Halecium pallens Jäderholm, 1904
|
publication ID |
https://doi.org/10.11646/zootaxa.3790.2.2 |
|
publication LSID |
lsid:zoobank.org:pub:BE6B199C-6E81-478A-8AC9-EB674B85FA35 |
|
DOI |
https://doi.org/10.5281/zenodo.4630891 |
|
persistent identifier |
https://treatment.plazi.org/id/3E6287E0-295A-FF97-2CA9-1A373EAEF910 |
|
treatment provided by |
Plazi |
|
scientific name |
Halecium pallens Jäderholm, 1904 |
| status |
|
Halecium pallens Jäderholm, 1904 View in CoL
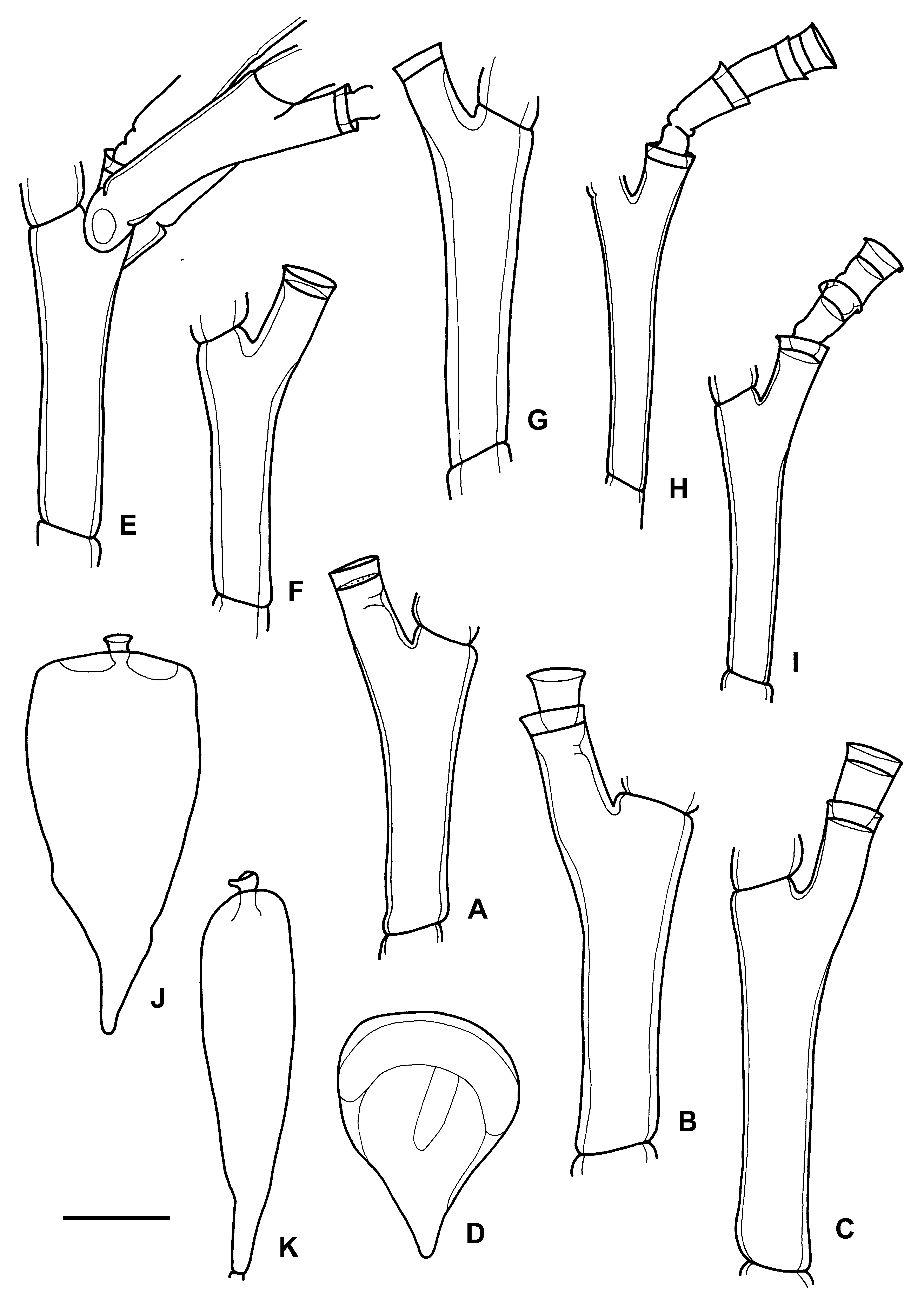
( Fig. 3 View FIGURE 3 E–K)
Halecium pallens Jäderholm, 1904 View in CoL : iv; 1905: 4, 12–13, 38, pl. 5 figs 1–3; Blanco, 1994a: 156; 1994b: 187; Vervoort & Watson, 2003: 86.
Not Halecium pallens View in CoL — Broch, 1948: 4, 7–8, fig. 1; Stepanjants, 1979: 105, pl. 20 fig. 2; Peña Cantero, 2004: 769; 2006: 935, fig. 3B; 2008: 455, fig. 1h, i; 2010: 766, fig.4; Peña Cantero & Vervoort, 2009: 85, fig. 1g, h (= H. banzare Watson, 2008 View in CoL ). Not Halecium pallens View in CoL — Galea & Schories, 2012a: 36, pl. 3A, B, fig. 3G–M. (=? H. banzare Watson, 2008 View in CoL ).
Material examined. Syntype, SMNH Type-7947 (old no. 436), Schwedischen Antarctic-Expedition 1901–1903, Stn 34, 05–06–1902, 54°11’S 36°18’W (mouth of Cumberland Bay, South Georgia), 252–310 m, one stem c. 130 mm high.
Diagnosis. Polysiphonic stems, at least 130 mm high. Branching regular, stem giving rise alternately, likely every third hydrotheca, to paired branches forming two distinct, longitudinal series, approximately arranged in one plane. Branches originating from hydrophore of primary hydrotheca. Hydrothecae alternately arranged in one plane. Hydrotheca at the end of long, free hydrophore lacking pseudodiaphragm, but with strong adcauline perisarc development. Hydrothecal diameter slightly increasing distally. Very slightly everted rim. Up to third-order hydrothecae present. Female gonotheca flattened, pear-shaped, with short distal neck. With acrocyst (up to eight eggs). Cnidome consisting of microbasic mastigophores? and microbasic euryteles?
Description. Completely polysiphonic stem, at least 130 mm high (probably a little higher since it is distally broken). Diameter slightly decreasing distally (c. 2 mm at base). Hydrorhiza much developed, rhizoidal, composed of numerous thin stolons.
Stem giving rise to side-branches, alternately arranged in approximately one plane and resting more or less perpendicular to it. Side-branches polysiphonic in part of their extension. Some much developed, reaching up to 38 mm long, and strongly polysiphonic. Side-branches paired ( Fig. 3 View FIGURE 3 E), although in many cases one lost, particularly at basal part. Paired side-branches particularly present at distal part of colony.
Stem branching regular, at a more or less constant interval; probably every third hydrotheca, although large polysiphonic development prevents from confirming this. Branching ocurring every third hydrotheca in sidebranches. These giving rise to short, little-developed secondary branches. Stem and branches divided into internodes by alternately arranged oblique nodes ( Fig. 3 View FIGURE 3 E–I).
Hydrothecae placed at the end of long free hydrophores ( Fig. 3 View FIGURE 3 E–I); ratio between adcauline length of hydrophore and diameter at diaphragm 1.0–1.5. Hydrophores straight, regularly diverging from internode ( Fig. 3 View FIGURE 3 E–I). Hydrothecae considerably surpassing distal node of internode.
Hydrotheca low, slightly widening distally, a little more at adcauline side ( Fig. 3 View FIGURE 3 F–I). Hydrothecal height slightly smaller at adcauline side. No pseudodiaphragm observed, although a strong perisarc development present at adcauline side of hydrophore ( Fig. 3 View FIGURE 3 F–I). Up to third-order hydrothecae present ( Fig. 3 View FIGURE 3 H–I).
Numerous female gonothecae present ( Fig. 3 View FIGURE 3 J–K), provided with distal, large acrocyst, with up to eight eggs. Gonotheca flattened, pear-shaped, with a short distal neck.
Measurements (in µm). Hydrothecae: diameter at aperture 125–145, diameter at diaphragm 100–115, height abcauline side 40–50, height adcauline side 30–40. Hydrophore: adcauline length 110–170. Internodes: length 650–800, diameter 100–160. Gonothecae: height 1800–2100, maximum diameter 820–970 (frontal view) and c. 440 (lateral view), length of distal neck c. 90, diameter at aperture c. 140. Cnidome: very abundant microbasic euryteles? with rounded ends [range 6.5–7 x 3 –3.5, mean 6.8±0.2 x 3.2±0.2 (n=10); ratio, range 2.0–2.3, mean 2.2±0.1 (n=10)] and abundant microbasic mastigophores? with sharp ends (6–7.5 x 2).
Remarks. As indicated above, Halecium banzare is similar to H. pallens . In fact, they are almost indistinguishable concerning the trophosome. They are similar in colony structure, with strongly polysiphonic stems, alternately branched every third hydrotheca, giving rise to paired side-branches. They also agree in the structure of internodes, with the hydrotheca placed at the end of a long free hydrophore, and in the shape of the hydrotheca. The only difference concerns the absence of pseudiaphragm in H. pallens , although a strong perisarc development is present at the adcauline side of the hydrophore. In material from Low Island previously assigned to H. pallens (cf. Peña Cantero 2013), the pseudodiaphragm is usually absent in the hydrothecae from the branches, although it is clearly visible in the stem hydrothecae. Although information about the cnidome in the type material of H. banzare is non-existent, it is known in material brought here to that species (see above) and there is no difference with that of H. pallens .
Both species are, however, clearly distinguishable by the shape and size of the female gonothecae. They are inverted conical, contain large scattered ova (seven in Watson’s figure) and are distinctly smaller (1027 µm high and 948 µm wide) in H. banzare , whereas they are pear-shaped, with a short distal neck, and a large acrocyst, with up to eight eggs, in H. pallens . It could also be possible that the described female gonothecae in H. banzare were incompletely developed, later developing the acrocyst to complete egg development, as it happens in H. pallens . Nevertheless, it should be indicated that in the Bentart material (see above), the female gonothecae are apparently completely developed, with the aperture in frontal view extending the entire diameter, from the widest part upwards. Therefore, they are different from those of H. pallens , which indicates the presence of two different species.
As indicated above, Antarctic material previously assigned to Jäderholm’s species could actually belong to H. banzare , being undistinguishable from Watson’s species.
On the other hand, Galea & Schories (2012a) assigned to H. pallens material from the sub-Antarctic Magellan Strait that has some distinct differences with Jäderholm’s species. According to these authors ( Galea & Schories 2012a: 36) “Pseudodiaphragm seen in most hydrophores; considerably hypertrophied in basalmost hydrophores, often present in secondary or even tertiary hydrophores; reaching abaxial side of hydrophore as a thick belt”, whereas a psedodiaphragm is absent in H. pallens . The material also differs from Jäderholm’s species in the shape and size of the female gonotheca. According to Galea & Schories (2012a: 36) the female gonotheca is “seemingly composed of two halves "fused" along a crease line following the perimeter of gonotheca”, there are “four to five eggs per gonotheca” and the “perisarc dissolved upon formation of acrocysts, leaving a large apical aperture for passage of egg mass”. In Jäderholm’s species the gonothecae is apparently not composed of two valves, there is no sign of any crease line along the perimeter of the gonotheca, there are up to eight eggs, and the gonothecal aperture is formed by a small distal neck, so that eggs and acrocyst must go out when they are small and little developed, contrary to what is clearly visible in the material of Galea & Schories (2012a). This material could belong to H. banzare , but it seems to have a different female gonotheca, since no acrocyst has been observed in any material assigned to H. banzare and the aperture is formed by the distal part of the gonothecae, contrary to the large apical aperture formed by the dissolution of the perisarc indicated by Galea & Schories (2012a). The material studied by these authors could be a new species to science.
Ecology and distribution. Halecium pallens was collected at a depth between 160 ( Jäderholm 1905) and 310 m ( Jäderholm 1904), off South Georgia ( Jäderholm 1904, 1905) and Shag Rocks ( Jäderholm 1905). Gonothecae in June ( Jäderholm 1904).
| SMNH |
Saskatchewan Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Halecium pallens Jäderholm, 1904
| Peña Cantero, Álvaro L. 2014 |
Halecium pallens Jäderholm, 1904
| Vervoort 2003: 86 |
| Blanco 1994: 156 |