Callibaetis floridanus, Banks, 1900
|
publication ID |
https://doi.org/10.5281/zenodo.5170691 |
|
publication LSID |
lsid:zoobank.org:pub:A4EC11F3-CEF9-4AC9-B221-5F8FD03EA391 |
|
persistent identifier |
https://treatment.plazi.org/id/465687EC-AA61-FFD0-B0D3-B496FEFDF3EF |
|
treatment provided by |
Felipe |
|
scientific name |
Callibaetis floridanus |
| status |
|
Holotype of Callibaetis floridanus View in CoL . Female imago, Florida, Biscayne Bay , A. T. Slosson . Holotype of Callibaetis completa . Female imago, Province Cienfuegos, Río Soledad , 2- VI, R. Salt .
Banks (1930) described Callibaetis completa from female imagos collected in Soledad, Cienfuegos Province (Central Region). Later Kluge (1991) redescribed the female and described for the first time the nymph and male imago from specimens collected in Río El Codillo and Río El Tártaro (Guamá, Santiago de Cuba Prov.). Callibaetis completa is now considered to be a synonym of C. floridanus ( Lugo-Ortíz and McCafferty 1996a) .
Nymphs of this species are easily recognized because the first and second pair of abdominal gills are triple-folded (3 apparent lamellae) and the 3 rd to 6 th pairs of gills are doubled. Tarsal claws of all legs have a double row of denticles, but these denticles are much longer on the first pair of legs. In Callibaetis adults the body is covered by reddish brown spots (pigmentation more intense in the female). In forewings there are two marginal intercalary veins in each space.
Ecology. Kluge (1991) and Lugo-Ortiz and McCafferty (1996a) report that nymphs of C. floridanus swim in small pools of still water that form in lowland streams and rivers. In the Alayo collection at IES are several specimens collected in the Zapata Swamp (Western Region). Nymphs swim through vegetation and appear to prefer warmer waters; the presence of double and triple gill lamellae is presumably an adaptation to low oxygen concentrations characteristic of stagnant waters. According to Naranjo (1986) and Cañizares (1998), the species has a narrow altitudinal range ( 5–110 m). Kluge (1991) also reported this species from the lowlands of Sierra Maestra. Other specimens have been collected from marshes, temporary ponds, and brackish waters in the cays north of Camagüey Province. In Florida, specimens have been collected from a wide variety of habitats including brackish waters and highly acidic waters ( Berner and Pescador 1988). This is the only ovoviviparous species known from Cuba. Berner (1941) reported the phenomenon of ovoviviparity for C. floridanus , C. pretiosus Banks , and other species of Callibaetis from Michigan and suggested that all or nearly all species of the genus are ovoviviparous.
According to Trost and Berner (1963), the life cycle is relatively short and both nymphs and adults have been collected throughout the year. These authors suggest that developmental time varies by season. The shortest time recorded from oviposition to emergence of the subimago was 27 days (19 August to 14 September) and the longest from 60 to 75 days. The nymphs pass through 9 to 11 nymphal instars (possibly more) before emerging to subimagos. The molt from subimago to imago occurs 7–9 hours later, and most mating takes place in the morning. The male imago dies within two days, but the female may survive up to two weeks. The longevity of the females is related to the ovoviviparity of the species ( Berner and Pescador 1988).
Nymphs reported here were collected in the period between January and July, and Kluge (1991) reports collections in October, November and December.
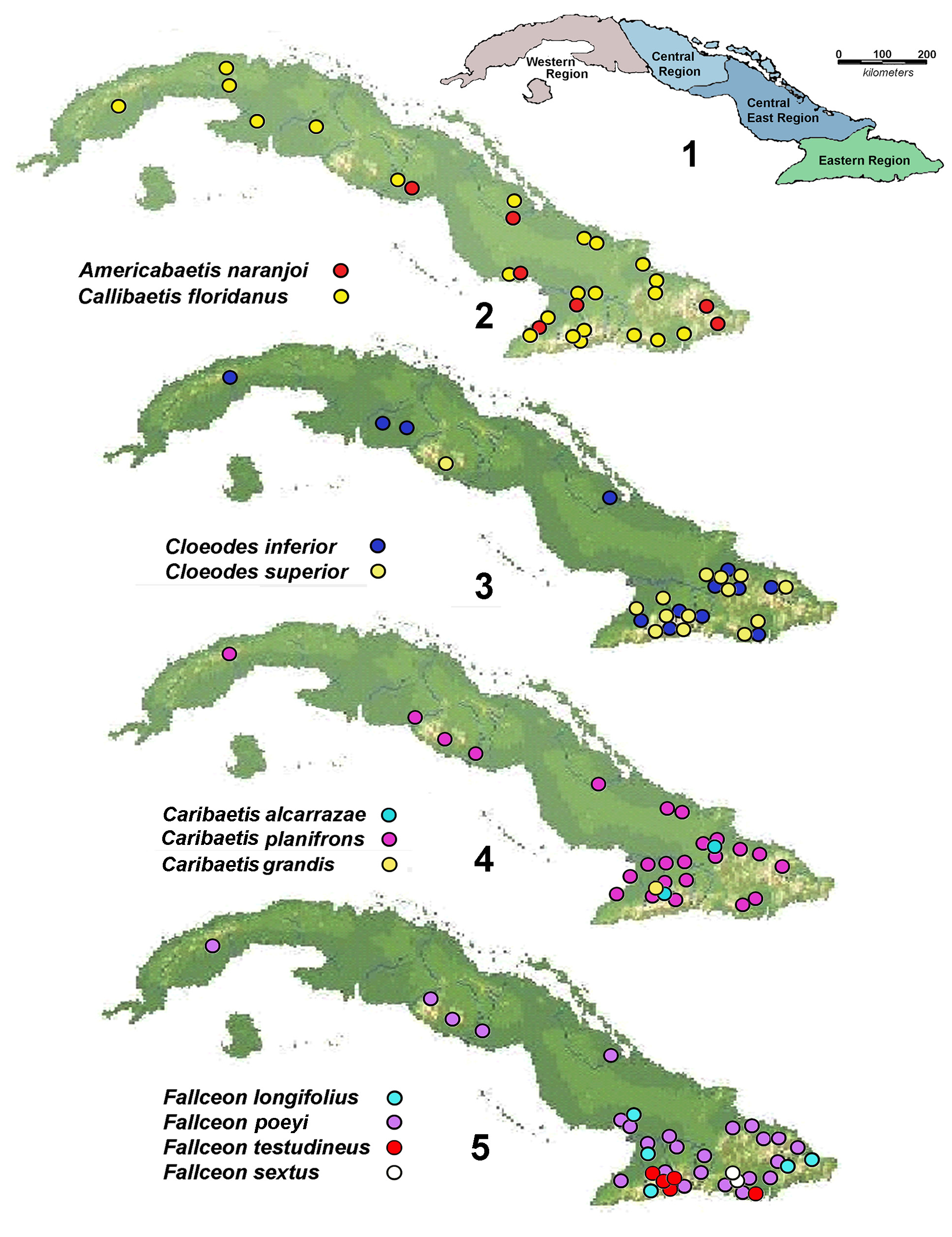
Geographic distribution. In Cuba, C. floridanus is reported throughout the island ( Banks 1930, Kluge 1991, Lugo-Ortiz and McCafferty 1996a). In our collections are specimens from the Central, Central- East, and Eastern Regions but no records from the far west of the island. However, in the collection of P. Alayo are records from Arroyo del Pinar ( Pinar del Río Prov.), Playa Larga (Ciénaga de Zapata, Matanzas Prov.), Guanimar (Alquizar, Havana Prov.), Río Quitacalzón (Casiguas, Havana Prov.) and Casimbas (Yateras, Guantánamo Prov). Outside of Cuba it is reported from the northeast, southeast and southwest of the United States, from Mexico (North America) to Belize, Costa Rica, Honduras, El Salvador and Guatemala (Central America) to Puerto Rico (Greater Antilles) and Guadeloupe (Lesser Antilles) ( Traver 1938, Berner and Pescador 1988, McCafferty and Waltz 1990, Kluge 1991, McCafferty and Davis 1992, Lugo-Ortiz and McCafferty 1996 a, Hofmann et al. 1999, McCafferty and Jacobus 2013) ( Fig. 2 View Figures 1–5 ).
| T |
Tavera, Department of Geology and Geophysics |
| VI |
Mykotektet, National Veterinary Institute |
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.