Sclerotinia cirsii-spinosissimi Senn-Irlet, 2016
|
publication ID |
https://doi.org/ 10.5281/zenodo.1040189 |
|
DOI |
https://doi.org/10.5281/zenodo.6090414 |
|
persistent identifier |
https://treatment.plazi.org/id/40752870-FFFB-2C0E-F3D6-FE3E02489FD3 |
|
treatment provided by |
Plazi |
|
scientific name |
Sclerotinia cirsii-spinosissimi Senn-Irlet |
| status |
|
Sclerotinia cirsii-spinosissimi Senn-Irlet View in CoL sp. nov.
— MycoBank 516616, Plate 1-3 View Plate 1 View Plate 2 View Plate 3 . View Plate 1 View Plate 2 View Plate 3
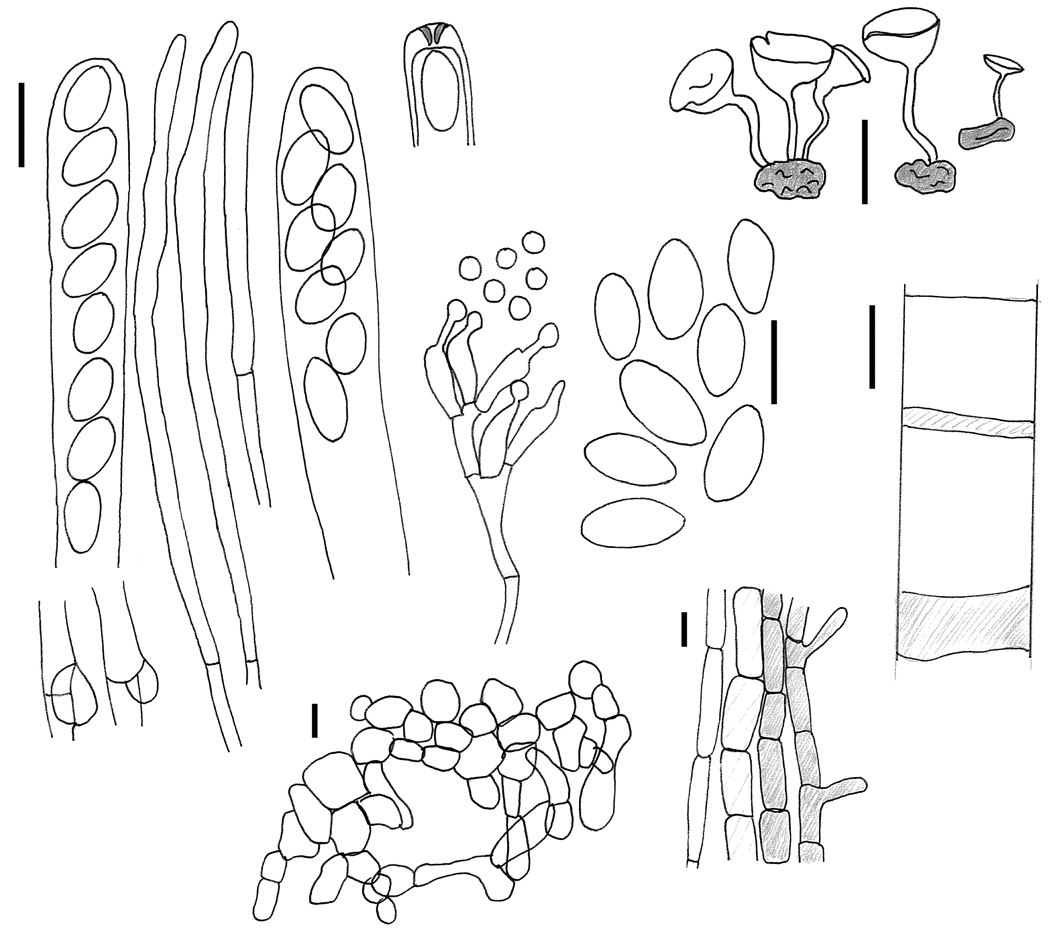
Apothecia solitaria, discus acetabuliformis, 5-12 mm diam, stipitatus, hymenio brunneo, stipes cylindraceus, colore simile disco, 5–20 × 0.8–1.5 mm. Asci cylindracei, † (120–)130–170 × 7–9 μm, octospori, poro jodo caerulescenti. Ascosporae uniseriatae, hyalinae, elipsoideae, inaequilaterales, † 9.5–11.8 × 4.7–6.6 μm. Microconidia 2–4 μm diam., globosae, in sporodochiis in hymenio vel in vitro. Sclerotia tuberoidea, 4–8 × 1.5–4 mm, extus nigra, intus albida.
Habitat in bracteas Cirsii spinosissimi in regio alpina, Helvetia.
Holotypus: Senn-Irlet 96/32 , Uri , Attinghausen , 31 Augusto 1996, in herbario Z-ZT conservatur .
Etymology — Refers to the species name of the plant host.
Apothecia arising singly or rarely in pairs or in triplet from a true sclerotium, embedded in fallen old, last-year bracts of Cirsium spinosissimum lying on the soil ( Plate 1a View Plate 1 ); receptacle 5–12 mm diam., 2–4 mm high, cupulate or discoid with a central depression; hymenium ochraceous to burnt Sienna, smooth; stipe cylindrical, often flexuous, 5–20 × 0.8–1.5 mm, tapering towards base, concolorous or paler than hymenium, glabrous to felted under hand lens.
Asci arising from croziers, cylindric–clavate, (*) 140–180 × 9– 10.5 Μm, (†) (120–) 130–140 × 7–9 Μm, with a tapering, blunt–end base, regularly 8–spored, all spores of about equal size, apex truncated-rounded,apical pore MLZ+ blue, IKI blueish, deep blue in MLZ after KOH ( Plate 2d View Plate 2 ). Ascospores (*) 11.3–15.3 (17) × 5.7–7.1 Μm (fresh in H2O), Q = 1.6–2.4, mean Q = 2.04, (†) (8) 9.5–11.8 (14.5) × 4.7–6.6 Μm, Q = 1.5–2.2 (in Congo red and 5% KOH) mean Q = 1.85, (†) uniseriate, young in ascus biseriate, hyaline, unicellular, ellipsoid and slightly inequilateral, eguttulate, with two (to four?) nuclei, content (†) cyanophilous (in Cotton blue), IKI wall hyaline, content yellow. Microconidial state with microconidia globose, hyaline, 2– 4 Μm diam., produced from phialides in sporodochia ( Plate 1f View Plate 1 ), superficial in hymenium of older apothecia from germinating ascospores, especially towards margin. Paraphyses filiform, septate, simple or sparsely branched, hyaline, in upper part 3–4 Μm wide, fresh with large pale brown guttules in the upper cells, without gelatinous covering, even in living state. Subhymenium 45–60 Μm, of densely septate, prismatic cells, brown-walled, reddish brown. Medullary excipulum of loosely interwoven, filiform, 2–5 Μm wide, colourless hyphae, forming a textura intricata, 40–250 Μm thick, without crystals, no blueing in IKI. Ectal excipulum 50–150 Μm, 3– 5 cells thick, of pale brown angular to prismatic cells, 20–35 × 10– 16 Μm in size, orientated perpendicularly to apothecial surface. No gelatinous matrix observed.
Stipe composed of a medullary excipulum forming a textura porrecta, composed of thin-walled, elongated, 5–8 Μm wide, hyaline cells, and an ectal excipulum, 8–20 Μm thick, of 2–3 rows of elongated, light brown, thin-walled cells, forming a textura angularis, outermost cells often with short outgrowths. Both textures arranged parallel to the stipe axis.
Pigment brownish, membranaceous, in subhymenium, and outer cells of the stipe, intracellular in paraphyses.
Stroma an irregular tuberoid sclerotium ( Plate 1b View Plate 1 ), globose to cylindrical, constricted and often furrowed, variable in shape, 4–8 × 1.5–4 mm, with scrobiculate, black outer rind and white inner context, developing within fallen, straw-like involucral bracts of Cirsium spinosissimum , but never incorporating remnants of plant material. Sclerotial rind two to four cells wide, of dark brown-walled, angular to prismatic cells, outermost cells heavily melanized, carbonaceous; uppermost cells pale brown, cells compact,(†) 12–20 × 6– 10 Μm, forming a textura angularis to textura oblita, sclerotial medulla of interwoven, hyaline hyphae, 4–6 Μm wide, forming a textura oblita-intricata, walls gelatinized, without apparent remnants of host tissue.
Ascospores germinate readily on PDA and MA, mycelium whitish, adhering to the agar surface, after 4–6 weeks applanate sclerotia are produced regularly spread over the whole surface of the petridish on PDA ( Plate 1c View Plate 1 ), half to three quarter immerged, irreguarly spread and larger (up to 12 × 7 mm) on MA.
18 days after inoculation scattered flocculose tufts produced small globular sporodochia, with conidia born on phialides on aerial mycelium, phialides with no obvious collarettes,conidia globose to slightly ovate, 2.5–4 Μm in diam., with one internal guttule.
The ITS sequences of the five isolates of Sclerotinia cirsii-spinosissimi collected from different locations in Switzerland were identical. Parsimony analysis revealed 44 of the 514 characters to be parsimony informative.The analysis yielded 530 most parsimonious trees (MPTs) which were reduced to 48 after reweighting (cf. HOLST- JENSEN et al., 1998). S. cirsii-spinosissimi was consistently placed in a cluster with S. borealis, which was supported by the strict consensus tree and a bootstrap value of 64% (Fig. 6). In 32 of the 48 MPTs, S. nivalis clustered with S. glacialis (52% bootstrap).
Substratum — Sclerotinia cirsii-spinosissimi , always on Cirsium spinosissimum .
Distribution — Europe, Alps.
Specimens examined. SWITZERLAND – Uri, Attinghausen-Geissberg, 1740 m alt., 31 August 1996, B. Senn-Irlet & R. Mürner (ZT, holotype, BSI 96/32, culture isolate, NCBI GQ848548 View Materials ); Spirigen-Kinzigpass, 2070 m alt., 19 August 2001, B. Senn-Irlet & R. Mürner (ZT, BSI 01/194); – Bern, Guttannen-Oberaar, 2330 m alt., 30 August 1994, B. Senn-Irlet (ZT, BSI 94/43); 15 August 1995, H.U. Aeberhard & B. Senn-Irlet (ZT, BSI 95/150, culture isolate, Sclerotinia spec 2 sensu HOLST- JENSEN et al., 1997), 8 August 1998, H.U. Aeberhard (ZT, 024.98), 2 September 2004 (ZT, BSI 04/130, NCBI EU330398 View Materials ), 17 August 2010, B. Senn-Irlet (ZT, BSI 10/63), 22 August 2010, H. Woltsche (ZT, BSI 10/93); – Graubünden, Bivio, near Leg Grevasalvas, 2460 m alt., B. Senn-Irlet (ZT, BSI 03/80, NCBI EU330399 View Materials ); – Ticino, Bedretto, Ponte di Paltano, 1840 m alt., 20 August 2002, H.Woltsche & B. Senn-Irlet (ZT, BSI 02/79); – Valais,Val d’Anniviers, Moiry, 21 August 2010, B. Senn-Irlet (ZT, BSI 10/83).
Sclerotinia nivalis Saito : JAPAN – Hokkaido, Makubedtsu-cho, on Arctium lappa , 15 May 1982, I. Saito (HAK, Holotype, 24055, NCBI EU 330400 View Materials ).
Habitat and ecology
Sclerotinia cirsii-spinosissimi was exclusively found in association with Cirsium spinosissimum , a widespread, abundant forb of the lower alpine zone of the Alps, forming clones ( URBANSKA, 1992) on pastures and near rivulets, especially at nutrient rich sites with forb vegetation and in scree vegetation, often at places indirectly favoured by cattle. The apothecia were always found on the involucral bracts from the almost intact, fallen inflorescenses from the previous year below the forb plant, profiting thus from a favourable humid microclimate.The accompanying macromycetes found were Peziza granularis Donadini, Ombrophila spec., Scutellinia spec., Tarzetta cu-
pularis (L.) Svrček, and Hemimycena ochrogaleata (J. Favre) M.M. Moser.
Involucral bracts of Cirsium spinosissimum lying beyond the forb plant on open grassland are often infected by Crocicreas calathicola (Rehm) S.E. Carpenter, a saprotrophic ascomycete with orange coloured apothecia. Sclerotinia cirsii-spinosissimi and Crocicreas calathicola were never found together suggesting that microclimatic conditions were required for germination and establishment, and humid conditions favoured Sclerotinia . A necrotrophic life-form is suggested as no signs of reduced plant growth or flowering has been observed.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |