Halecium antarcticum Vanhöffen, 1910
|
publication ID |
https://doi.org/10.11646/zootaxa.3790.2.2 |
|
publication LSID |
lsid:zoobank.org:pub:BE6B199C-6E81-478A-8AC9-EB674B85FA35 |
|
DOI |
https://doi.org/10.5281/zenodo.4630873 |
|
persistent identifier |
https://treatment.plazi.org/id/3E6287E0-294C-FF89-2CA9-1D073EBBFD90 |
|
treatment provided by |
Plazi |
|
scientific name |
Halecium antarcticum Vanhöffen, 1910 |
| status |
|
Halecium antarcticum Vanhöffen, 1910 View in CoL
Halecium banzare Watson, 2008 View in CoL
Halecium brevithecum Watson, 2008 View in CoL
Halecium delicatulum Coughtrey, 1876 View in CoL * (= in part Halecium antarcticum Vanhöffen, 1910 View in CoL ; in part H. pseudodelicatulum View in CoL sp. nov.)
Halecium elegantulum Watson, 2008 View in CoL
Halecium exaggeratum Peña Cantero, Boero & Piraino, 2013 View in CoL
Halecium frigidum Peña Cantero, 2010 View in CoL
Halecium incertus Naumov & Stepanjants 1962 View in CoL
Halecium interpolatum Ritchie, 1907 View in CoL
Halecium jaederholmi Vervoort, 1972 View in CoL
Halecium macrocaulus Watson, 2008 View in CoL (= Halecium incertus Naumov & Stepanjants, 1962 View in CoL )
Halecium ovatum Totton 1930 View in CoL (= Halecium interpolatum Ritchie, 1907 View in CoL )
Halecium pallens Jäderholm, 1904 View in CoL
Halecium pseudodelicatulum View in CoL sp. nov.
Halecium pseudoincertus View in CoL sp. nov.
Halecium secundum Jäderholm, 1904 View in CoL
Halecium tenellum Hincks, 1861 View in CoL * (=? Halecium exaggeratum Peña Cantero et al., 2013 View in CoL )
Halecium tubatum Watson, 2008 View in CoL species inquirenda
Halecium View in CoL sp.1 Peña Cantero, 2008 (= Halecium exaggeratum Peña Cantero et al., 2013 View in CoL ) Halecium View in CoL sp.2 Peña Cantero, 2008 (= Hydrodendron arboreum Allman, 1888 )
Halecium antarcticum Vanhöffen, 1910: 317 View in CoL , fig. 34; Vervoort & Watson, 2003: 85; Watson, 2008: 166 View Cited Treatment –167, fig. 2A–C.
? Halecium antarcticum View in CoL — Billard, 1914: 7 –8, fig. 5; Totton, 1930: 144, fig. 4.
? Halecium gracile View in CoL — Billard, 1906: 10 –11.
? Halecium delicatulum View in CoL — Naumov & Stepanjants, 1962: 94 –95, fig. 16; 1972: 52; Stepanjants, 1979: 105, pl. 20 fig. 4a–b; Branch & Willians, 1993: 11, fig.
? Halecium antarcticum View in CoL — Blanco, 1984: 7 –10, pl. 4 figs 8–11, pl. 5 figs 12–13 (in part, part belongs to H. exaggeratum View in CoL ).
? Halecium pallens View in CoL — Stepanjants, 1979: 105, pl. 20 fig. 2 (in part, part belongs to H. banzare View in CoL ).
Material examined. Syntype ZMB Cni 14885*, Deutschen Südpolar–Expedition 1901–1903, Gauss Station, 65°21’S, 86°06’E (Davis Sea), 385 m, one stem c. 45 mm high, with male gonothecae. Australian Antarctic Expedition Aurora Australis II, Stn BTC 25, 65.87’S 89.28’E (Davis Sea), 522.4 m, 06–01–2010, numerous strongly polysiphonic stems, with ripe female gonothecae, up to 80 mm high, on tube of polychaete, axis of dead gorgonian and Eudendrium sp.
Diagnosis. Polysiphonic, irregularly branched stems, up to 80 mm high. Branches originating from hydrophore of primary hydrotheca. Hydrothecae alternately arranged in one plane. Hydrotheca at the end of long, free hydrophore. Hydrothecal diameter increasing distally; rim slightly everted. Up to fifth-order hydrothecae present. Male gonotheca laterally flattened, maximum width by the middle. Female gonotheca terebratulid brachiopod-shaped; abcauline wall longer. Aperture wide, at distal part of gonotheca. With acrocysts with up to seven eggs. Cnidome consisting of microbasic mastigophores? and microbasic euryteles?
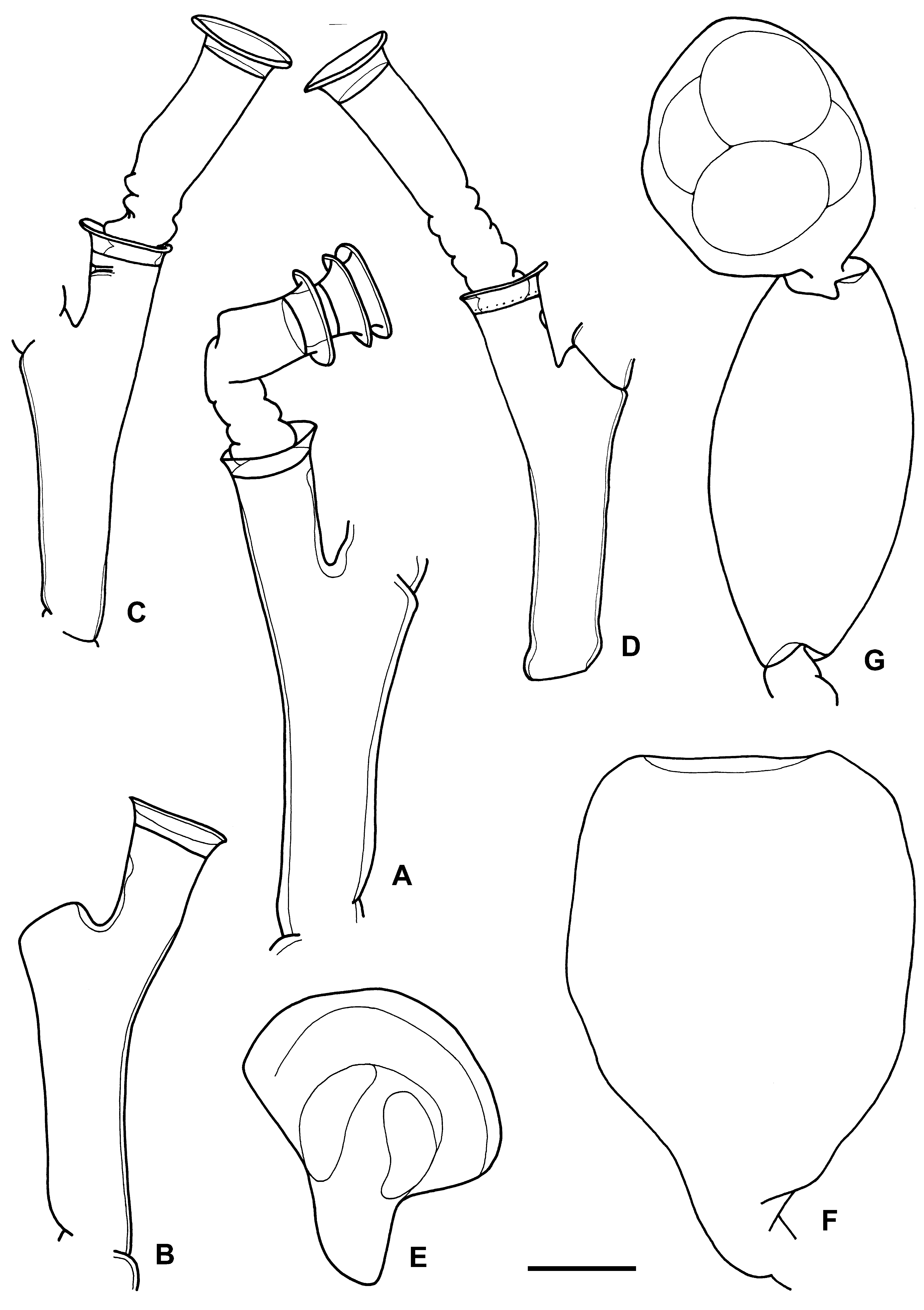
Description ( type material). Stem c. 45 mm high, polysiphonic, except for the most distal 6 mm. Stem giving rise irregularly to primary branches, some of these much developed, also polysiphonic, and originating monosiphonic second-order branches. Branches originating from hydrophore of primary hydrotheca. Stem and branches divided into internodes by alternately arranged oblique nodes ( Fig. 1 View FIGURE 1 A, B). Hydrothecae alternately arranged in one plane and placed at the end of long hydrophores; ratio between adcauline length of hydrophore and diameter at diaphragm 1.3–1.4. Abcauline side of hydrophores straight or with a slight basal convexity; adcauline side straight ( Fig. 1 View FIGURE 1 A, B). Hydrothecae considerably surpassing distal node of internode ( Fig. 1 View FIGURE 1 A, B). No distinct pseudodiaphragm present.
Hydrothecae widening distally, much less in stem primary ones ( Fig. 1 View FIGURE 1 A), with slightly everted rim, much more in lower-order hydrothecae ( Fig. 1 View FIGURE 1 A) and those from branches ( Fig. 1 View FIGURE 1 B). Up to fifth-order hydrothecae present.
Male gonotheca laterally flattened, c. 680 µm high, and 640 µm wide in frontal view ( Fig. 1 View FIGURE 1 E). Maximum width by the middle, strongly decreasing both distally, to form a rounded part, and basally, up to a clear inflexion point from where it diminishes very slightly until reaching its origin, forming a sort of basal pedicel. By inflexion point gonothecal wall passing from convex to concave.
Measurements (in µm). Hydrothecae: diameter at aperture 240–260, diameter at diaphragm 160–200, height 45–70, adcauline length of hydrophore 130–275. Cnidome: very abundant microbasic mastigophores?, with sharp ends [range 7–7.5 x 2 –2.5, mean 7.3±0.2 x 2.1±0.2 (n=10); ratio, range 3.0–3.8, mean 3.5±0.3 (n=10)], and scarce microbasic euryteles?, with blunt ends [range 6.5–7 x 3 –3.5, mean 6.7±0.2 x 3.2±0.2 (n=10); ratio, range 1.9–2.3, mean 2.1±0.1(n=10)].
Description of female gonothecae from Stn BTC 25. Gonotheca flattened in lateral view, terebratulid brachiopod-shaped, with abcauline wall longer. Adcauline wall not reaching distal part of gonotheca, instead curved inwards. Gonothecae with acrocyst with c. 7 eggs (checked) ( Figs 1 View FIGURE 1 G, 2B). When acrocyst goes out, gonotheca becoming more or less fusiform in lateral view because distal part of adcauline wall deploys, reaching distal part of gonotheca ( Figs 1 View FIGURE 1 G, 2A). When acrocyst is shed, gonotheca recovering shape of terebratulid brachiopod. Gonothecal aperture wide, occupying large extension of distal part of gonotheca ( Fig. 1 View FIGURE 1 F). Gonothecae 1300–1450 µm high and 750–800 µm wide, in frontal view, and c. 490 µm, in lateral view. Gonothecal aperture c. 450 µm in frontal view.
Remarks. The holotype of H. antarcticum has only male gonothecae and, consequently, it is not possible to be completely sure about the assignation of the female material studied here. However, I am considering the material from the Australian expedition to be the female colony of H. antarcticum based on geography and cnidome. In addition, it agrees with the type material in the irregularly branched, polysiphonic stems and in the shape and size of hydrothecae ( Fig. 1 View FIGURE 1 C, D). Pseudodiaphragm is present in some hydrothecae ( Fig. 1 View FIGURE 1 C).
Watson (2008) found gonothecae that she described as “probably male, inserted in hydrophore on distal monosiphonic branches; shape variable from flattened clavate to ovoid with a low apical dome with flattened top”. She later indicated, however, that sex could not be determined. In my opinion, the gonothecae studied by Watson are probably female. As stated above, male gonothecae are present in the type material studied. They are considerably smaller than those described by Watson ( 1176–1568 µm in length and 882–910 µm in maximum width), which, on the other hand, match the size of the female gonothecae found in the Australian material. The apical dome with flattened top described by Watson (2008) could correspond to remnants of the acrocyst; she remarked on the fragility of the gonothecal perisarc, with few gonothecae undamaged and only two showing a flattened apex.
Previous records of H. antarcticum based on infertile material, or just with immature gonothecae, such as those by Billard (1914) and Totton (1930), are not reliable because of the similarity with Halecium pseudodelicatulum sp. nov. (see below).
Concerning Blanco’s (1984) material of H. antarcticum, Peña Cantero (2013) pointed out that this record is based on material from different localities and seems to consist of specimens of several species. In fact, Peña Cantero (2013) indicated that the material represented in pl. 4 figs 10 and 11 and pl. 5 fig. 13 could belong to H. frigidum . Instead, I here consider it conspecific with H. exaggeratum (see below). The remaining material of Blanco (1984) could either belong to H. antarcticum or to H. pseudodelicatulum sp. nov.
Naumov & Stepanjants (1962) considered H. antarcticum and H. pallens conspecific with H. delicatulum , taking into account “the great resemblance in the structure of the colonies … and the fact that the differences found in the structure of the gonothecae of these species have an ontogenetic character” (Naumov & Stepanjants 1965: 98). Later, Stepanjants (1979), after studying new material, considered H. delicatulum and H. pallens different species, but still considered H. antarcticum conspecific with H. pallens . Due to this fact, it is not possible to take into account Stepanjants’s (1979) records without revising all her material to separate those specimens belonging to H. antarcticum from those of H. pallens .
Although Halecium delicatulum View in CoL has been reported to occur in nearly all oceans ( Schuchert 2005), it seems that different species are included under that name. Schuchert (2005), for example, pointed out the distinct differences present in Halecium parvulum View in CoL var. magnum Millard, 1957 , considered as H. delicatulum View in CoL by Millard (1975), and concluded that it should be considered a different species because it occurs sympatrically with H. delicatulum View in CoL in South Africa. Galea & Schories (2012b) also stated that it is likely that the binomen Halecium delicatulum View in CoL has been used as a common denominator for several haleciid hydroids from various oceans.
In spite of previous Antarctic records, it seems that H. delicatulum View in CoL is not present in this area. There are sound reasons to believe that the Antarctic material belongs to other species. Galea & Schories (2012b) indicated that the gonothecae from New Zealand material are provided with two prominent, very characteristic, "ears" flanking the aperture and suggested avoiding the use of the binomen H. delicatulum View in CoL for Antarctic haleciids, unless it is indisputably shown that their female gonothecae are morphologically identical to those described in New Zealand specimens.
Female gonothecae with the characteristic “ears” of H. delicatulum View in CoL have never been reported from Antarctic waters. Actually, it seems that the Antarctic material similar in trophosome structure to H. delicatulum View in CoL actually belongs to different species, in particular to H. antarcticum View in CoL and to H. pseudodelicatulum View in CoL sp. nov. (see below). The female gonotheca in H. delicatulum View in CoL is also characterized by having a small aperture, situated in a depression between the peaks or “ears”, contrary to the large aperture found in female gonothecae from Antarctic material.
Ecology and distribution. Halecium antarcticum View in CoL seems to be a shelf species, having been found at depths from 45 ( Watson 2008) to 522 m (present material).
Although reported all around Antarctica, it is known for sure only from East Antarctica, in particular from the Davis Sea ( Vanhöffen 1910; present material), and from Commonwealth Bay, George V Coast, and off Mawson Coast ( Watson 2008).
| ZMB |
Museum für Naturkunde Berlin (Zoological Collections) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Halecium antarcticum Vanhöffen, 1910
| Peña Cantero, Álvaro L. 2014 |
Halecium antarcticum
| Blanco 1984: 7 |
Halecium pallens
| Stepanjants 1979: 105 |
Halecium delicatulum
| Branch 1993: 11 |
| Stepanjants 1979: 105 |
| Naumov 1962: 94 |
Halecium antarcticum
| Totton 1930: 144 |
| Billard 1914: 7 |
Halecium antarcticum Vanhöffen, 1910 : 317
| Watson 2008: 166 |
| Vervoort 2003: 85 |
| Vanhoffen 1910: 317 |
Halecium gracile
| Billard 1906: 10 |