Spinaptychus, CLARKE, 2004
|
publication ID |
https://doi.org/10.1206/0003-0090(2004)286<0001:MPTASO>2.0.CO;2 |
|
persistent identifier |
https://treatment.plazi.org/id/4302B56E-FFF4-FFBD-FF7B-71457DFAB60A |
|
treatment provided by |
Felipe |
|
scientific name |
Spinaptychus |
| status |
|
Diagnosis of Ichthyornis dispar
Nine autapomorphies are considered to currently diagnose Ichthyornis dispar . They are illustrated in figure 4. Marsh (1872b) commented on only a single character in his original note recognizing and naming Ichthyornis dispar , the ‘‘ type species’’ of the name ‘‘ Ichthyornis ’’. He noted that Ichthyornis dispar differed ‘‘widely from all known birds, in having biconcave vertebrae’’ (emphasis original, Marsh, 1872b: 344) and considered that the ‘‘rest of the skeleton presents no marked deviation from the ordinary avian type’’ ( Marsh, 1872b: 344). He recognized the cervical, thoracic, and caudal vertebrae to be ‘‘biconcave’’ (i.e., amphicoelous). However, in 1880 he considered this morphology unique to his ‘‘Odontotormae’’ (which also included Apatornis ) and to ‘‘separate them widely from all birds recent and extinct, and point back unmistakably to a very low ancestry, even below the reptiles’’ ( Marsh, 1880: 119). Thus, apparently, while considering this character diagnostic originally of Ichthyornis and later of ‘‘Odontotormae’’, he also considered it essentially plesiomorphic and even listed it as one of a list of characters that would be expected in the ‘‘ancestral type of the class of Birds’’ ( Marsh, 1880: 188). As discussed below, the morphology of the vertebral centrum articular surfaces (although of only the cervical vertebrae and not best described as a ‘‘biconcave’’ articulation) are currently found to diagnose Ichthyornis .
Subsequent to Marsh’s work, essentially the only proposed apomorphic ‘‘character’’ of Ichthyornis was from the humerus. Harrison (1973: 123; followed by Martin, 1983, and Olson, 1985) proposed ‘‘the humerus of Ichthyornis as a taxonomically isolating character’’ considering a dorsally projecting deltopectoral crest (appendix 1, character 112) and the lack of a bicipital crest to be autapomorphic of Ichthyornis . Harrison (1973) considered Archaeopteryx to lack a dorsally projecting deltopectoral crest and to share a anteriorly projecting crest condition similar to that of extant birds. However, a dorsally projecting (appendix 1, character 112) deltopectoral crest is developed in other basal avialans (e.g., Confuciusornis sanctus , Cathayornis yandica , Concornis lacustris , Neuquenornis volans , Gobipteryx minuta , and Apsaravis ukhaana ) and is optimized as plesiomorphically present in Ichthyornis . A weakly developed bicipital crest is also plesiomorphic for Avialae. Furthermore, what constitutes the ‘‘strongly developed crest’’ ( Harrison, 1973) illustrated for a neoavian remains difficult to assess, as it appears to be a complex of distinct characters. Certainly, the ‘‘small’’ bicipital crest, seen in nonavialan theropods (e.g., Deinonychus antirrhopus ; Ostrom, 1969) and all other basal avialans (e.g., Confuciusornis sanctus , Enantiornithes , Apsaravis ukhaana ) is also a plesiomorphy retained in Ichthyornis . What characters comprise the derived ‘‘strongly developed’’ condition and when these characters arose remain to be investigated.
Each of the nine characters identified here as diagnosing Ichthyornis are commented on in more detail in the body of the Anatomical Description and/or in appendix 1. The figures of the anatomy of Ichthyornis that illustrate the morphologies discussed in this diagnosis are numbered to correspond to the organization of the body of the anatomical description. Several of these characters are proposed as local autapomorphies and not as features known only from Ichthyornis (e.g., the position of the quadrate pneumatic foramen, development of a condylar pterygoid articulation on the quadrate, and an internal index process on Phalanx II: 1). As opposed to those characters unique to Ichthyornis , these characters’ optimizations as locally autapomorphic will be affected by resolution of basal neoavian relationships. Characters found to be ancestral for Neoaves could make characters currently found to be derived for Ichthyornis ambiguously optimized. After surveying galloanserines and palaeognaths, considered to represent the two most basal divergences within the crown clade, as well as more basal avialans, a determination of the primitive condition was hazarded for some characters not included in the phylogenetic analyses. Of the following proposed diagnosic characters of Ichthyornis , those that were evaluated in the phylogenetic analyses and additionally commented on in Part II (Results) are indicated with an asterisk:
1. Quadrate: single large pneumatic fora men located on the anteromedial surface of the corpus of the quadrate, lying close to the pterygoid articulation (‘‘1’’ in fig. 4). This condition is not encompassed by either character 39 or 40 (appendix 1) as it was not observed in any of the other included taxa (although per the recommendation in Part II, Methods, it will be included in subsequent analyses). The anteromedial position of the foramen (close to the pterygoid articulation) is considered a local autapomorphy of Ichthyornis (contra Witmer, 1990) with this condition also observed in some Neoaves ( Witmer, 1990).
2*. Cervical vertebrae: amphicoelous or ‘‘biconcave’’. The conformation of the articular surfaces of the cervical vertebral centra in Ichthyornis is unambiguously optimized as derived (‘‘2’’ in fig. 4). While amphicoelous cervical articulations are developed in nonavialan theropods and Archaeopteryx , the cervical articular surfaces are heterocoelous in Patagopteryx deferrariisi , Hesperornis regalis , Baptornis advenus , Apsaravis ukhaana , Lithornis , and Aves. Other basal avialan taxa (e.g., Confuciusornis sanctus ) exhibit an intermediate condition (appendix 2, character 52).
The cervical articulations in Ichthyornis (described in detail in the Anatomical Description) are optimized not as homologous with the amphicoely in more basal theropods, but as a unique transformation of a heterocoelous conformation. However, both the anterior and posterior articular surfaces of all cervical vertebrae are ovoid and flat with a central concavity (fitting the definition of amphicoely). By contrast, heterocoelous articulations involve an anterior surface that is broadly concave mediolaterally but convex dorsoventrally and a posterior surface convex mediolaterally and concave dorsoventrally.
3*. Caudal vertebrae: anterior free caudal vertebrae with welldeveloped prezygapophyses clasping the dorsal surface of preceding vertebra (‘‘3’’ in fig. 4; appendix 1, char acter 66:2). As Marsh (1880) noted, in Ichthyornis a reverse of the typical zygapophysial articulation is developed with elongate pre zygapophyses clasping the dorsal surface of the preceding vertebra in the anterior caudal vertebrae. The postzygapophyses, by contrast, are extremely weakly developed. They are flat facets on the posterodorsal surface of the neural arch that are in contact with the ventral surface of the prezygapophysis of the succeeding vertebra. By contrast, welldeveloped pre and postzygapophyses (appendix 1, character 66:0) are present in the anterior caudal vertebrae of the outgroups and Confuciusornis sanctus . Both the pre and postzygapophyses are short (even apparently noncontacting in the avian taxa included in this analysis as well as in Hesperornis regalis ; appendix 1, character 66:1) but show no sign of a reverse articulation. In Ichthyornis , the pre and postzygapophyses are short relative to the outgroup condition, but have a reverse articulation developed. This conformation is also observed within Neoaves (e.g., Charadriiformes such as Vanellus melanopterus ). And, while its distribution deserves further scrutiny, it is not present in any taxa included in this analysis other than Ichthyornis . Even if development of a reverse articulation is found to be ancestral to Neoaves, it would remain most parsimoniously optimized as an autapomorphy of Ichthyornis .
4*. Scapula: The presence of an extremely diminutive acromion process (‘‘4’’ in fig. 4) is unambiguously optimized as an autapomorphy of Ichthyornis . The acromion in Ichthyornis is minute (fig. 37); it does not extend anteriorly beyond the bosslike articular surface for the coracoid (appendix 1, character 103:0). The acromion also does not extend anterior to this articulation in Chauna torquata in what is optimized as a separate evolution of this morphology. In all other included taxa for which this character could be scored, the acromion extends well anterior to the articular surface for the coracoid (see appendix 1, character 103, regarding outgroup condition and Hesperornithes).
5. Humerus, bicipital crest, pitshaped fossa for muscular attachment located directly at the distal end of the bicipital crest (‘‘5’’ in fig. 4): The condition in Ichthyornis is cur rently not seen in any other avialan taxa. Considering the fossa/scar seen in Enantiornithes , Apsaravis ukhaana , Ichthyornis , and Aves on the bicipital crest (see appendix 1, character 115) as potential homologues is different from specifying a transformation series for this morphology. The latter would require that the directly distal position seen only in Ichthyornis is a necessary intermediate condition between an anterodistal position seen in more basal taxa (see appendix 1, characters 115, 116) and the posterodistal position in Aves. As the condition in Ichthyornis dispar is seen in no other taxon, it is used in the diagnosis. However, because this optimization is currently ambiguous (due to missing data in Limenavis patagonica and Gansus yumenensis and of uncertain homology with the highly transformed condition in Hesperornithes), this character is not used in the definition of the clade name ‘‘ Ichthyornis ’’ although it is preserved in the holotype of Ichthyornis dispar (YPM 1450) .
6*. Ulna: the dimensions of the dorsal condyle (appendix 1, character 132) are such that the length of the trochlear surface along the posterior surface of the distal ulna is approximately equal to the width of the trochlear surface taken across its distal end (‘‘6’’ in fig. 4). While these dimensions are also seen developed within Neoaves ( Clarke and Chiappe, 2001) as well as apparently in Gobipteryx minuta ( Kurochkin, 1996; although this morphology is extremely poorly preserved in that taxon), this character is unambiguously optimized as a local autapomorphy of Ichthyornis . All included taxa for which this character could be scored have a dorsal condyle with the posterior extent of the trochlear surface less than its distal width. In the outgroup taxa, the trochlear surface has no extension up the posterior edge of the ulna (see appendix 1, characters 131, 132).
7. Radius: an oval scar located on the posteroventral surface of the distal radius, in the center of a depression (depressio ligamentosa in Aves; Baumel and Witmer, 1993). The depression is also seen in Ichthyornis , but no conspicuous oval scar is developed in the included Aves. The ovoid scar (‘‘7’’ in fig. 4) was not observed in any other avialans. However the posteroventral surface of the distal radius is not visible in Apsaravis ukhaana and not preserved in Patagopteryx deferrariisi . A scar appears to be absent in Baptornis advenus and Confuciusornis sanctus .
8. Carpometacarpus: A large tubercle is developed close to the articular surface for the first phalanx of the second digit where the deep tendinal groove for the m. extensor digitorum communis ends as this tendon passes distally to insert on the first phalanx in the crown clade ( Stegmann, 1978). This robust tubercle (‘‘8’’ in fig. 4) is not present in any of the included Aves, YPM 1734, Limenavis patagonica , or more basal taxa where this portion of metacarpal II is preserved (e.g., Neuquenornis volans or Confuciusornis sanctus ). Some Charadriiformes ( Stegmann, 1978) have a tubercle in approximately the same position as Ichthyornis . Stegmann (1978) related this feature to the attachment of part of the lig. digitometacarpale, part of which constrains the passage of the m. extensor digitorum communis.
9*. Phalanx II:1: the presence of an internal index process ( Stegmann, 1978; appendix 1, character 152:1). An internal index process (‘‘9’’ in fig. 4) is seen within Neoaves (e.g., Charadriiformes ; Stegmann, 1978) but not in any of the avian taxa included in the phylogenetic analyses (i.e., the galloanserine or palaeognath exemplars used; see Part II, Material and Methods). This process is not present in Iaceornis marshi (YPM 1734; see below), Limenavis patagonica ( Clarke and Chiappe, 2001) , Apsaravis ukhaana (Norell and Clarke, 2001) , or more basal taxa (e.g., Confuciusornis sanctus, Chiappe et al., 1999 ; see also appendix 1, character 152:1).
THE SPECIES QUESTION
Across the specimens here referred to Ichthyornis dispar (table 1), there is variation in size and in morphology. Future consideration of whether there is more than one species of Ichthyornis would depend on rejection of other explanations of the variation among these specimens. As mentioned, these possible explanations include: (1) anagenetic change in a single lineage, (2) sexual dimorphism, and (3) differences due to ontogenetic stage. I have concluded that these explana tions of the variation across material referred to Ichthyornis cannot currently be rejected. Variation in size and morphology will first be described, and then intralineage explanations of this variation will be compared to the explanation of observed variation by the presence of distinct species.
VARIATION IN SIZE
There is a considerable range in the size of individuals represented by specimens referred to Ichthyornis , but the different sizes do not occur with the same frequency across the YPM material. Only two specimens (i.e., YPM 1738 and YPM 1765) are significantly smaller than the Ichthyornis dispar holotype (YPM 1450). And only four other specimens (i.e., YPM 1460, YPM 1462, YPM 1766, and YPM 9148) are approximately the same size as or just slightly smaller than the Ichthyornis dispar holotype. That is, of 77 specimens referred to Ichthyornis , only two specimens, or 2.6%, are notably smaller than the Ichthyornis dispar holotype, four specimens, or 5.2%, are the same size, and 92.2% are larger.
Those YPM specimens slightly larger than the Ichthyornis dispar holotype include, for example, YPM specimens 1730, 1733, 1749, 1756, and 1764. However, the majority (85.7%) of the YPM material referred to Ichthyornis is significantly larger than the Ichthyornis dispar holotype. Most of these specimens were previously referred to ‘‘ Ichthyornis victor ’’ and include, for example, YPM specimens 1447, 1452, 1457, 1461, 1720, 1721, 1722, 1725, 1729, 1737, 1741, 1742, 1747, 1748, 1750, 1755, 1757, 1762, 1763, 1773, and 1775.
The data presented in table 3 as well as figures 5–9 are not suggestive of the presence of distinct size classes, but rather a near continuum of differently sized individuals. A more detailed morphometric analysis would rigorously explore whether there are distinct size classes to be discriminated.
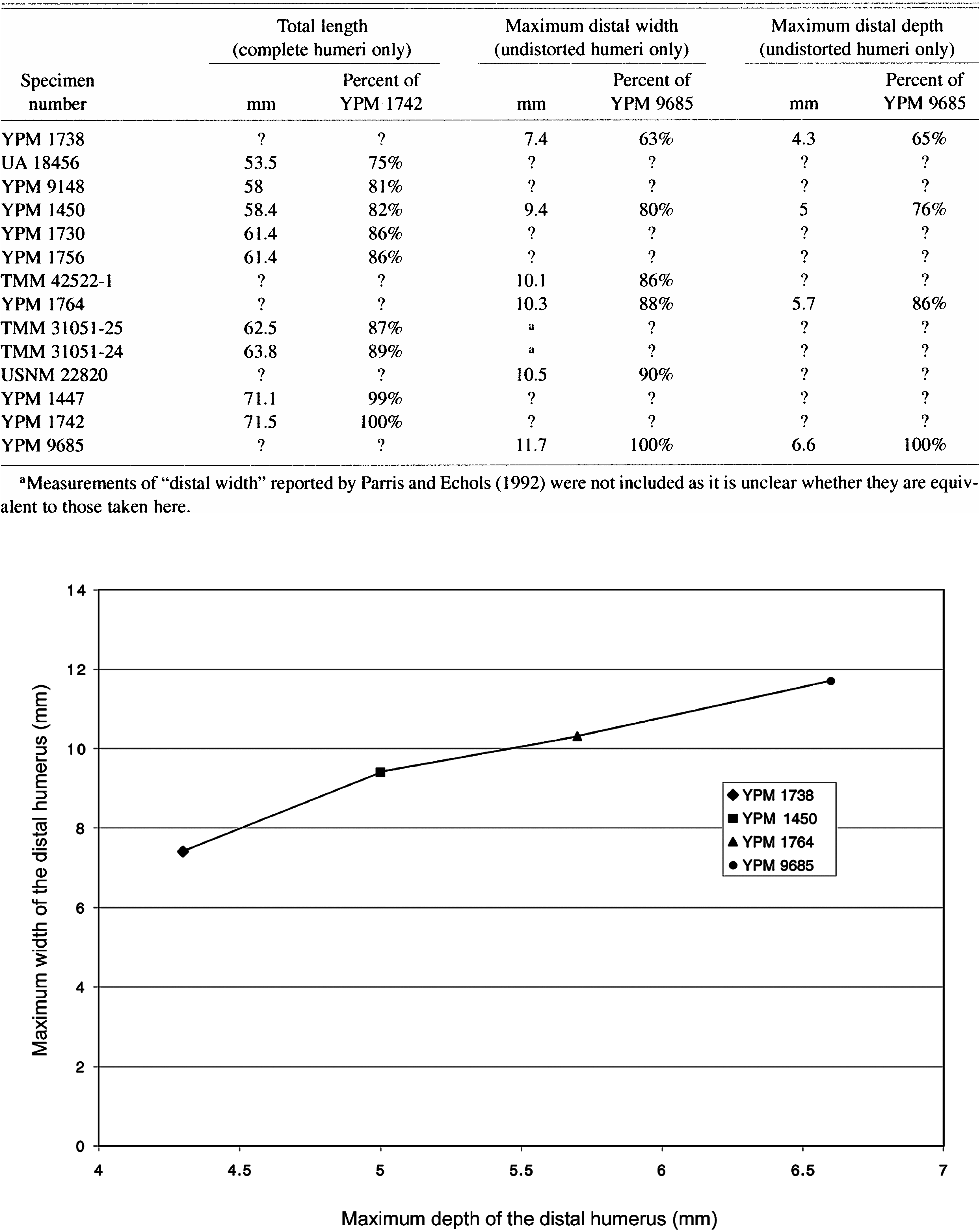
The width and depth of the distal humerus were compared for the only four undistorted YPM specimens (fig. 5). The smallest undistorted distal humerus (YPM 1738) measured (table 3) is one of the two YPM specimens significantly smaller than the Ichthyornis dis
TABLE 3 Size of Specimens Referred to Ichthyornis : Measurements of the Humerus in mm and as a Percentage of the Largest Individual
par holotype (YPM 1450). The undistorted humerus of YPM 1764 is slightly larger than the Ichthyornis dispar holotype; it plots between the holotype of Ichthyornis dispar (YPM 1450) and the largest undistorted specimen, YPM 9685 (table 3; fig. 5). YPM 9685 is approximately the same size as the majority of preserved YPM specimens. Based on comparison of the distal humeral dimensions of the two smallest undistorted humeri to the largest (YPM 9685), one of the two smallest specimens in the YPM material (YPM 1738) is 63–65% of the largest depending on what measurement is compared (table 3). By contrast, the Ichthyornis dispar holotype (YPM 1450) is 76–82% of the largest specimen compared (YPM 9685, or YPM 1742 in the case of total humeral length; table 3).
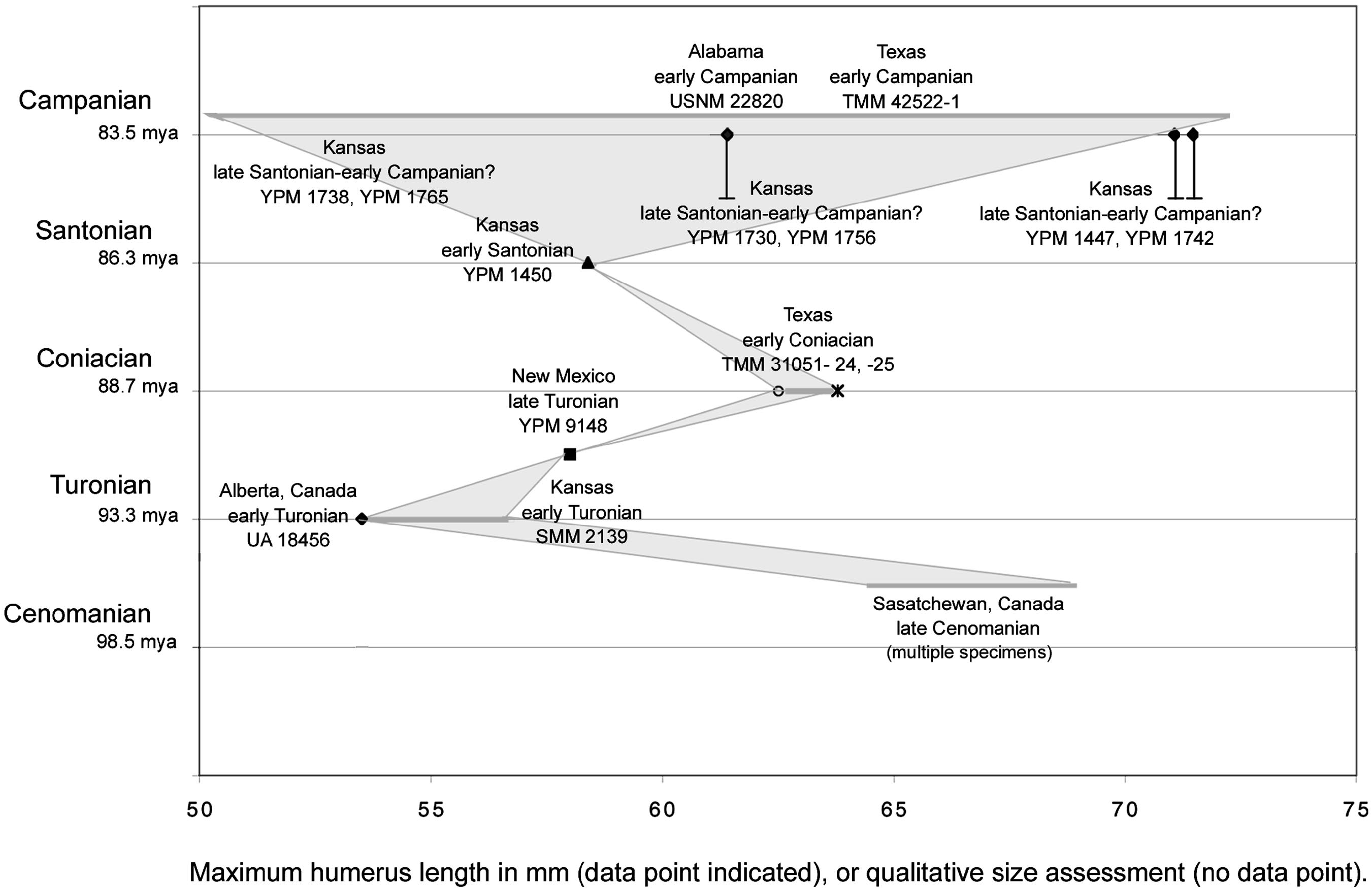
Placing the size of specimens in the context of time allows the investigation of anagenetic change as an explanation for some or all of the size differences among the material. Specimens referred to Ichthyornis have been described from the late Cenomanian through the early Campanian, an interval of more than 10 million years. The specimens referred from the late Cenomanian ( Tokaryk et al., 1997) are all significantly larger than the Ichthyornis dispar holotype. However, the three next oldest (Turonian) specimens referred to Ichthyornis , one from the early Turonian of Alberta ( Fox, 1984) and Kansas ( Martin and Stewart, 1982), as well as from the late Turonian of Texas ( Lucas and Sullivan, 1982), are all notably smaller than or approximately the same size (figs. 6, 7) as the holotype of Ichthyornis dispar (YPM 1450) . The oldest specimen is the smallest of the three (figs. 6, 7). Two slightly later specimens (TMM 31051–24, TMM 31051–25) from the early Coniacian of Texas ( Parris and Echols, 1992; table 3) are slightly larger than YPM 1450. All of this material is smaller than the majority of the YPM material, for which more precise age estimates are generally not possible.
Unfortunately, there is almost no detailed stratigraphic information for the YPM Ichthyornis material collected from the Smoky Hill Chalk Member. Only a few YPM specimens referred to Ichthyornis have locality information more detailed than, for example, ‘‘Wallace County, Kansas’’ (YPM VP Catalogue). The entire Smoky Hill Chalk Member of the Niobrara Formation is upper Coniacian–lower Campanian in age ( Stewart et al., 1990), and specimens of Ichthyornis have been estimated to be present in all recognized intervals of the Chalk (Stewart, 1990). However, Bennett (1990) concluded that, historically, nearly all of the fossil vertebrates, including the YPM material, were collected in the upper part of Smoky Hill Chalk Member from between Marker Unit 15 and 20 of Hattin (1982). Stewart (1990) estimated the in terval containing Hattin’s (1982) Marker Units 8–10 to be upper Santonian in age. Marker Units 15–20 must therefore be, at the earliest, from later in the upper Santonian through the early Campanian. Thus, the majority of the YPM material would appear to be from the late Santonian–early Campanian.
By contrast, the holotype of Ichthyornis dispar (YPM 1450) appears to be early Santonian in age: Bardack (1965) specified the locality of the holotype within Rooks County (see Ichthyornis dispar below), and this locality was mapped in the Cladoceramus undulatoplicatus by Stewart (1988). The ‘‘Zone of Cladoceramus undulatoplicatus ’’ was given as early Santonian in age by Stewart (1990: 22). The top of this zone was dated more precisely by 40 Ar/ 39 Ar as 84.88 ± 0.28 Ma, which is in the late Santonian ( Obradovich, 1993). Thus, more than 80% of the YPM Ichthyornis material is significantly larger, and inferred as younger, than the holotype of Ichthyornis dispar .
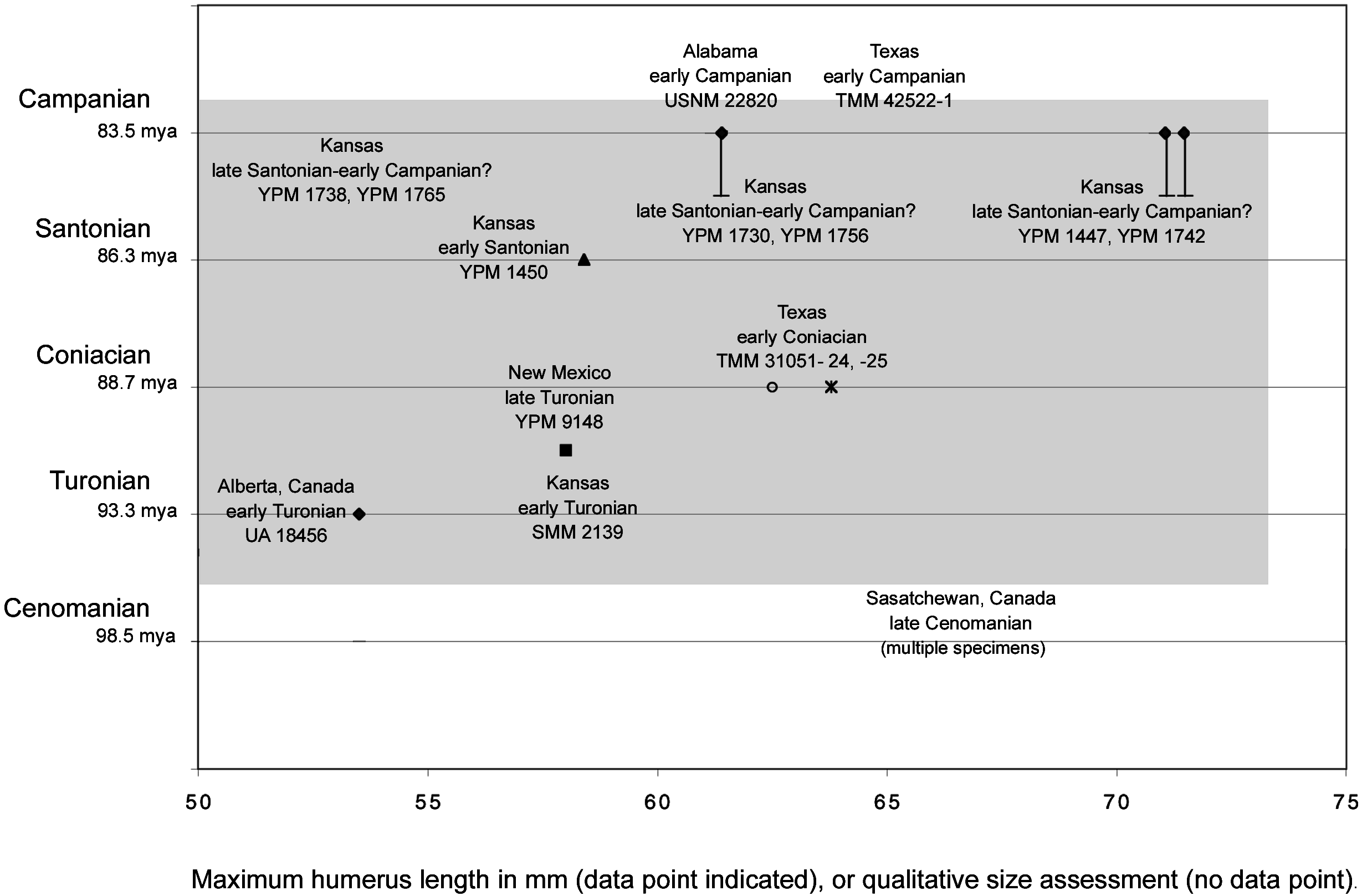
Figures 5–7 View Fig View Fig View Fig summarize the data discussed above. Shading in figure 6 illustrates an explanation of the sizethroughtime data as anagenetic change, and figure 7 illustrates this data as differential sampling through time of a consistent size range. Measurements of the six complete humeri in the YPM collection were taken (table 3). These measurements, taken from the material itself, differ from those given in Marsh (1880) and Lucas and Sullivan (1982). Other published measurements of complete humeri referred to Ichthyornis from the other localities mentioned above were also included (i.e., Fox, 1984; Parris and Echols, 1992). Further, qualitative estimates of the size of several additional specimens referred to Ichthyornis that do not include complete humeri but have comparatively good constraint on their age were also included. For example, a proximal carpometacarpus (SMM 2139) referred to Ichthyornis (although it preserves no apomorphies) from the early Turonian of Kansas ( Martin and Stewart, 1982) was included. It is approximately the size of the Ichthyornis dispar holotype (YPM 1450; Clarke, personal obs.). The comparative sizes of the holotype of Ichthyornis antecessor from the Campanian of Alabama ( Olson, 1975) and two specimens referred to that taxon from the Campanian of Texas ( Parris and Echols, 1992) plotted in figures 6 and 7 were based on comparisons of published measurements for the distal humeri that comprise these specimens to comparable measurements of complete YPM humeri.
There is also slightly more precise locality data for one of the four specimens the size of the Ichthyornis dispar holotype. This specimen, YPM 1460, was collected at Twin Butte Creek, a wellknown locality representative of the upper part of the Smoky Hill Chalk Member ( Stewart et al., 1990). Bennett (1990) measured seven stratigraphic sections along Twin Butte Creek and identified the interval represented to be between Mark er Unit 15 and 19 of Hattin (1982); this is the same interval as the majority of vertebrates from the Chalk ( Bennett, 1990) and, presumably, the majority of the YPM Ichthyornis material. Indeed, YPM 1209 is approximately the size of 80% of the YPM material and also was also collected at Twin Butte Creek (e.g., Marsh, 1880).
Stewart (1990: 22) reported Ichthyornis ‘‘c.f. Ichthyornis anceps ’’ and a ‘‘smaller species’’ as present in his late Coniacian ‘‘ Spinaptychus n. sp. ’’ zone. This reference is cryptic, as the holotype of ‘‘ Ichthyornis anceps ’’ is a specimen from a large individual comparable in size to most of the YPM material. However, the one specimen referred to ‘‘ Ichthyornis anceps ’’ is from an individ ual midsize between the Ichthyornis dispar holotype and the size of most of the YPM material. All that can be gleaned from this information is that individuals of different sizes are present at this earlier time as well (i.e., lower in the Smoky Hill Chalk Member). However, measurements of these individuals were not reported in Stewart (1990).
In sum, there appears to be no evidence of individuals as large as more than 80% of the YPM Ichthyornis material from older deposits from the Smoky Hill Chalk Member (figs. 6, 7). There is some evidence that smaller individuals (i.e., the size of the Ichthyornis dispar holotype), in low abundance, are present in the upper part of the Smoky Hill Chalk Member where most of the material is large. The only known Cenomanian specimens ( Tokaryk et al., 1997) are significantly larger than the Ichthyornis dispar holotype, while all of the Turonian specimens ( Lucas and Sullivan, 1982; Fox, 1984; Clarke, personal obs.) are smaller than or the same size as the early Santonian Ichthyornis dispar holotype.
VARIATION IN MORPHOLOGY
YPM specimens showing morphological differences from the holotype of Ichthyornis dispar were divided into two kinds: (1) difference in specimens otherwise supported by other evidence as part of Ichthyornis and (2) difference in specimens not supported by other evidence as part of Ichthyornis . For the four YPM specimens not supported as part of Ichthyornis (table 2), noted differences were used to identify specimens for inclusion as separate terminal taxa in the phylogenetic analyses (Part II). A description of these four specimens (listed in table 2) and evaluation of their taxonomic status is provided below in the Taxonomic Revision.
Subtle variation that appears to represent intraspecific variation is seen among specimens referred to Ichthyornis . A large ovoid scar on the distal end of the anterior surface of the deltopectoral crest (e.g., YPM 1461, YPM 1720, YPM 1742) and a smaller scar just anterodorsal to the humeral head appear to be more pronounced in larger specimens (e.g., YPM 1742) as does a scar on the distal radius, character 7 from the diagnosis of Ich thyornis. These scars are commented on in more detail in the Anatomical Description. In addition, Olson (1975) reported a nutrient foramen just proximal to the dorsal condyle of the humerus in Ichthyornis dispar and Ichthyornis antecessor . It is also developed in other YPM humeri (e.g., YPM 1748, YPM 1764) but may not be present in all (e.g., YPM 9685) and can vary slightly in position and development. In the Ichthyornis dispar holotype, the foramen is conspicuous on the right but not on the left humerus. These subtle differences were observed in multiple YPM specimens.
The only other morphological variation observed in YPM material referred to Ichthyornis was observed only in single specimens. Nine YPM specimens show such differences. These specimens, the morphological differences noted, and the basis for referral to Ichthyornis dispar are listed in table 4. Unfinished bone at the ends of the ulna that comprises YPM 1740 suggests that it may be an immature individual. It is intermediate in size between the Ichthyornis dispar holotype and the large specimens that are the majority of the YPM material. YPM 1774 is a large coracoid (i.e., the size of the majority of the material) with what appears to be a pathology; the glenoid facet has an irregular boss of bone on its posterolateral edge and slopes smoothly into the corpus.
EVIDENCE FOR THE PRESENCE OF DISTINCT ONTOGENETIC STAGES
There is some evidence for the presence of individuals of different ontogenetic stages in the YPM material referred to Ichthyornis . In the Ichthyornis dispar holotype, septa between most of the posterior alveoli of the mandible are thickened and completely formed. By contrast, in several specimens larger than the Ichthyornis dispar holotype, these septa are thin or apparently absent (e.g., YPM 1735, YPM 1749, SMM 2503). However, in both the Ichthyornis dispar holotype and in these larger specimens, most of the bones of the mandible appear to be fused.
In YPM 1733, a midsized specimen larger than the Ichthyornis dispar holotype but smaller than the majority of the YPM material, the intercentrum of the atlas is complete ly fused to the body of the axis: No suture is visible. However, in the larger YPM 1775, which is the same size as the majority of the YPM Ichthyornis material, this suture is incompletely obliterated. Finally, as mentioned above, YPM 1740 is an ulna intermediate in size between the Ichthyornis dispar holotype and larger individuals, and is possibly subadult, based on the bone surface texture of its ends.
All specimens (with the exception of YPM 1740) appear to be adult. Muscle scars are well developed on all bones and some, as noted, appear more pronounced in the material larger than the Ichthyornis dispar holotype. All known carpometacarpi, tibiotarsi, and tarsometatarsi are completely fused. All skull bones, with the possible exception of the frontoparietal contact, are fused in both the Ichthyornis dispar holotype and the apparently just slightly larger YPM 1728.
CONCLUSIONS
Several possible conclusions can be drawn about the number of species represented in the Ichthyornis material from the variation in size, morphology, and ontogenetic stage. First, it must be emphasized again that the specimens referred to Ichthyornis do not sample contemporaneous individuals. Specimens referred to Ichthyornis have been described from the late Cenomanian–lower Campanian, a period of approximately 15 million years. Furthermore, even if the majority of the YPM specimens are only from the upper Santonian–lower Campanian, this interval still represents several million years. The individual represented in the holotype of Ichthyornis dispar , collected from the lower Santonian, may have existed as many as a million years earlier than the majority of YPM specimens.
Explanation of some of the observed variation in size by anagenetic change may be supported by apparent size variation across time (fig. 6; although see an alternative explanation of the data as differential sampling through time [fig. 7]). While the distribution of referred material from the Cenomanian is all significantly larger than the early Santonian holotype of Ichthyornis dispar , all Turonian referred specimens are smaller than or the
TABLE 4 Nine YPM Specimens Exhibiting Morphological Differences from the Holotype of Ichthyornis dispar and/or Other Specimens Referred on the Basis of Apomorphy
same size as the Ichthyornis dispar holotype. The latest specimens (late Santonian–early Campanian) include the largest specimens, which are significantly larger than the holotype of Ichthyornis dispar . If the Cenomanianreferred material is borne out as Ichthyornis , there may be a sharp decrease in size across the Cenomanian–Turonian boundary. This may be consistent with anagenetic change tracking environmental degradation associated with a global biotic event that has been recognized in marine environments (Raup and Stepkoski, 1986) and isotopic ( 813 C and 818 O) fluctuations ( Kyser et al., 1993).
However, within the comparatively short period of time represented in the Smoky Hill Chalk Member, there is variation in size: A small individual, approximately the size of the Ichthyornis dispar holotype, and a larger one the size of the majority of referred specimens are copresent in the upper part of the Smoky Hill Chalk Member, and two specimens in the YPM Smoky Hill material are smaller than the earlier (Turonian) material. Anagenetic change within a single lineage, thus, does not appear sufficient to explain all observed variation in size.
This ‘‘residual’’ variation may be explained by the presence of more than one species of Ichthyornis or as intraspecific variation. At this time, the latter explanation cannot be rejected. Indeed, because of the distribution of morphological differences among the YPM specimens referred to Ichthyornis , and because other Mesozoic avialan species show gross differences in size among referred individuals ( Houck et al., 1990; Chiappe et al., 1999), this hypothesis is currently considered the more strongly support ed.
Two forms of intraspecific variation are specifically considered here: difference due to ontogenetic stage and due to sexual dimorphism. Generally, there is little information on intraspecific variation in size or morphology (polymorphism) for Mesozoic avialan species to contextualize the variation seen in Ichthyornis because most of these species are known from single specimens. However, for the few species that are known from multiple specimens, considerable variation in size among individuals has been described. For example, both Archaeopteryx lithographica ( Houck et al., 1990) and Confuciusornis sanctus ( Chiappe et al., 1999) are known from multiple specimens that show a considerable range in size. In Archaeopteryx lithographica , the largest (Solnhofen) exemplar is roughly twice the size of the smallest (Eichstätt; Houck et al., 1990). In Confuciusornis sanctus, Chiappe et al. (1999) noted that the length of the humerus from the smallest specimen sampled was approximately 60% the length of the humerus of the largest specimen considered. The smallest humerus of the YPM specimens (table 3) is approximately 63% percent of the largest while that of the Ichthyornis dispar holotype is approximately 80% of the largest individual measured (table 3). However, only 2 specimens of the total 77 referred are this small, while most of the variation is between 80% and 100% of the largest specimen. Thus, while size variation as extreme as that observed in Confuciusornis sanctus is represented in the YPM material, the majority of the variation is less extreme than in that taxon or in Archaeopteryx .
Houck et al. (1990) explained the variation in Archaeopteryx as a growth series by presenting morphometric data, indicating that the known specimens scale allometrically. These authors further suggested that all Archaeopteryx specimens might represent subadult individuals, as all lack the compliment of fusions seen in adult coelurosaurs ( Houck et al., 1990). Chiappe et al. (1999) also considered the size variation in Confuciusornis sanctus to represent a growth series. Other authors, with reference to the Ichthyornis material, have considered individuals even slightly different in size to be parts of distinct species (e.g., Olson, 1975). Apparently, this is because a comparatively minute difference in size is all that appears to distinguish some crown clade species, and size within species is often assumed to be near invariant for adults of crown clade species. It has also been generalized that birds reach adult size in weeks to months after hatching ( de Ricqlés et al., 2001). However, because Ichthyornis lies outside the avian crown clade it cannot, without additional evidence, be justifiably inferred to have grown like a living bird ( Gauthier and de Queiroz, 2001). That certain muscle impressions in the YPM Ichthyornis material appear more strongly developed in larger specimens appears consistent with these larger individuals being more mature. Furthermore, plots (figs. 5, 8, 9) of the differently sized individuals in Ichthyornis appear closer to a continuum than to support the presence of distinct size classes.
As noted above, nearly all of the Ichthyornis specimens appear adult or nearly adult as indicated by the fused elements, muscle impressions, and bone texture that subadult Aves often lack. Wang et al. (2000) conducted a statistical analysis of several hundred specimens of Confuciusornis sanctus and concluded that all of these specimens were of adult individuals. These observations would appear inconsistent with the interpretation of the variation in size in both taxa as representing a growth series.
Some histological studies have been taken to indicate that a shift to an avian pattern of rapid sustained growth occurred phylogenetically after Patagopteryx deferrariisi (as well as Enantiornithes and Confuciusornis sanctus ), and before Hesperornis regalis and Ichthyornis ( Chinsamy et al., 1998) . This conclusion was based on the apparent absence in Hesperornis regalis and Ichthyornis of lines of arrested development (LAGs) and on the presence of highly vascular primary bone ( Chinsamy et al., 1998). At least one LAG was interpreted as present in Patagopteryx deferrariisi and a sampled enantiornithine ( Chinsamy et al., 1995). However, this hypothesis has been critiqued more recently, as the significance of LAGs, as well as their known distribution, has been reappraised ( de Ricqlés et al., 2001). Furthermore, the notion that an avian growth pattern is seen in Hesperornis regalis and Ichthyornis is considered speculative ( Castanet et al., 2000). Complicating the interpretation of the data from histological studies, the single humerus of Ichthyornis so far sampled has been considered possibly subadult ( Chinsamy et al., 1998; de Ricqlés et al., 2001). Indeed, that this element was apparently not assessed to be subadult (which would make it less than ideal for sampling) based on morphology alone is suggestive that other Ichthyornis specimens, previously assumed to be fully mature individuals, may also be subadult.
Rapid rates of growth during some part of ontogeny have been associated with highly vascularized primary bone that is seen not only in basal avian taxa ( Castanet et al., 1996, 2000) but in nonavialan dinosaurs ( Horner et al., 2001). However, it has been postulated that histological evidence from basal avialans suggests that some part of their ontogeny involved a slow growth phase and that they may have taken longer to reach adult size ( de Ricqlés et al., 2001). This conclusion is consistent with the size ranges described for Archaeopteryx and Confuciusornis sanctus ( de Ricqlés et al., 2000, 2001) and might explain the size variation observed in Ichthyornis . We appear to be early in developing understanding of the evolution of dinosaur ontogeny, and further study of the histology of noncrown clade avialans is necessary to document the changes that occur in growth pattern ( de Ricqlés et al., 2001). Planned future histological work investigating samples from Ichthyornis should provide key insights into whether small and large individuals are, indeed, all adult. This data is essential to resolving the number of species represented in the YPM Ichthyornis material and to determining when, phylogenetically, the growth pattern seen in Aves (in which adult size is achieved in a matter of weeks to months) arose. Currently, the explanation of the variation in size present among the YPM Ichthyornis material as distinct growth stages, perhaps within a protracted slow growth phase, fits histological data and is consistent with data from more basal Mesozoic avialans. If minute variation in size is considered to distinguish distinct species, it is not currently clear where the lines distinguishing these size classes could be meaningfully drawn.
While the variation in size among the YPM specimens appears largely explained by some anagenetic change and by individuals at different growth stages, it may not explain the three cases described above: (1) the axis is preserved in two specimens, and the larger of the two (YPM 1775) has an open suture that is closed in a slightly small er specimen (YPM 1733); and (2) the septa separating the posterior alveoli of the dentary tooth row in the Ichthyornis dispar holotype are fully formed while in larger specimens (e.g., YPM 1735), they appear slightly more weakly developed or absent. Finally, unfinished bone at the ends of an ulna (YPM 1740) suggest it is from a very young individual, but the ulna is larger than that of the Ichthyornis dispar holotype. These data may suggest the presence of at least two species. However, we do not have data that any of the mentioned specimens represent nearly contemporaneous individuals. Furthermore, to compare the case of the axis and the ulna: The first case involves a specimen that falls nearly exactly intermediate in size between the Ichthyornis dispar holotype and the majority of the YPM Ichthyornis material and that appears adult relative to a larger individual. In the case of the ulna, a specimen that is, again, squarely intermediate in size appears to be from a very young individual. Thus, the signal from these cases is not clear. Even if two species were present, intermediarysized material could potentially belong to either, and for other specimens, ontogenetic clues are not available. It is also possible that this variation is anagenetic at a fin er scale than that discussed, with small scale climatic variation causing fluctuations in adult size or growth rates. On the basis of geochemical (%CaCO 3, %Al, %organic carbon) logs, gammaray logs, and bedding couplets, Pratt et al. (1993) identified four cycles in Niobrara Formation (Fort Hays Limestone and Smoky Hill Chalk Members) with estimated periodicities of 1.7 m.y. (30m cycles), 280 k.y. (5m cycles), and 100 k.y. (2m cycles) and 41 k.y. for the bedding couplets. The 41 k.y. and the 100 k.y. periodicities are close to expected periodicities of the precession and eccentricity of the earth’s orbit ( Pratt et al., 1993) and may reflect cyclic variation in the depositional environment. Pratt et al. (1993) concluded that proximate causes of such cycles transcribed in the sediment record were changes in regional climate and paleooceanographic conditions, both of which would be expected to impact the local fauna; food supply and surface water temperatures have been shown to relate to intraspecific differences in adult body size in an array of avian taxa (e.g., Graves, 1991; Leafloor et al., 1998). However, even if the YPM specimens are assumed to represent contemporaneous individuals (contra limited stratigraphic information available), an explanation other than the presence of more than one species lineage is plausible.
Sexual dimorphism in size is a widespread phenomenon in Aves, having arisen many times and ranging from minor to extreme difference in size. Although sexual size dimorphism appears highly homoplastic across Aves, the presence of dimorphism in size in palaeognaths and galloanserines (del Hoyo et al., 1992 –1999) suggests it may be ancestral to Aves. Furthermore, although dimorphism in size has not been investigated for Confuciusornis sanctus , there is evidence of sexual dimorphism in plumage ( Chiappe et al., 1999). A variety of nonavialan dinosaurs has also been described as possibly sexually dimorphic in the development of particular morphologies or as having gracile and robust morphs (e.g., Chapman et al., 1997). Sexual dimorphism in growth rates and growth duration is frequently documented in birds (reviewed in Teather and Weatherhead, 1994). Recent studies have found that in avian species in which males are larger than females, they may reach adult size after females ( Badyaev et al., 2001). The growth rates of different traits and the duration of different growth phases varied in their relative time of onset between males and females ( Badyaev et al., 2001).
The difference in size between the Ichthyornis individuals discussed is comparatively minimal. In the more extreme of the two cas es discussed, the axis with the closed suture is approximately 85% the size of that of the larger individual. This would yield a difference between these individuals in a ratio of larger/smaller of 1.18. This is well below the most extreme instances of dimorphism in Aves where, for example, in one icterid ( Passeriformes ) the male/female ratio for wing measurements is 1.33 ( Webster, 1997), a value approached by a variety of other avian taxa (e.g., Tetrao urogallus , Galliformes : 1.30 for the wing and Otis tarda: Gruiformes : 1.27 for the wing; K. Zyskowski, personal commun.). Sexual size dimorphism in the extinct dodo ( Raphus cucullatus ) and solitaire ( Pezophaps solitaria ; Livezey, 1993: table V) is also close to or exceeds size difference among Ichthyornis specimens (e.g., ratio of humerus length in ‘‘males’’/‘‘females’’: 1.10 and 1.30, respectively) and these differences were similarly considered by some early workers to be evidence of distinct species ( Livezey, 1993).
The morphological variation noted in single specimens (table 4) is not considered sufficient to merit the recognition of distinct species. Discovery in additional specimens of any of the morphological variants described, especially if associated with additional differences from the morphologies of the holotype of Ichthyornis dispar , may form the basis for recognizing distinct species of Ichthyornis . Variation in a variety of morphological characters has been noted across Confuciusornis sanctus specimens (e.g., in the development of the sternal midline ridge, fusion of the dentaries, and possible presence of uncinate processes; Chiappe et al., 1999). These differences, like those observed in the Ichthyornis material, may be related to the presence of individuals of differing ontogenetic stages or sexes among the material. To assume that in a sample of specimens representing individuals from across millions of years there would be no variation, even as minor as that described, would seem unrealistic.
Two previously named species referred to the genus Ichthyornis ( Marsh, 1880) are not part of the clade Ichthyornis (see Part II, Results). Apatornis celer Marsh, 1873a ( Marsh, 1873b) is recognized as a valid taxon. The holotypes of Ichthyornis tener Marsh, 1880 , Ichthyornis lentus Marsh, 1877b ( Marsh, 1880) , and Apatornis celer Marsh, 1873a ( Marsh, 1873b) , are differentiated from Ichthyornis dispar and were discovered more closely related to or part of Aves or placed as part of Aves in the phylogenetic analyses (Part II, results). The species epithets ‘‘ tener ’’, ‘‘ lentus ’’, and ‘‘ celer ’’ as well as the name ‘‘ Apatornis ’’ are converted in the context of a system of phylogenetic taxonomy ( de Queiroz and Gauthier, 1990, 1992; Cantino and de Queiroz, 2000). Two new clades are named to which tener and lentus are designated internal specifiers. The one specimen previously referred to Apatornis celer (YPM 1734) cannot be compared to the holotype of Apatornis celer (YPM 1451) and is designated as the holotype of a new species. The five remaining previously named species of Ichthyornis are junior synonyms of Ichthyornis dispar , as mentioned above. Descriptions are provided of the holotypes of all previously named and newly identified species. Commentary is provided on the prior referral of specimens to these species. With reference to previouslynamed species that are recognized as junior synonyms of Ichthyornis dispar , the descriptions and commentary given support the synonymy of these species and serve as a resource for future work.
Most specimens previously referred to species of Ichthyornis and Apatornis cannot be compared to the holotypes of these species. Indeed, many of the referrals appear to have been largely arbitrary. Even if some of the species names synonymized with Ichthyornis dispar are found to be valid, almost without exception, the previous referral of specimens to these taxa will not. Size appears to have been the basis for the referral of the majority of specimens to Ichthyornis victor ; however, even this criterion appears to have been inconsistently applied. For example, Marsh (1880) referred YPM 1733 to Ichthyornis victor , while a specimen of approximately the same size (YPM 1764) was referred to Ichthyornis dispar ( Olson, 1975) .
SPECIES RECOGNIZED AS JUNIOR SYNONYMS
OF ICHTHYORNIS DISPAR MARSH 1872b
Graculavus anceps ( Marsh, 1872a) was named several months before Ichthyornis
dispar (1872b) and was later referred to Ichthyornis ( Marsh, 1880) . Graculavus anceps is not the namebearer of the taxon ‘‘ Graculavus ’’ and, thus, the name of the species was changed to Ichthyornis anceps ( Marsh, 1880) . The specimen number of the holotype was not given in the original publication but was later specified ( Marsh, 1880).
HOLOTYPE SPECIMEN: YPM 1208 About YPM is a poorly preserved distal end of a left carpometacarpus (fig. 10A).
LOCALITY AND HORIZON: Marsh (1872a) indicated that the holotype was collected on the North Fork of the Smoky Hill River. Marsh (1880: 198) later specified that he collected the holotype in 1870 on the North Fork ‘‘about twelve miles east of Fort Wallace, Kansas.’’ Bardack (1965) gave its provenance as Section 11 or 13, Township 13 S, Range 36 W. Again, it is unclear how this more precise information was ascertained.
DISCUSSION: A brief description of the specimen was provided when it was named as the holotype specimen of Graculavus anceps ( Marsh, 1872a) . Graculavus was a genus Marsh (1872a) allied with extant cormorants ( Phalacrocoracidae ). Graculavus anceps was supposed to be differentiated from Graculavus velox , the type species of Graculavus , by the articular surface for the ‘‘external digit [metacarpal II] broader and nearly flat’’, that of the ‘‘internal digit [metacarpal III] considerably smaller and oval in outline’’, and the ‘‘intervening tubercle [which would be part of the articular surface for metacarpal II] more prominent’’ ( Marsh, 1872a: 364). This specimen corresponds in all preserved morphologies, including those mentioned in Marsh’s (1872a) differentia above, to those preserved in the holotype of Ichthyornis dispar . The specimen also has a diagnostic character preserved in the holotype of Ichthyornis dispar (Diagnosis, character 8). For these reasons, and because it differs only in size from the holotype of Ichthyornis dispar , it is recognized as a junior synonym of that taxon. Size is not considered sufficient to diagnose distinct species in this analysis.
If, however, Ichthyornis victor (a taxon here also synonymized with Ichthyornis dispar , see below) were determined in the future to be a valid species with one of its an cillary diagnostic features being larger size, Ichthyornis anceps must be a junior synonym of that taxon. Marsh (1880) published measurements of the holotype specimen that appear to have been intended to discriminate it based on size from Ichthyornis victor . The difference in the ‘‘greatest diameter of the distal end’’ ( Marsh, 1880:156) was given as 6.75 for YPM 1208 and 7.6 for that referred to Ichthyornis victor (YPM 1724; Marsh, 1880). However, YPM 1208 is badly crushed and missing most of the distal portion of metacarpal III, making this comparison meaningless. Indeed, if the anteroposterior width of the relatively undistorted midpoint of the distal end is compared instead, the specimens are nearly identical in size (3.4 mm for YPM 1208 vs. 3.5 mm for YPM 1724). The dorsal process of the holotype is broken, leading to an apparent, but artifactual, difference in the development of the anterodorsal edge of the trochlea of metacarpal II.
REFERRED SPECIMENS: The only specimen referred to Ichthyornis anceps is YPM 1749 ( Marsh, 1880: 124). As YPM 1749 consists only of a partial humerus and mandible that cannot be compared to the carpometacarpus holotype of Ichthyornis anceps , this referral is considered baseless.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.