthographus Germar 1824, p. 424 View in CoL . Type material: from Buenos Aires, Argentina, probably in Halle , Germany (not seen) .
Naupactus xanthographus: Schoenherr 1833, p. 571 View in CoL ; Schoenherr 1840, p. 7; von Dalla Torre et al. 1936, p. 24 (catalogue); Blackwelder 1947, p. 795 (checklist); Hustache 1947, p. 39 (in key); Wibmer and O ’ Brien 1986, p. 62 (checklist).
Redescription. Female ( Figure 1a, c View Figure 1 )
Body length 12 – 16 mm. Vestiture brown or grey-brown, with pattern of whitish or whitish/yellow stripes on pronotum and elytra; pronotum with two pairs of longitudinal yellowish or whitish stripes, one on disc and another on margins; elytra with whitish stripe along suture, yellow stripes along anterior third of 5° interval and along 7 – 8° intervals, the latter obliquely curved towards 3 – 4° intervals on posterior third; middle half of 3° interval with small white macula (typical form) or long stripe (Brazilian populations). Rostrum 1 – 1.15× as long as wide at apex; lateral carinae strong, slightly convergent towards forehead. Eyes round, convex. Scape reaching to slightly exceeding anterior margin of pronotum; funicular article 2, about twice as long as article 1. Pronotum subcylindrical, 1.10 – 1-20× as wide as long; sides slightly divergent towards base; disc smooth, flat. Elytra navicular, 1.55 – 1.60× as long as wide; sides strongly curved and narrowed towards apex; apical tubercles large; intervals about 3× as wide as punctures of striae. Front femora 3.05 – 3.35× as long as wide and 1.10 – 1.25× as wide as hind femora. Front tibiae with large mucro and 8 – 10 small denticles on inner margin; middle tibiae with smaller mucro and minute denticles; hind tibiae lacking mucro and denticles; corbel of hind tibiae broad, squamose. Ventrite 5 slightly shorter than ventrite 2.
Female genitalia
Sternite VIII as in Figure 3a View Figure 3 . Ovipositor slightly shorter than abdomen, lacking rows of long setae on posterior third, on external side of baculi ( Figure 3c View Figure 3 ). Spermatheca ( Figure 3e View Figure 3 ) subcylindrical, small (0.63 mm), strongly sclerotized on proximal half of body; nodulus and ramus almost indistinct; spermathecal duct about 6× as long as spermatheca.
Male ( Figure 1b View Figure 1 )
Body length 11 – 13 mm. Smaller and more slender than female. Rostrum about 1.25× as long as wide. Pronotum about as wide as long; disc slightly convex; sides not divergent towards base. Elytra about twice as long as wide; sides slightly curved. Hind tibiae with row of denticles on inner margin, the distal one very large, with the appearance of a proximally mucro displaced. Ventrite 5, 1.70 – 2× as long as ventrite 2, apex slightly inwardly bent.
Male genitalia ( Figure 4a, b View Figure 4 )
Body of penis 1.4 – 1.5× as long as penis apodemes; apex arrow-shaped, rounded and slightly protruding beyond lateral points; ostium about as long as wide; endophallus with distinct spiny area but lacking internal pieces.
Material examined
ARGENTINA. Buenos Aires: Bragado, 1 November 2006, G del Rio (1f MLP- IBOL); Capital Federal, 26 March 1979, O Salomon (1f USNM); idem, O de Ferraris (2f MLP); Flores, 21 April 1912 (1m MLP); Florencio Varela, 23 October 1949, O de Ferraris (1m MLP); Haedo, February 1926, Harrington (1f 1m USNM); Hurlingham, Biocontrol Lab., 20 January 1972 (1f MLP); Isla Martín García, 1 May 1935, M Viana (1m MLP); idem, November 1937, J Costa (1m mLP); José C Paz, 13 January 1940, PA Berry (1m USNM); idem, 1937, Maldonado (8f MLP); La Plata, 23 November 1977, S Tuler (1m MLP); idem, AR Bezzi (6f 5m MLP); idem, 12 April 2014, A Lanteri (1m MLP); Lomas de Zamora, 1 November 1919 (1f MLP); Luján, 18 December 1938 (1f 1m USNM); Pacheco, 22 October 1925, Bridarolli (1m MLP); Parque Pereyra Iraola, 11 January 1991, A Marvaldi (2f 1m MLP); Pilar, 18 September 1938, Castillo (1m MLP); Punta Lara (2f MZSP); idem, 10 April 1938, Maldonado (3f 2m MLP); Quilmes, 13 February 1991, A Marvaldi (1f 1m MLP); A Martínez (2f MLP); San Antonio de Areco, 16 December 1942, PA Berry (1f 1m USNM); idem, 22 December 1938, PA Berry (1m USNM); San Fernando, 25 November 1932, Arroyal (2f 1m MLP); San Isidro, April 1957, Molinari (1f USNM); idem, III March-April 1954, IV April-May 1955 (3f 1m MLP); San Nicolás, 33° 21 ʹ S, 60° 13 ʹ W, 18 m, 8 March 2006, D Carpintero (1f MLP, IBOL); Sierra de la Ventana, 20 October 1965, Ronderos (2f MLP); Tigre, 12 May 1950, M Viana (1f MLP); idem, 28 September 1968, CW O ’ Brien (1m MLP); Villa Calzada, 1 April 1940 (1f 1m MLP); Villa Elisa, 15 December 1979, O Flint (1m USNM); Zelaya, 18 February 1968, O Flint (1f USNM); idem, 24 April 1927, Kraliuk (1m USNM). Catamarca: no loc. (3f 6m MLP). Córdoba: Bajo Grande, 14 August 1939, Maldonado (1f MLP); Camino 60 cuadras, 29 January 1953 (1f USNM); Capilla del Monte, 1000 m, 31 January 1981, C Bordón (1f USNM); Cruz del Eje, 28 April 1950, Maldonado-Bruzzone (1m MLP); El Sauce, January 1962, M Viana (1f 1m MLP); Las Rabonas, 27 February 2997 (1m MLP); Puente Río de los Espinillos, Embalse Los Molinos, 31° 29 ʹ 40 ʺ S, 64° 34 ʹ 35 ʺ W, 925 m, 27 February 2006, D Carpintero (3m MLP, IBOL); Río Cuarto, Arroyo del Gato, 28 February 1941, M Birabén (1f MLP); Río Primero, 21 May 1953 (1f USNM); Villa Nueva, 1 January 1939 (1m MLP). Corrientes: Santo Tomé (1m MLP). Chaco: Roque Saenz Peña (1f MLP). Entre Ríos: Colón (1f MLP); Concordia, Salto Chico, February 1951, Nuñez-Regueiro (1m MLP); Gualeguaychú (1f MLP). La Pampa: General Pico, 21 September 1942, PA Parker (1f USNM; 1f MZSP). La Rioja: Chilecito, 20 February 1939, Birabén-Scott (3f 4m MLP). Mendoza: Agrelo, Luján, 10 January 1947, s/papa, Torres (2f 1m MLP); Las Heras, 15 March 1945, Torres (1f 2m MLP); Pedro Vidal, Tupungato, 14 January 1947, Torres, s/papa (21f 33m MLP); Mendoza, C Bruch (2f 2m MLP); no loc. (12 ex MNHN). San Juan: San Juan, December 1938, PA Berry (3f USNM). San Luis: Concarán, 11 February 1960, Vidal-Trotta (7f 9m MLP); San Luis, 1 January 1934, Vignatti (1f MLP); San Pablo, 13 March 1960, Vidal-Trotta (1m MLP); Sierras de San Luis, 1 February 1933, Vignatti (3f 1m MLP); Suyuque, 1 December 1933, Vignatti (2f MLP). Santa Fe: Esperanza, 1 December 1989, P Stock (2m MLP); Rosario (3m MZSP). Santiago del Estero: Beltrán, October 1942, MLP); Maldonado (1fDto. Capital, 2 February 1940 (1m MLP); Kenti Taco, Sumapampa, 25 November 1944, Maldonado (1m MLP); Santiago del Estero, Wagner (15f 15m MLP). Tucumán: San Miguel de Tucumán, Moznette (1f USNM). BRAZIL. Río Grande do Sul: Nova Padua, 21 September 2000, on videira var. Niagara rosada (1f 1m MZSP). Santa Catarina : Florianópolis, 19 January 2004, A Cordeiro (1f 2m MZSP); Nova Teutonia, November 1945, 300 – 500 m, 27° 11′ S, 52° 23 ʹ W, F Plaumann (6f MZSP, 9f 1m CWOB, 1m MLP); idem, 18 July 1939, F Plaumann (1f 1m MLP); idem, October 1935 B Pohl (2f 1m MZSP); idem, 20 October 1962, F Plaumann (3f MNRJ); idem November 1980, F Plaumann (2f 1m MZSP); November 1981, F Plaumann (1f MZSP); idem, March – May 1966, F Plaumann (5f MZSP). CHILE. Atacama: Copiapó, 3 December 1960, Oakley (1m USNM). Región Metropolitana: Linderos, 12 November 1942 (1f 1m USNM); San Bernardo, 29 November 1960, on nectarine, Oakley (1f USNM). Valparaíso: Llay Llay, 27 April 1948, on grape (1f USNM); idem, 26 November 1960, Oakley (1f 1m USNM); Santa María, March 1967, Latta (1f 1m USNM); Valparaíso, 6 February 1905 (1f USNM). PARAGUAY. Itapúa: Hohenau, Richter (6f MLP). Paraguarí: Ybycuí (25 km SE), Ybycuí National Park, 12 – 24 April 1980, PJ Spangler et al. (1f 1m USNM). URUGUAY. Artigas: Isla Rica, 9 February 1978, L Zolessi, E Morelli & F Rodríguez (2m URUC). Colonia: Barra Arroyo El Chileno, 11 January 1970, GJ Wibmer (1m CWOB). Montevideo: Millán, 13 April 1958, MA Monné (1f URUC); Montevideo, Palermo, 18 March 1922, Marshall (1f 1m USNM); idem, 28 April 1940, PA Parker (1m USNM); idem, 26 April 1943, PA Berry (1f USNM); Montevideo, Tremoleras (8f 3m MLP); Sayago, 2 May 1932 (1f MLP); idem, 25 February 1967, MS Moratorio, CS Morey & MA Monné (1m CWOB); Colón, 10 March 1931, Montoro Cuarch (1f MLP); idem, 15 March 1929 (1f MLP); Montevideo, Pocitos, 22 October 1932, C Carbonell (1m URUC); idem, 4 May 1958, R Praderi (1f CWOB); Punta Carreta, 4 January 1959, M Lozaudo (1m URUC). Soriano: Colonia Concordia, 8 July 1959, JC Zorrilla & MA Monné (2m URUC). Treinta y Tres: Río Olimay, November 1958, MA Vignoli (1f URUC).
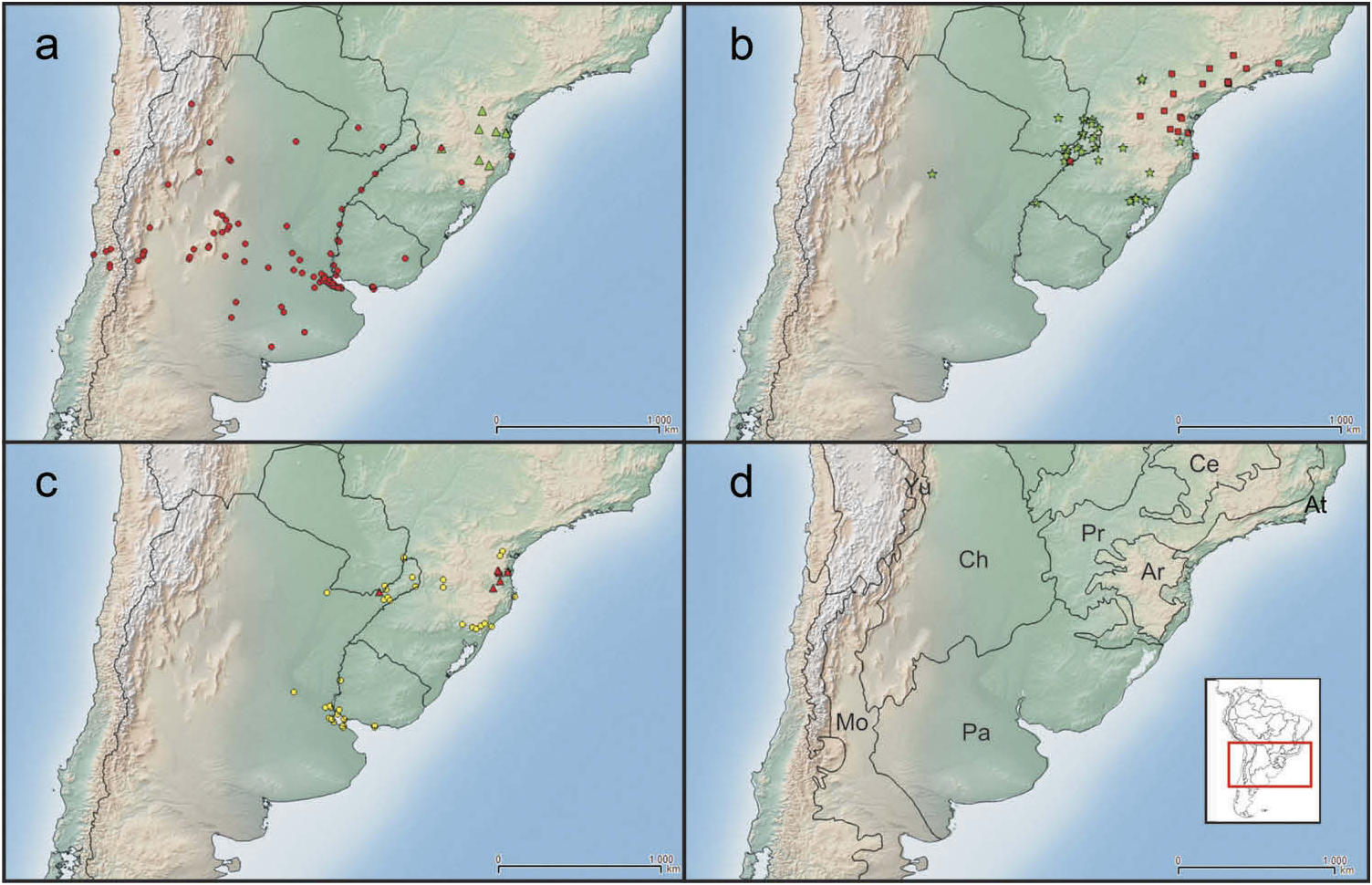
Geographic distribution ( Figure 5a, d View Figure 5 )
Naupactus xanthographus is distributed mainly in the Pampean biogeographic province. It was recorded for Argentina (Buenos Aires, Catamarca, Córdoba, Corrientes, Chaco,
Entre Ríos, La Pampa, La Rioja, Mendoza, Misiones, San Juan, San Luis, Santa Fe, Santiago del Estero and Tucumán), southern Brazil (Rio Grande do Sul and Santa Catarina), Paraguay (Itapúa and Paraguarí), and Uruguay (Artigas, Colonia, Montevideo, Soriano and Treinta y Tres) . It was introduced in Chile in 1942 ( Durán 1944), where is currently widespread from Atacama to Valparaiso, and also occurs in Juan Fernández islands .
D ’ Araujo e Silva et al. (1968) cited N. xanthographus for Paraná and Sāo Paulo ( Brazil); however, we did not find any material from these Brazilian states. We suspect that it could be a misidentification with either N . navicularis or N. mimicus .
Host plants
One of the main native hosts of N. xanthographus is Erythrina crista-galli L. ( Fabaceae ), native tree from north-eastern and central-western Argentina, eastern Bolivia, southern Brazil and a great part of Paraguay ( Burkart 1952; Izaguirre and Beyhaut 1998). The distribution of the weevil follows, approximately, the range of this host plant, which has been introduced in central Chile as ornamental and also in south-eastern USA.
About 50 agricultural plants have been cited as hosts of N. xanthographus ; the most important is Vitis vinifera L. ( Vitaceae ), because of the damage produced in Chile, Argentina (Mendoza) and Brazil (Rio Grande do Sul) ( Caballero 1972; González 1982; Ripa 1986a; González et al. 1992; Lanteri et al. 2002a; Ripa and Larral 2008). Other fruit plants damaged by N. xanthographus in Chile are Prunus persica var. nectarine , Prunus armeniaca L., Pyrus communis L., Malus domestica Borkh. (Rosaceae) , Juglans regia L. ( Juglandaceae ), Persea americana Mill. (Lauraceae) , and Citrus × sinensis Osbeck ( Rutaceae ) ( Elgueta 1993; Artigas 1994). In Argentina it is usually associated with alfalfa ( Lanteri 1994), garden plants, e.g. Ligustrum sp. (Oleaceae) ( Lanteri et al. 2002a), berries and cherries, e.g. Prunus avium L., Prunus cerasus L. ( Rosaceae ) and Ribes sp. (Grossulariaceae) in some valleys of Patagonia (del Río et al. 2010), potatoes in Mendoza, and most recently, with soybean in the central area of the country. An olfactometer bioassay for the analysis of the behavioural and nutritional ecology of this weevil demonstrated that starved males and females showed no preference to volatiles of grapes; however, non-starved males and females preferred grape volatiles ( Vera et al. 2016).
Biology
Due to its economic importance, N. xanthographus is one of the best-studied South American broad-nosed weevils ( Caballero 1972; González 1982; Ripa 1986a; González et al. 1992; Ripa and Larral 2008). The adults feed on the aerial parts of the plants, mainly leaves, flower buds, and in some instances, fruits (e.g. grapes in vineyards). The females lay eggs in clusters ( Marvaldi 1999), usually in crevices of the upper parts of the plants (trunks, large branches, rolled leaves) but not in calices of orange fruits, such as N. cervinus Boheman, 1840 ( Olivares et al. 2014). Egg laying includes 12 – 50 eggs ( Caballero 1972).
First instar and mature larvae of N. xanthographus have been described and compared with other Entiminae larvae by Loiácono and Díaz (1992), Marvaldi and Loiácono (1994), and Marvaldi (1998). The larvae cause damage to the rootlets and roots of the plants and in some instances reach the phloem; the life cycle is completed in 12 – 16 months ( González 1983; Ripa 1986b).
Several natural enemies are known for N. xanthographus , including pathogenic fungi and nematodes, and parasitic wasps ( Lanteri et al. 1998). Centistes sp. (Braconidae) is a parasite of adults; Fidiobia asinus Loiácono (Platygastridae) and Grassator viator De Santis (Eulophidae) are parasites of eggs ( De Santis 1948; Loiácono 1982). Mejías (2003) investigated the biological control of N. xanthographus by means of Beauvaria bassiana (Bals.) Vuill., Metarhyzium anisopliae (Metchnikoff) Sorokin and Steinernema sp. in Chile. Several chemical control strategies have been applied and are still under study in this country ( Ripa 1985, 1987; Sazo and Gerstle 1989; Ripa and Berhó 1990; Pinto and Zaviezo 2003). Moreover, a PCR-based diagnostic system has been developed to identify N. xanthographus at the egg stage ( Aguirre et al. 2015).
Remarks
Naupactus xanthographus shows a remarkable sexual dimorphism. Males are smaller and more slender than females, legs and antennae are longer, and the hind tibiae bear a row of denticles on the inner margin, the distal one with the appearance of a proximally displaced mucro . The species also shows geographic variation . The typical form (type material from Buenos Aires, Argentina) is widespread throughout the Pampas of Argentina and Uruguay, and it is also present in central Chile and Paraguay . The specimens from Brazil differentiate from those of the typical form because they are slightly smaller, narrower and with different colour pattern . The scaly vestiture is greyish-whitish instead of brownish, with more extended white stripes on the elytra, especially along the 3° interval, and the yellow stripes characteristic of the typical form are lacking ( Figure 1c View Figure 1 ) . Moreover , in the Brazilian populations we did not find males .
Naupactus xanthographus was recovered as the sister species of N. navicularis in a cladistic analysis using molecular data (COI sequences), in which N. dissimilis and N. mimicus were not included ( Scataglini et al. 2005). The characters of the male genitalia (apex of penis arrow-shaped) suggests that N. dissimilis and N. mimicus might be closer to N. xanthographus than N. navicularis .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Naupactus xanthographus (Germar)
| Lanteri, Analia A. & del Río, María G. 2017 |
Naupactus xanthographus: Schoenherr 1833 , p. 571
| Blackwelder RE 1947: 795 |
| Hustache A 1947: 39 |
| von Dalla Torre KW & van Emden M & van Emden FI 1936: 24 |
| Schoenherr CJ 1840: 7 |
| Schoenherr CJ 1833: 571 |
Leptocerus xanthographus
| Germar EF 1824: 424 |