Cheleocloeon Wuillot & Gillies 1993
|
publication ID |
https://doi.org/10.11646/zootaxa.4067.2.2 |
|
publication LSID |
lsid:zoobank.org:pub:A98D3968-422A-4BC3-B078-A922ADC64344 |
|
DOI |
https://doi.org/10.5281/zenodo.6091583 |
|
persistent identifier |
https://treatment.plazi.org/id/471CEC26-4F29-FF86-21B6-0F4EFD49EE5F |
|
treatment provided by |
Plazi |
|
scientific name |
Cheleocloeon Wuillot & Gillies 1993 |
| status |
|
Genus Cheleocloeon Wuillot & Gillies 1993 View in CoL View at ENA
( Figs 1–133 View FIGURES 1 – 6. 1 – 5 View FIGURES 7 – 18. 7 – 15 View FIGURES 19 – 29. 19 – 23 View FIGURES 30 – 57 View FIGURES 58 – 63 View FIGURES 64 – 70 View FIGURES 71 – 78 View FIGURES 79 – 86. 79 – 85 View FIGURES 87 – 94 View FIGURES 95 – 98. 95 – 96 View FIGURES 99 – 102 View FIGURES 103 – 106 View FIGURES 107 – 112. 107 – 109 View FIGURES 113 – 116 View FIGURES 117 – 119 View FIGURES 120 – 121 View FIGURES 122 – 127 View FIGURES 128 – 133 )
Group dimorphicum of the genus Afroptilum: Gillies 1990: 99 View in CoL , 100, 119. Genus Cheleocloeon Wuillot & Gillies 1993: 213 View in CoL .
Hierarchical name: Cheleocloeon View in CoL /g1.
Type species: Cheleocloeon yolandae Wuillot (in Wuillot & Gillies) 1993.
Systematic position. Following the formal ranks of classification, the genus Cheleocloeon belongs to the family Baetidae Leach 1815 . In the rank-free classification developed by Kluge (2004) the taxon Cheleocloeon /g1 belongs to the following subsequently subordinated taxa: Tetramerotarsata Kluge 1997 > Liberevenata Kluge 1997 > Turbanoculata Kluge 1997 > Anteropatellata Kluge 1997.
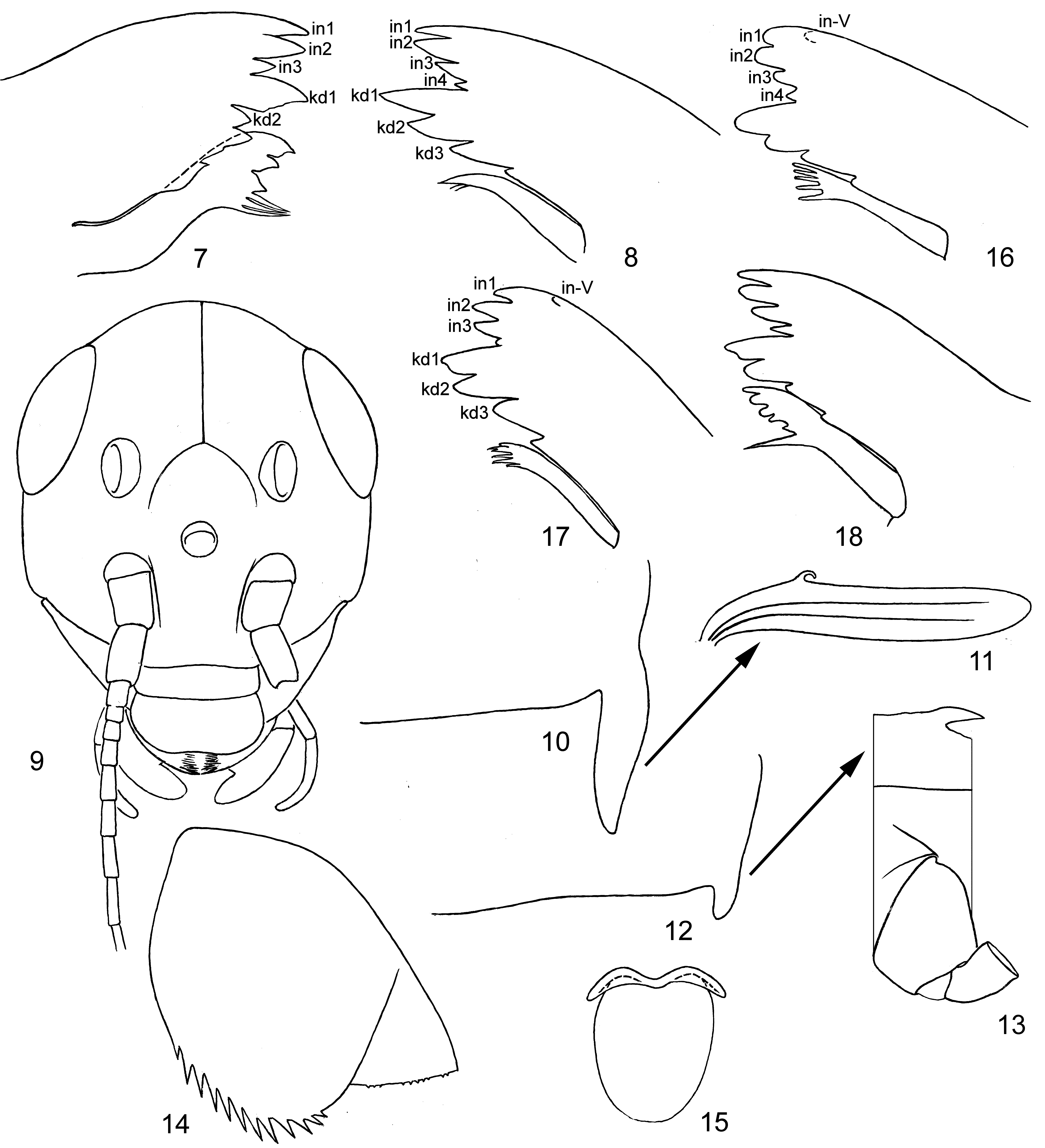
In all species of Cheleocloeon , the patella-tibial suture is equally developed in the fore and other legs of the larva, and female subimago and imago, that is characteristic for Anteropatellata ( Figs 19–22, 25, 26 View FIGURES 19 – 29. 19 – 23 ).
The taxon Anteropatellata is divided into the following taxa: (1) Baetovectata Kluge & Novikova 2011 (the largest baetid taxon with world-wide distribution); (2) Cloeon /fg1, or tribe Cloeonini Newman 1853 (the large taxon distributed in Holarctic, Afrotropical, Oriental and Australian Regions); (3) Centroptilum /g1, or the genus Centroptilum Eaton 1869 [with a singe Holarctic species Centroptilum luteolum (Müller 1776) ]; (4) Baetopus /g1, or the genus Baetopus Keffermüller 1960 , s.l. (with several species in Holarctic and Oriental Regions); (5) Afrobaetodes /g1, or the genus Afrobaetodes Demoulin 1970 (with several species in Afrotropical Region) and (6) Cheleocloeon /g1, or the genus Cheleocloeon Wuillot & Gillies 1993 (with several species in Afrotropical Region, northern Africa and Arabia). The genus Anafroptilum Kluge 2012 (with several species in the Holarctic and Oriental Regions) has questionable systematic position, because its patella-tibial suture is always absent on fore legs, as in true Protopatellata Kluge & Novikova 2011, but other characters agree with that of the taxon Cloeon /fg1, which belongs to Anteropatellata.
In Cheleocloeon , as well as in all other Turbanoculata except for Baetovectata, the fore wing has no more than one marginal intercalary in each space.
Annotated diagnosis. Male imago of Cheleocloeon has a peculiar structure of the penis, which represents an autapomorphy of this taxon [see (23)]; other characters are either plesiomorphies, or characters which could repeatedly appear in some non-related taxa, so that the larval stage of Cheleocloeon is characterized only by combination of these characters.
(1) Larval frons. Bases of antennae are widely separated, frons between them is flat ( Fig. 9 View FIGURES 7 – 18. 7 – 15 ) (unlike carinate in some other taxa).
(2) Larval frontal suture. Frontal suture is nearly semicircular, with angle blunt and arms evenly arched ( Fig. 9 View FIGURES 7 – 18. 7 – 15 ).
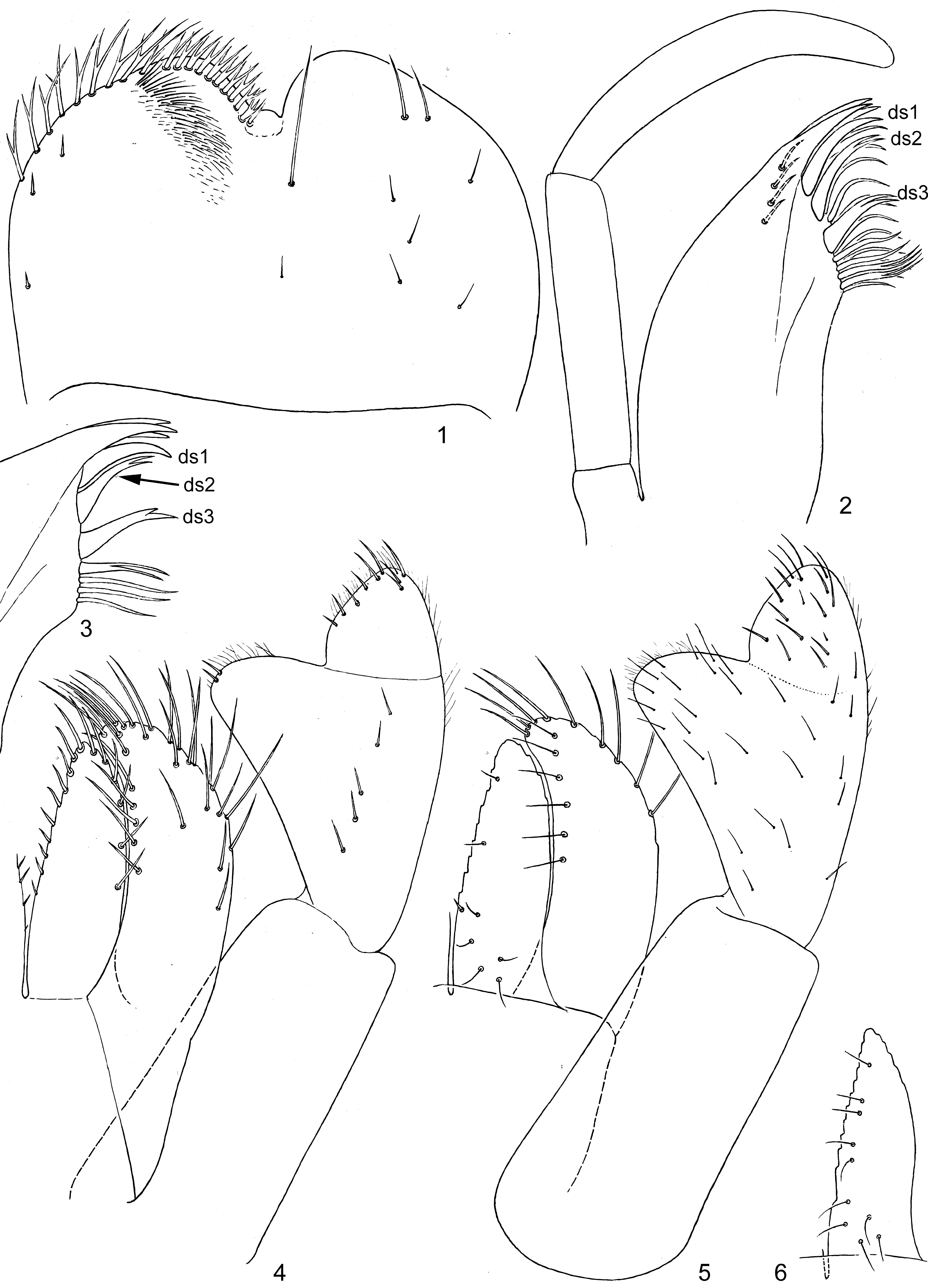
(3) Labrum. Labrum has an usual form, outer surface with submedian pair of long setae and a few (often two) antero-lateral setae on each side ( Fig. 1 View FIGURES 1 – 6. 1 – 5 ; Gattolliat et al. 2009: Figs 1–3 View FIGURES 1 – 6. 1 – 5 , 35 View FIGURES 30 – 57 ); in some individuals these setae are absent. Smaller setae are irregularly scattered of outer surface of labrum; some of them can be subequal to the submedian and antero-lateral setae. Therefore the structure of the labrum is not a good diagnostic character for Cheleocloeon , but it allows it to be distinguished from other taxa, whose antero-lateral setae form regular rows.
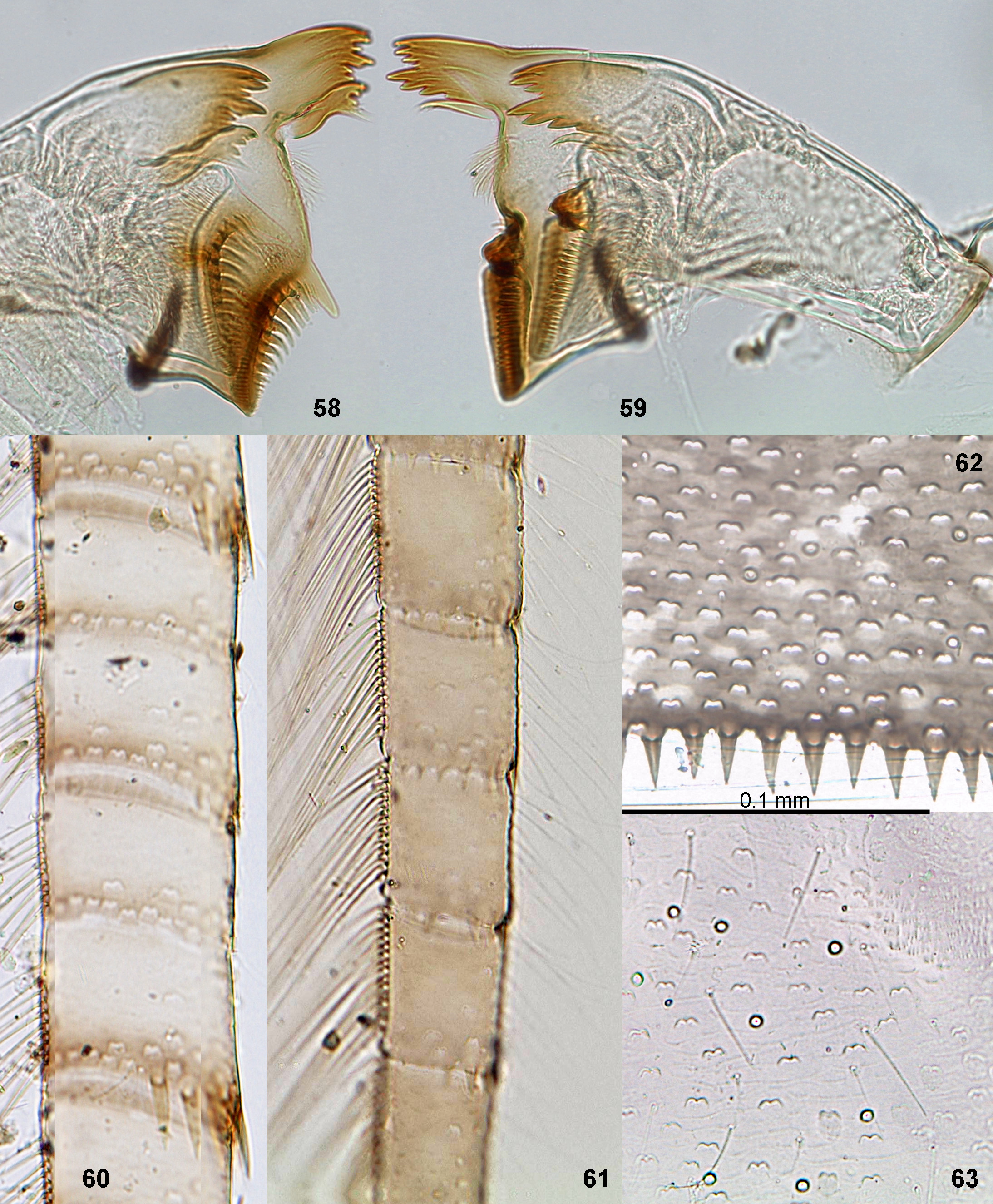
(4) Mandibles ( Figs 7, 8, 16–18 View FIGURES 7 – 18. 7 – 15 , 58, 59 View FIGURES 58 – 63 ; Wuillot & Gillies 1993: Figs 4, 5 View FIGURES 1 – 6. 1 – 5 , 13, 14 View FIGURES 7 – 18. 7 – 15 ; Gattolliat et al. 2009: Figs 7–9, 13–15 View FIGURES 7 – 18. 7 – 15 ). On both mandibles, incisor and kinetodontium are moderately fused together. Left incisor is terminated by 3 prominent denticles with distal denticle the longest, without small ventral distal denticle. Left kinetodontium has 1st (most distal) denticle the longest, 2nd shorter than 3rd denticle. Left prostheca is terminated by 3–4 stout short denticles and 2–3 slender longer denticles (individual variability). Right incisor is terminated by 4 prominent denticles, with or without a small ventral distal denticle. Right kinetodontium is terminated by 4 denticles gradually getting shorter from most distal to most proximal. Right prostheca is stick-like, directed medially, apically either narrowed, or widened (species-specific character). Margin between prostheca and mola bears dense setae-like processes.
(5) Maxillae ( Figs 2, 3 View FIGURES 1 – 6. 1 – 5 ). Maxilla of the « Cloeon - type », i.e. with long slender canines and dentisetae; 1st dentiseta is simple, 2nd and 3rd dentisetae are bifid; 1st and 2nd dentisetae are pressed to canines, 3rd dentiseta is separated. Medio-dorsal setal row, continued proximal of dentisetae, consists of 4–6 setae, which are either all simple, or some of them are bifid (individual variability); in some individuals the first seta of this row, being adjacent to the 3rd dentiseta, is bifid, thicker than others and resembles dentiseta, so that maxilla appears to have 4 dentisetae (while only 3 true dentisetae are present in Baetidae ).
(6) Maxillary palp. Maxillary palp is 2-segmented, with 2nd segment often arched ( Fig. 2 View FIGURES 1 – 6. 1 – 5 ).
(7) Labium ( Figs 4–6 View FIGURES 1 – 6. 1 – 5 ). Labium of the « Cloeon - type », i.e., glossa and paraglossa have subequal width and length, paraglossa is crescent-shaped. Glossa has the following setal rows: median row (regular row of spine-like setae along median margin); dorso-lateral row (regular row along lateral margin, with setal bases visible in dorsal view). Ventral side of glossa bears setae directed ventrally; they are located irregularly in the proximal part of the glossa and form a ventro-median row (sparse row parallel to median margin). The median, the dorso-lateral and the ventro-medial rows stretch along most of the length of the glossa.
Paraglossa has following setation: latero-apical setae (longest ones) form a regular sparse row along lateral and apical margin and irregularly located on the dorsal side; ventro-median row (regular row of long slender straight setae attached on ventral side and directed mainly medially); dorso-median row (regular row of thicker setae attached on dorsal side and directed medially-distally). The ventro-median and the dorso-median rows are stretched along most of the length of the paraglossa.
Similar labial structure occurs in some other baetid taxa, both among Protopatellata and Anteropatellata (e.g., most Cloeonini, Centroptilum , Anafroptilum ).
(8) Labial palp ( Figs 4, 5 View FIGURES 1 – 6. 1 – 5 ). Shape of the labial palp is characteristic for Cheleocloeon : 2nd segment has prominent disto-median projection with distal margin convex. 2nd segment retains muscle running from its base to base of 3rd segment (shown by dotted stripe on figures by Wuillot and Gillies (1993: Figs 6 View FIGURES 1 – 6. 1 – 5 , 15 View FIGURES 7 – 18. 7 – 15 ). Two forms of labial palp are distinguishable among Cheleocloeon : (1) with apex of projection of 2nd segment pointed (in Ch. yolandae , Ch. carinatum , Ch. littorale and Ch. sp. 3) ( Wuillot & Gillies 1993: Figs 6 View FIGURES 1 – 6. 1 – 5 , 15 View FIGURES 7 – 18. 7 – 15 ) and (2) rounded (in other species— Ch. clavifolium sp. n., Ch. lancetofolium sp. n., Ch. truncifolium sp. n., Ch. madagascariense , Ch. dimorphicum , Ch. excisum , Ch. soldani and Ch. sp. 2) ( Figs 4, 5 View FIGURES 1 – 6. 1 – 5 ). Some non-related taxa also have disto-median projections of 2nd segment of maxillary palp, but their shape is often different from that of Cheleocloeon .
(9) Postsubalar sclerite ( Figs 89, 92 View FIGURES 87 – 94 ). Postsubalare (which is present in imago and subimago and in both these stages has contrasting brown cuticular coloration) has posterior-dorsal corner stretched to a rather wide process with shallowly convex dorsal margin. This is different from Cloeonini and some other taxa, whose posterior-dorsal postsubalare process is small or narrow, with concave dorsal margin.
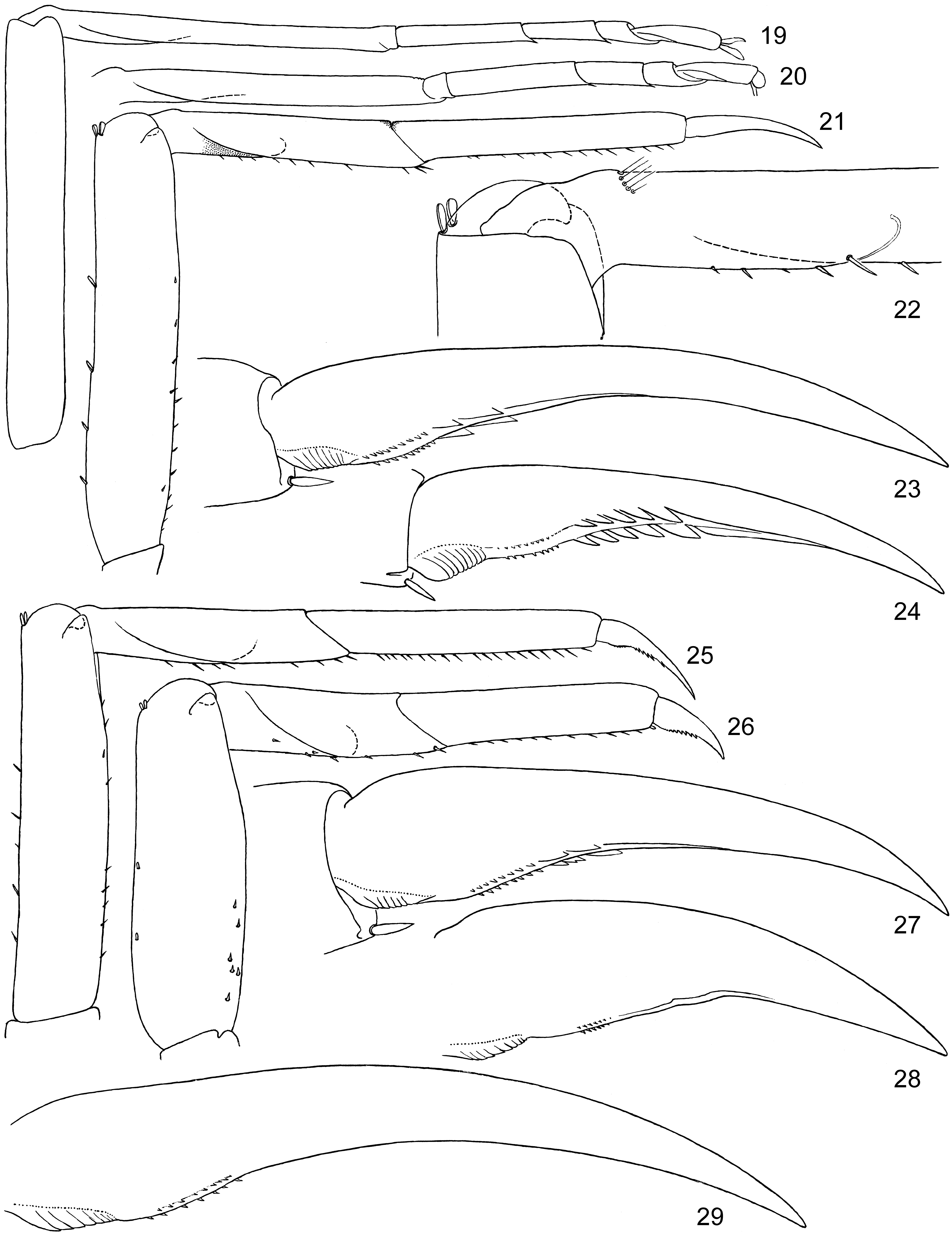
(10) Larval leg setation ( Figs 21, 25, 26 View FIGURES 19 – 29. 19 – 23 ). Outer side of femur bears sparse row of small stout setae, apically terminated by 2 small stout setae located close together. The same in many other baetid taxa; possible plesiomorphy.
While setation of legs is rather uniform among Cheleocloeon , leg shape varies from slender, with femur parallel-sided ( Fig. 21 View FIGURES 19 – 29. 19 – 23 ) to rather stout, with femur thickened ( Fig. 26 View FIGURES 19 – 29. 19 – 23 ).
(11) Patella-tibial suture ( Figs 19–22, 25, 26 View FIGURES 19 – 29. 19 – 23 ). This suture is equally developed on all legs of larva of both sexes and on all legs of female subimago and imago, being absent only on fore legs of male subimago and imago (i.e., the typical condition for Anteropatellata).
This character is important here, because Cheleocloeon was confused with both some Anteropatellata (e.g., Centroptilum ) and with some Protopatellata (e.g., Afroptilum and Bugilliesia ). Gillies wrote that Cheleocloeon has patella-tibial suture on middle and hind leg only ( Gillies 2001: 32); this conclusion was based on Ch. sigiense , which does not belong to Cheleocloeon (see below). Gattolliat et al. (2009) wrote in their larval diagnosis of Cheleocloeon , that the patella-tibial suture is absent. Probably, this statement was caused by the fact that larval legs of Cheleocloeon have colorless translucent cuticle, so the patella-tibial suture can be poorly visible on a background of living tissues; it is well visible on empty larval exuviae. The anteropatellate condition of patellatibial suture is present in all species examined.
When Wuillot and Gillies (1993) described Ch. yolandae and Ch. carinatum , they did not know about this character and did not pay special attention to it. Distal border of dark macula, shown by dots on fore legs of both these species ( Wuillot & Gillies 1993: Figs 8, 17 View FIGURES 7 – 18. 7 – 15 ) correspond to the patella-tibial suture. According to my examination of larval exuviae from Zambia, in both species patella-tibial suture is present on all legs; female subimago and imago of Ch. yolandae also have patella-tibial suture equally developed on all legs ( Figs 19–22 View FIGURES 19 – 29. 19 – 23 ).
In the descriptions of Ch. excisum ( Barnard 1932; Lugo-Ortiz & McCafferty 1997a) patella-tibial suture was not mentioned; actually it is equally developed on all legs of larva (as in Fig. 25 View FIGURES 19 – 29. 19 – 23 ).
According to the original description of Ch. soldani , “tibio-patellar suture absent” (Gattolliat & Sartori 2008: 52). Actually this suture is equally developed on all legs of larva ( Fig. 25 View FIGURES 19 – 29. 19 – 23 ).
All three new species, Ch. clavifolium sp. n., Ch. lancetofolium sp. n. and Ch. truncifolium sp. n. have patellatibial suture equally developed on all legs of larva and equally developed on all legs of female subimago and imago. Larvae of unnamed species reported below, also have patella-tibial suture equally developed on all legs ( Fig. 26 View FIGURES 19 – 29. 19 – 23 ).
(12) Imaginal and subimaginal tarsi. Tarsus of middle and hind leg is relatively long, about 0.7–0.9 of tibia length; proximal tarsal segment (result of complete fusion of primary 1st+2nd tarsomeres) is much longer than others, about 0.4–0.5 of total tarsal length. All examined species have 2 apical spines on middle and hind legs of both sexes and on fore leg of female (on 2nd and 3rd primary tarsomeres).
(13) Microlepides on subimaginal tarsi. All tarsal segments of all legs of both sexes are covered by pointed microlepides (examined for both sexes of Ch. clavifolium sp. n., Ch. lancetofolium sp. n. and Ch. truncifolium sp. n., males of Ch. soldani and Ch. sp. 1, females of Ch. yolandae and Ch. sp. 2). The same in Protopatellata, Cloeonini, Centroptilum, Baetopus, Afrobaetodes and some Baetovectata; in contrast to this, in some Baetovectata tarsi are partly or completely covered by blunt microlepides (Kluge & Novikova 2014: Figs 11–17 View FIGURES 7 – 18. 7 – 15 ). Such character pattern testifies that this is a plesiomorphy of Cheleocloeon .
(14) Larval claw ( Figs 23, 24, 27–29 View FIGURES 19 – 29. 19 – 23 ). Claw is slender, slightly bent, with 2 rows of denticles, among which minute denticles are located close to base of claw and larger denticles, if present, occupy more distal part of claw. In some species only minute proximal denticles are present. Larger denticles can be either well-expressed, or very delicate, thin-walled, translucent and poorly visible; they can be absent on individual claws. This is true for all species of Cheleocloeon (Gattolliat & Sartori 2008: 52) .
In the generic diagnosis of Cheleocloeon , based on two species, Ch. yolandae and Ch. carinatum, Wuillot and Gillies (1993) wrote that “claws long with minute teeth at base”. Actually, on the larval exuviae of Ch. yolandae examined by me, all six claws have, besides two rows of minute denticles near base, 2+2 larger denticles ( Fig. 23 View FIGURES 19 – 29. 19 – 23 ); they are so delicate and translucent, that they can be easily overlooked.
On the larval exuviae of Ch. carinatum examined, only minute denticles are present, larger denticles are absent.
In the descriptions and figures of Ch. excisum by Barnard (1932: 226, Fig. 15 View FIGURES 7 – 18. 7 – 15 k) and Lugo-Ortiz & McCafferty (1997a: 286, Fig. 9 View FIGURES 7 – 18. 7 – 15 ), the claws are described as “with three to four poorly developed denticles”. Actually each claw has two rows of denticles, each row consists of minute denticles proximally and 4–7 larger denticles more distally (similar to Fig. 24 View FIGURES 19 – 29. 19 – 23 ).
According to the original description of Ch. soldani , claw “with one row of 6 reduced teeth proximally and 7 stout teeth apically” (Gattolliat & Sartori 2008: 52). Actually it has two equal rows of such denticles ( Fig. 24 View FIGURES 19 – 29. 19 – 23 ).
All three new species, Ch. clavifolium sp. n., Ch. lancetofolium sp. n. and Ch. truncifolium sp. n. have at least two rows of minute denticles near base of claw ( Figs 27–29 View FIGURES 19 – 29. 19 – 23 ).
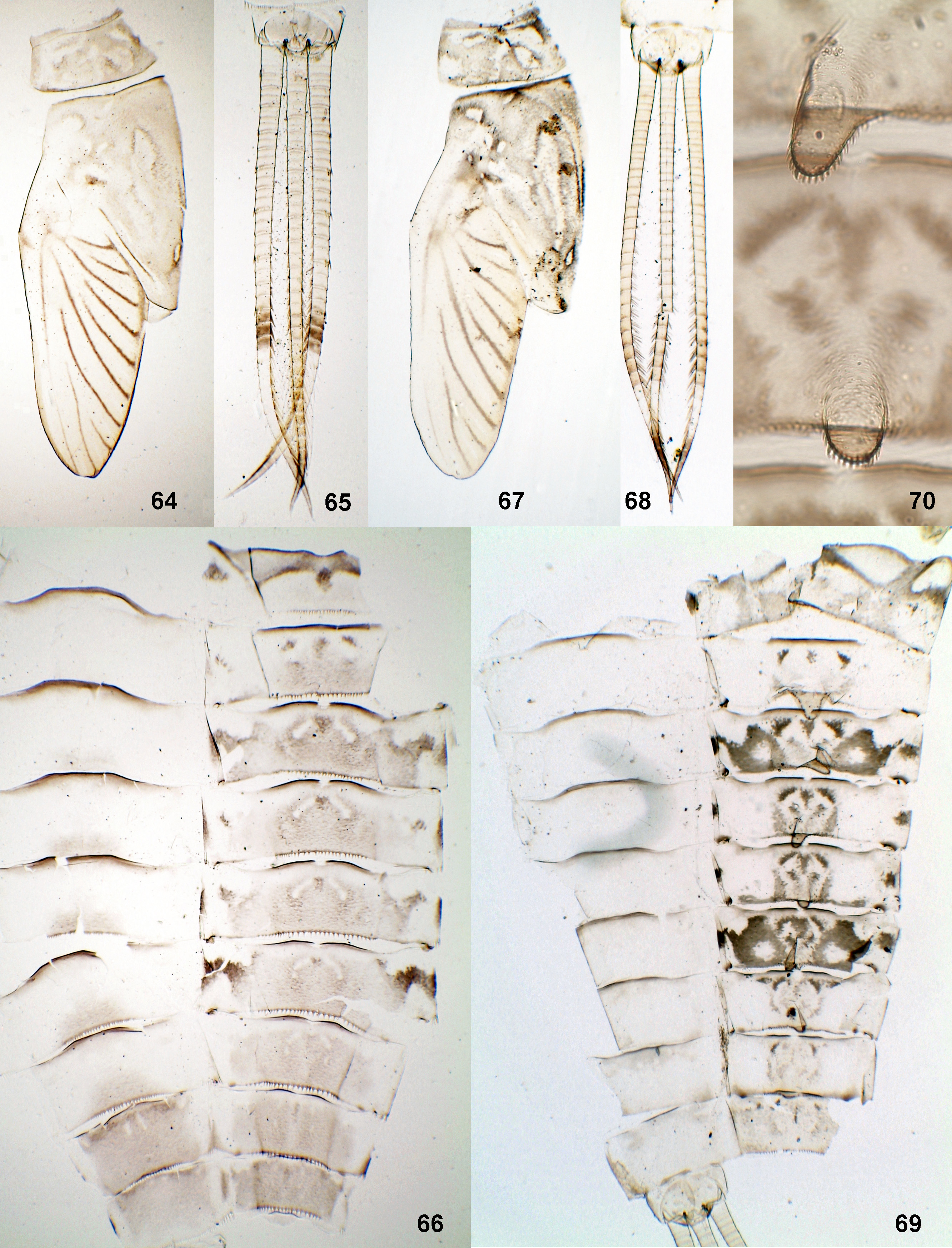
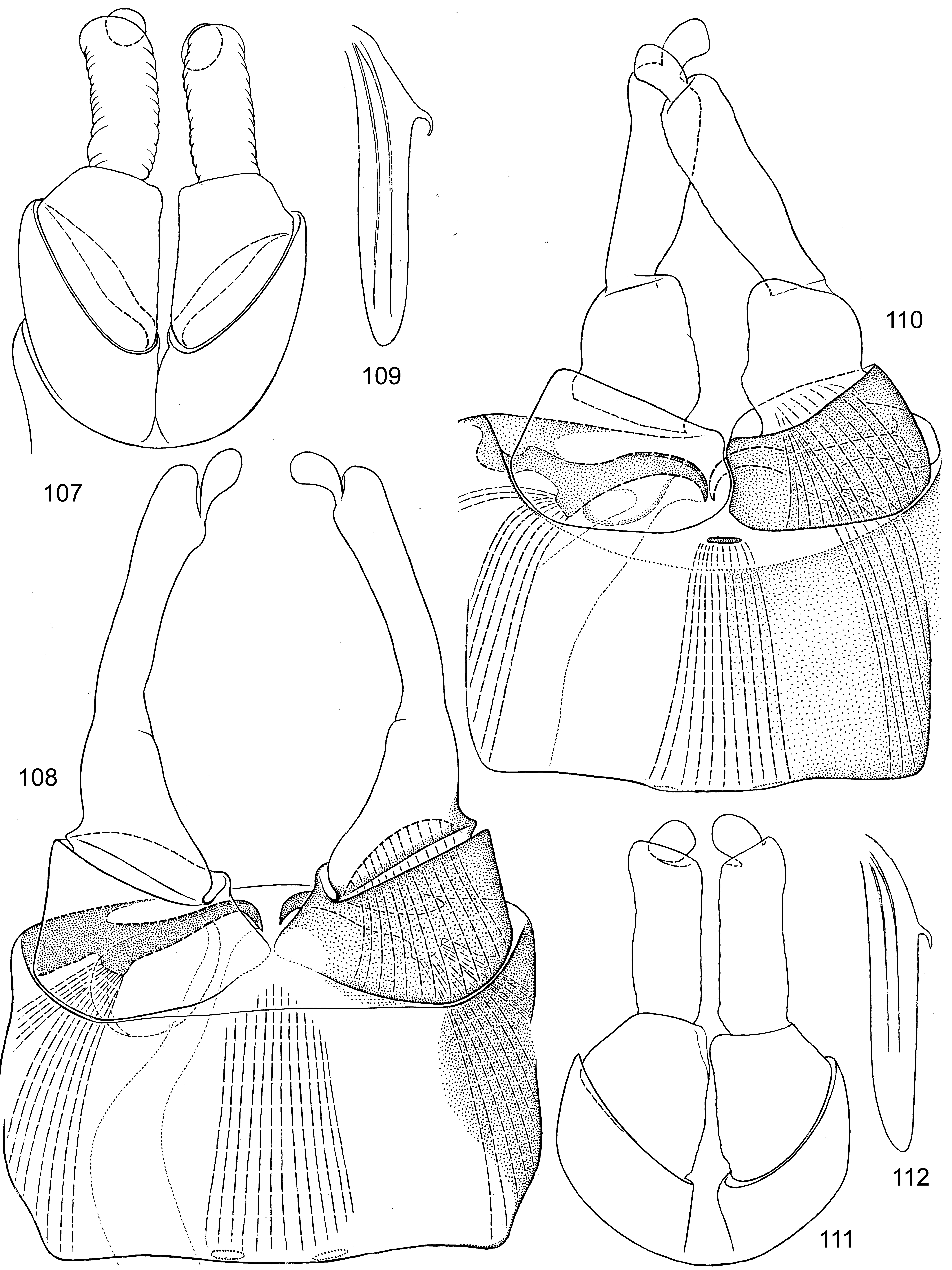
(15) Hind wing ( Figs 10–13 View FIGURES 7 – 18. 7 – 15 , 109, 112 View FIGURES 107 – 112. 107 – 109 ). Hind wing, if present, is of the « Centroptilum - type »: narrow, with 2 longitudinal veins only, with hooked costal projection ( Fig. 11 View FIGURES 7 – 18. 7 – 15 ); larval hind protopteron is narrow, slightly S-bent, narrowed toward apex ( Fig. 10 View FIGURES 7 – 18. 7 – 15 ). Females never have hind wings; males either have them, or not.
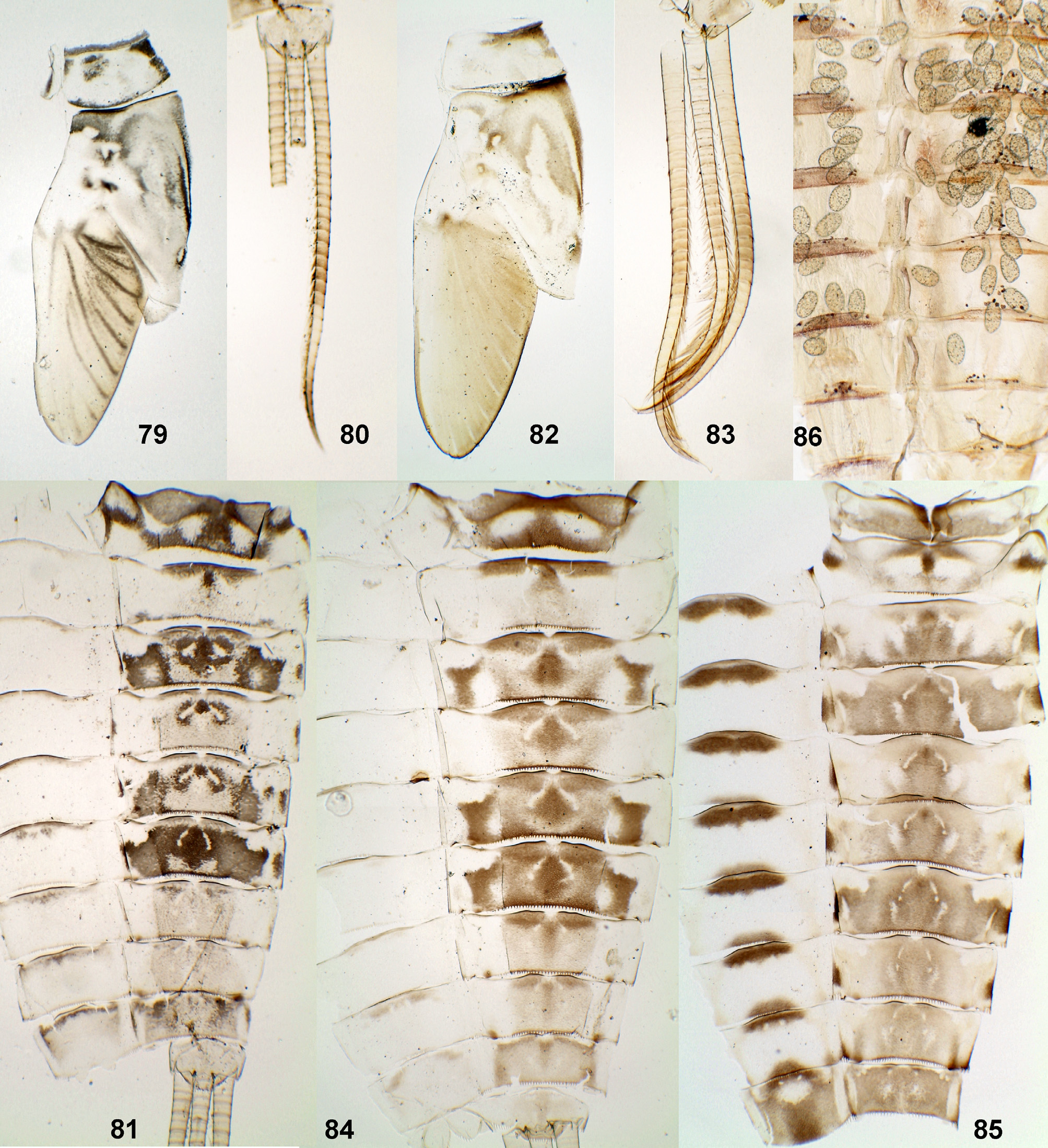
Ch. clavifolium sp. n., Ch. lancetofolium sp. n. and Ch. truncifolium sp. n. have hind wings in male; their female imagoes have vestiges of hind wings, which represent very small immovable membranous projections, whose length is equal to length of vestige of larval protopteron ( Fig. 13 View FIGURES 7 – 18. 7 – 15 ); vestige of larval protopteron is rather wide and projected posteriad of posterior margin of metanotum ( Fig. 12 View FIGURES 7 – 18. 7 – 15 ). The same vestiges of larval protopteron are present in female larvae of Ch. excisum , Ch. soldani, Ch. sp. 2 and Ch. sp. 3.
C. dimorphicum , according to the original description, also has hind wings in male, but its female imago has no vestiges of hind wings, and larval protoptera are “absent or considerably reduced, empty and rudimentary” ( Soldán & Thomas 1985: 182, Fig. 10 View FIGURES 7 – 18. 7 – 15 ).
Ch. yolandae and Ch. carinatum have no hind wings in male; at least females have no any vestiges of hind protoptera.
Contrary to the statement that females of Cheleocloeon never have developed hind wings, two species with hind wings in the female were attributed to Cheleocloeon . One of them, falcatum Crass 1947 [ Centroptilum ], is known from imagoes only, and does not belong to Cheleocloeon (see below). Another species, mirandei Lugo- Ortiz & McCafferty 1997 [ Cheleocloeon ] is known as a single female larva; recently it was moved to Bugilliesia ( Gattolliat et al. 2009) .
(16) Denticles on larval abdomen. Posterior margins of abdominal terga (at least terga II–X) bear pointed denticles, which can be larger ( Fig. 62 View FIGURES 58 – 63 ) or smaller. In Ch. carinatum , whose abdominal terga bear unpaired projections, these denticles border the margin of each projection ( Fig. 70 View FIGURES 64 – 70 ). Posterolateral spines are absent on all abdominal segments (as in most Baetidae ). Lateral margins of all abdominal segments have no denticles (in contrast to Cloeoninae and Anafroptilum ). Posterior margin of tenth abdominal tergum is semicircular with even denticles, as on previous terga (lateral denticles are not enlarged, in contrast to Procloeon ). Paraprocts bear pointed denticles ( Fig. 14 View FIGURES 7 – 18. 7 – 15 ).
(17) Scales of larval abdomen. Abdominal terga, sterna and caudalii bear translucent scales; socket of such scale is W-shaped, i.e. with two concavities; each concavity is covered by a short cover, which is not projected distad of the concavity ( Figs 15 View FIGURES 7 – 18. 7 – 15 , 62, 63 View FIGURES 58 – 63 ). Scales with two covers are present in various taxa of Baetidae , but not in Cloeonini, Centroptilum or Anafroptilum .
(18) Setae of larval abdomen. Abdominal terga and sterna, besides scales [see (17)] have only sparse, irregularly situated simple setae, which do not form rows or groups ( Fig. 63 View FIGURES 58 – 63 ).
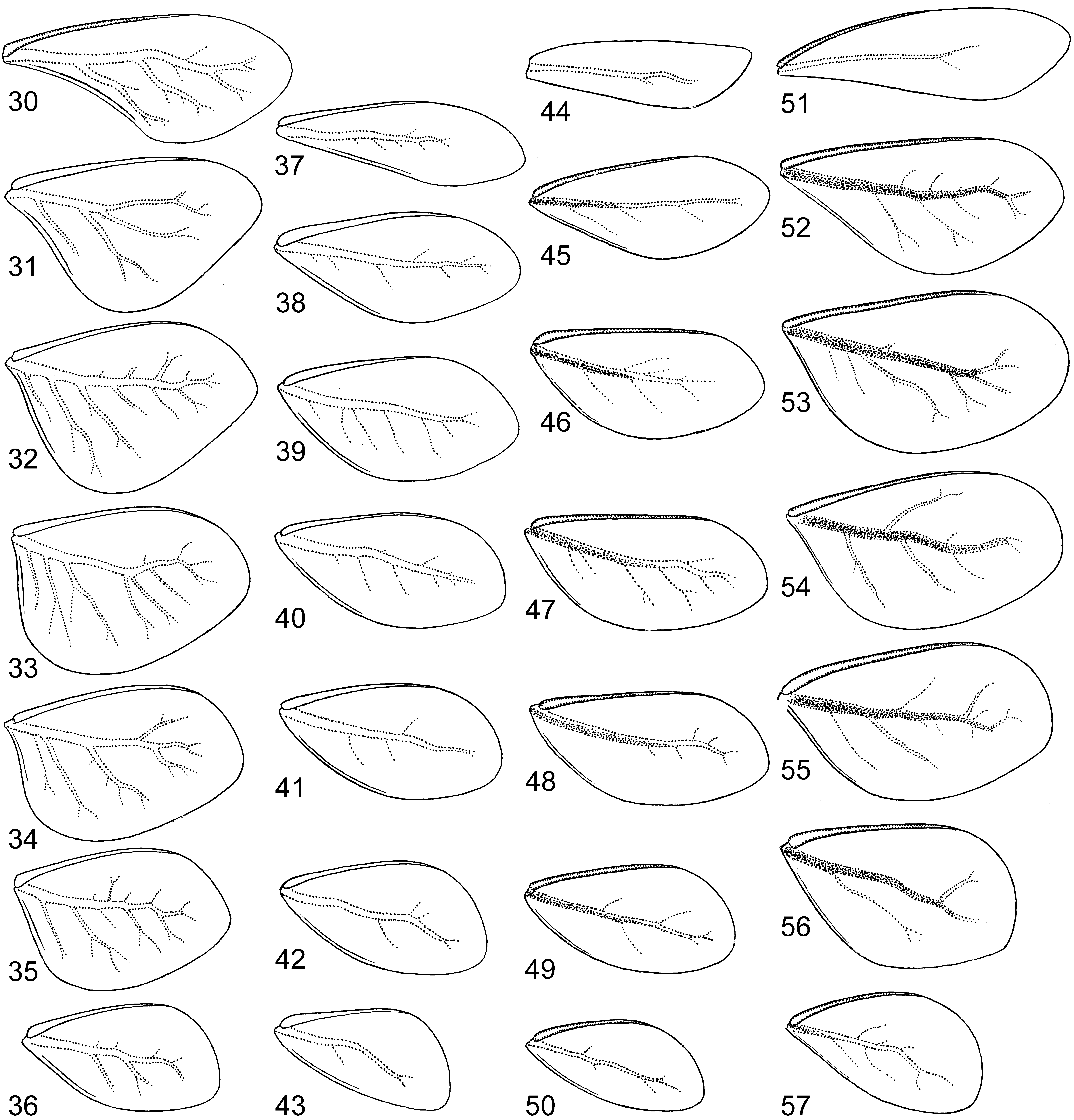
(19) Structure of tergalii ( Figs 30–57 View FIGURES 30 – 57 ). Costal and anal ribs can be rather long, but not reaching apex of tergalius, so that distal margin of tergalius is not bordered by rib. First tergalius is narrower than others, more or less petiolate; its length can be either less, or equal, or greater than length of other tergalii.
(20) Mobility of tergalii. Tergalii are able to make rhythmical respiratory movements. Wuillot and Gillies (1993) wrote about Ch. yolandae and/or Ch. carinatum : “In living nymphs, while at rest, the first gill is held above the body like a banner and actively vibrated. In contrast, the remaining gills are held passively, close to the abdomen”. Actually other tergalii are also able to make respiratory vibration. I observed respiratory vibrations of Ch. yolandae , Ch. excisum , Ch. clavifolium sp. n. and Ch. lancetofolium sp. n..
(21) Larval caudalii ( Figs 60, 61 View FIGURES 58 – 63 , 65, 68 View FIGURES 64 – 70 , 75 View FIGURES 71 – 78 , 80 View FIGURES 79 – 86. 79 – 85 , 83). Paracercus is always as long as cerci; cerci and paracercus up to the apex bear well developed primary swimming setae. Each swimming seta is thick and sometimes darkened in proximal part and contrastingly thinner and always colorless in distal part. Distal part of each cercus bears sparse longitudinal row of delicate secondary swimming setae ( Fig. 61 View FIGURES 58 – 63 ). These features are not reported in literature, but observed by me for Ch. yolandae , Ch. carinatum , Ch. clavifolium sp. n. Ch. lancetofolium sp. n., Ch. excisum , Ch. soldani, Ch. sp.2 and Ch. sp.3.
Position of denticles on posterior margins of segments of caudalii have species-specific variability: In most species ( Ch. clavifolium sp. n., Ch. lancetofolium sp. n., Ch. truncifolium sp. n., Ch. excisum , Ch. soldani, Ch. sp.2 and Ch. sp.3) enlarged pointed denticles form short transverse rows on each 4th or each 2nd segment on lateral side of each cercus ( Fig. 60 View FIGURES 58 – 63 ) and on dorsal side of paracercus, occasionally also on ventral side of paracercus. In Ch. yolandae such denticles are present only on cerci (one individual examined); in Ch. carinatum large denticles are absent (one individual examined).
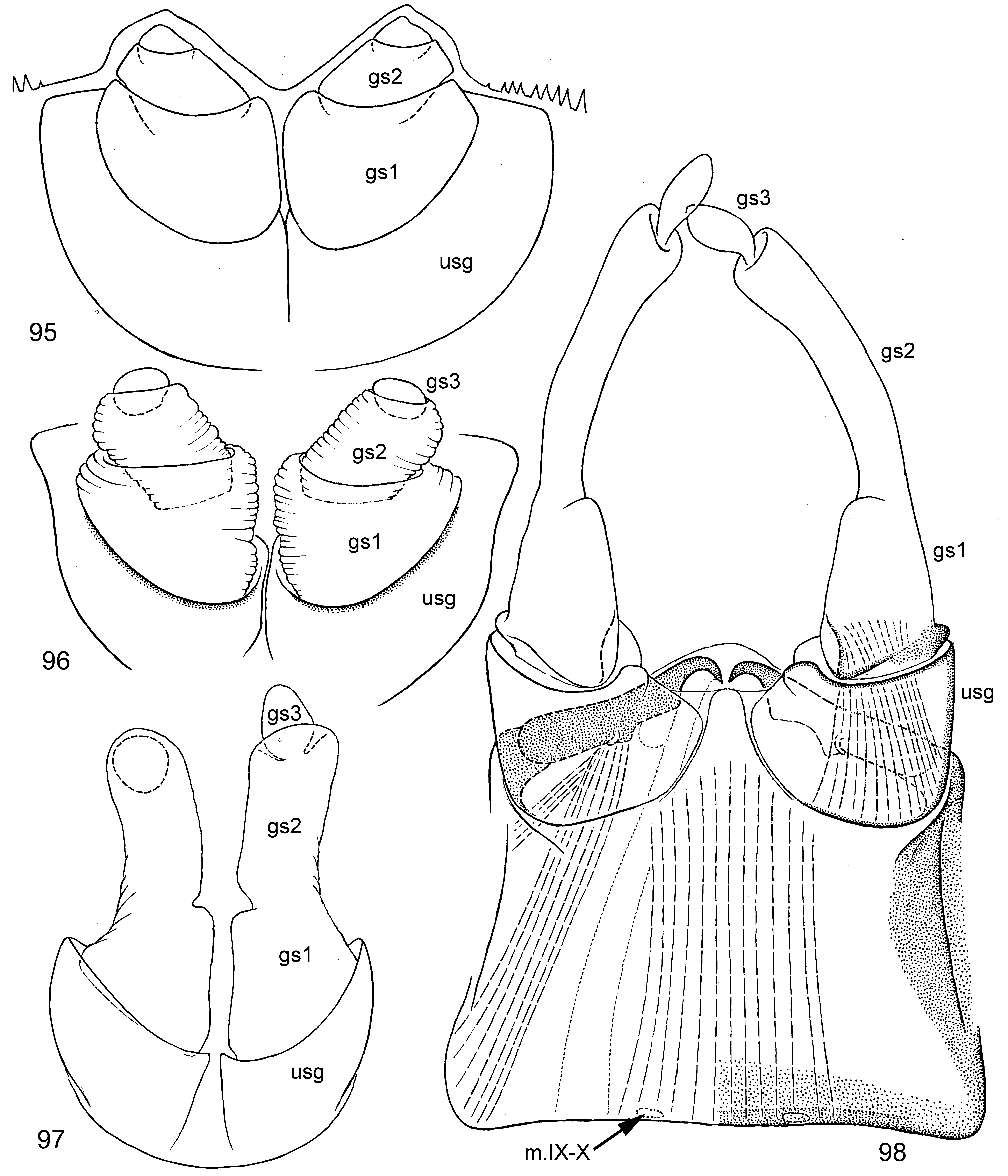
(22) Pose of developing gonostyli ( Figs 95, 96 View FIGURES 95 – 98. 95 – 96 ). Pose of developing subimaginal gonostyli folded under larval cuticle is of the « Cloeon - type », i.e. with 2nd segments diverging laterally. This is a plesiomorphic character, common with most Protopatellata, Cloeonini, Centroptilum, Baetopus and Afrobaetodes ; but it allows Cheleocloeon to be distinguished from Bugilliesia and other Rhithrocloeoninae, whose gonostyli have a quite different pose ( Kluge 2012b, 2015). The pose of developing gonostyli is examined for Ch. excisum , Ch. soldani , Ch. clavifolium sp. n., Ch. lancetofolium sp. n., Ch. truncifolium sp. n. and Ch. sp. 2.
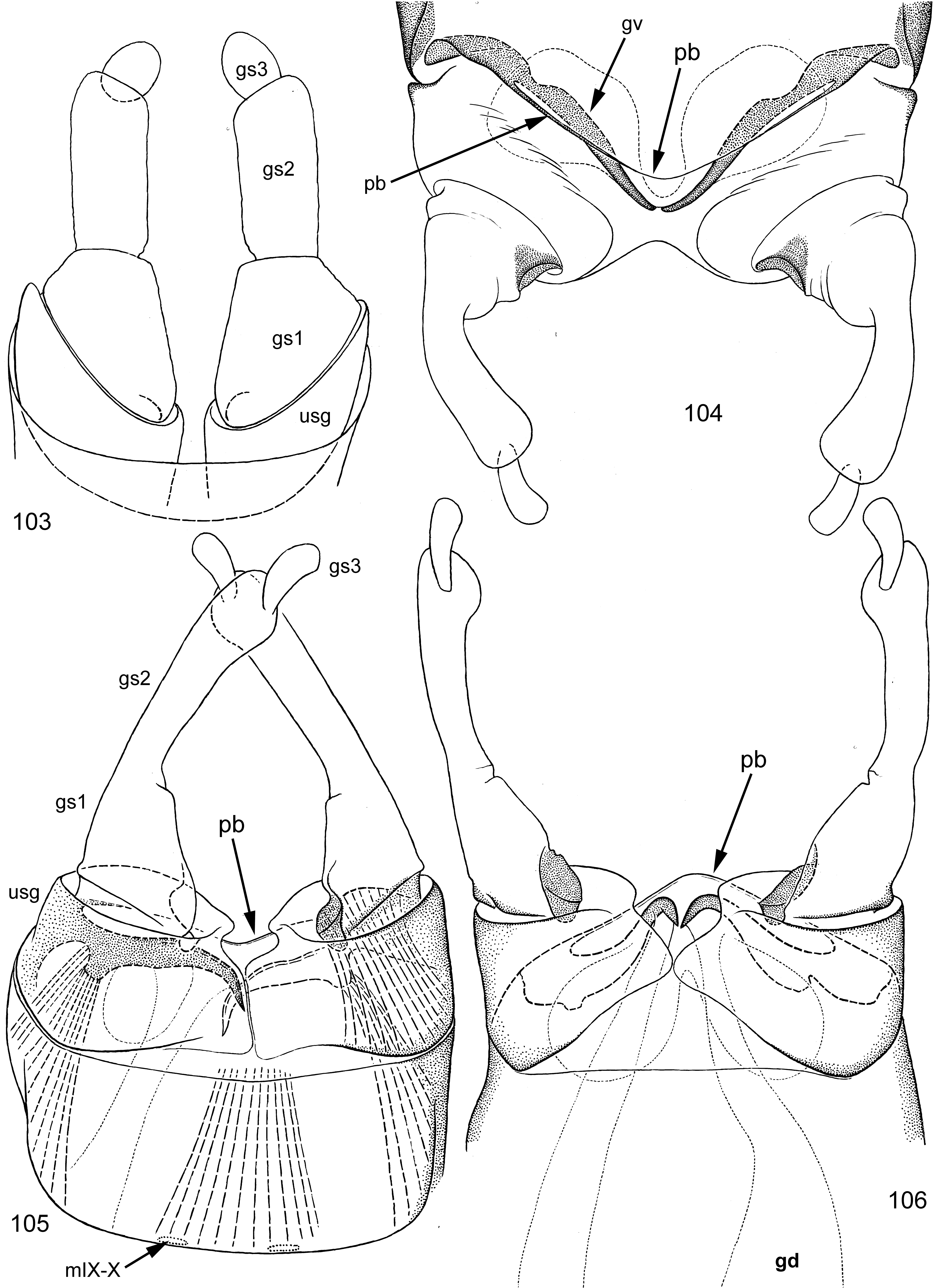
(23) Genitals of male imago ( Figs 98–110 View FIGURES 95 – 98. 95 – 96 View FIGURES 99 – 102 View FIGURES 103 – 106 View FIGURES 107 – 112. 107 – 109 ). Gonovectes are movable, wide, well-sclerotized and pigmented, with distal margin strengthened; apex of each gonovectis (where gonoduct is attached), being directed medially, is pointed and hooked so that its point is directed cranially (i.e. into the body). Protuberance at mid-length of gonovectis, served for attachment of gonovectal muscle, is prominent, located at the same plane as the hooked apex and has the same direction (cranially, i.e., into the body) ( Fig. 101 View FIGURES 99 – 102 ). When gonovectes are turned caudally, their hooked apices are visible between unistyligers ( Figs 99 View FIGURES 99 – 102 , 106 View FIGURES 103 – 106 ); when they are turned cranially, they are invisible from ventral view ( Fig. 105 View FIGURES 103 – 106 ). Penial bridge is slightly sclerotized in lateral parts and membranous medially ( Figs 101 View FIGURES 99 – 102 , 104 View FIGURES 103 – 106 ). This structure of gonovectes and penial bridge is uniform in all species examined ( Ch. clavifolium sp. n., Ch. lancetofolium sp. n., Ch. truncifolium sp. n. and Ch. sp. 1). Unpaired styligeral muscle is well developed, so that unistyligers can be brought together ( Fig. 105 View FIGURES 103 – 106 ) and turned apart ( Fig. 106 View FIGURES 103 – 106 ). Median sclerite served for insertion of the styligeral muscle, is either present ( Fig. 110 View FIGURES 107 – 112. 107 – 109 ), or not (such sclerite occurs also in some Protopatellata). Bases of unistyligers are either contiguous ( Figs 106 View FIGURES 103 – 106 , 108 View FIGURES 107 – 112. 107 – 109 ), or narrowly separated ( Fig. 98 View FIGURES 95 – 98. 95 – 96 ), but never widely separated. Gonostylus has 2nd segment widened apically and 3rd segment small and clavate (this is similar to Cloeonini, Centroptilum and Anafroptilum ). Shape of 1st segment of gonostylus is species-specific; in certain species ( Ch. clavifolium sp. n. and Ch. soldani ) it has a sclerotized and pigmented concavity on median side close to the base ( Figs 99, 100 View FIGURES 99 – 102 , 104–106 View FIGURES 103 – 106 ; Gattolliat & Sartori 2008: Fig. 7 View FIGURES 7 – 18. 7 – 15 ).
Genitals of the type species, Ch. yolandae , have not been examined by me, and structure of their internal parts (gonovectes and penial bridge) is not described; shape of their external parts (gonostyli and unistyligers) well agree with the description given above ( Wuillot & Gillies 1993: Fig. 2 View FIGURES 1 – 6. 1 – 5 ).
In contrast to this, genitals of the species described as Cheleocloeon sigiense Gillies 2001 and genitals of the species originally described as Centroptilum falcatum Crass 1947 and subsequently attributed to Cheleocloeon by Lugo-Ortiz and McCafferty (1998), differ from genitals of Cheleocloeon by widely separated unistyligers ( Gillies 2001: Fig. 3 View FIGURES 1 – 6. 1 – 5 ; Crass 1947: Fig. 20 View FIGURES 19 – 29. 19 – 23 b). These two species do not belong to Cheleocloeon : the first of them belongs to Protopatellata and the second one to Cloeonini (see below).
Structure of gonovectes is unique for Cheleocloeon , not found in any other taxa. In all Cloeonini, Baetopus and Anafroptilum , gonovectes are immovably fused by their apices with penial bridge (e.g., Kluge 2012a: Fig. 45 View FIGURES 30 – 57 ; Kluge, Tiunova & Novikova 2014: Figs 30, 31 View FIGURES 30 – 57 ). In Centroptilum gonovectes are movable, pointed and hooked; in contrast to Cheleocloeon , their points are directed not cranially, but caudally ( Grandi 1960: Fig. XI.3). In Afrobaetodes gonovectes are also movable and hooked; in contrast to Cheleocloeon , the protuberance which serves for muscle attachment and the hooked apex are directed to opposite sides (unpublished data). In all Baetovectata gonovectes are also movable, but slender, sharply bent and deeply evaginated into the body. In various taxa of Protopatellata, gonovectes are either fused with penial bridge (e.g. in Rhithrocloeoninae, Indocloeon and others) or movable, but always different from that of Cheleocloeon .
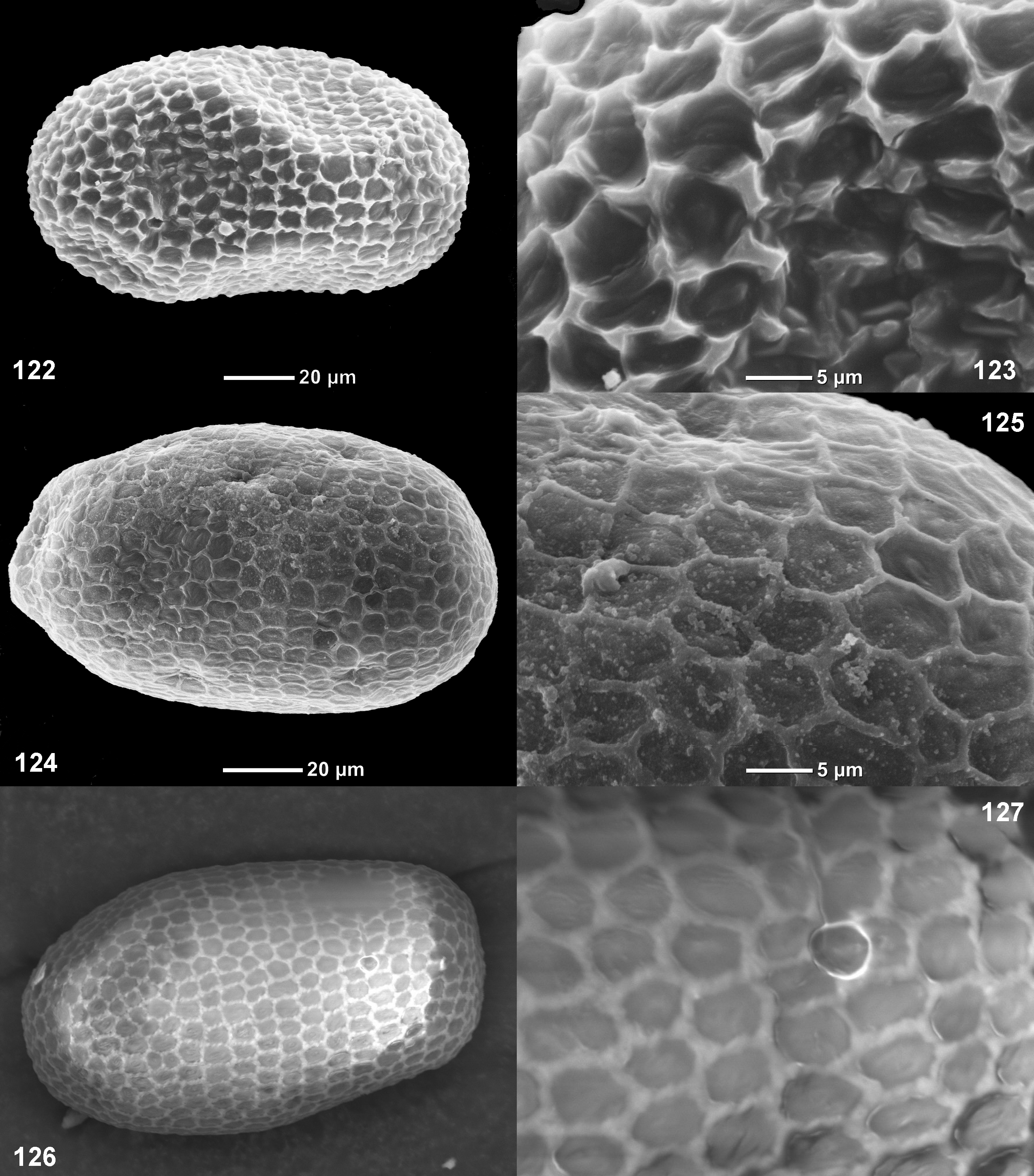
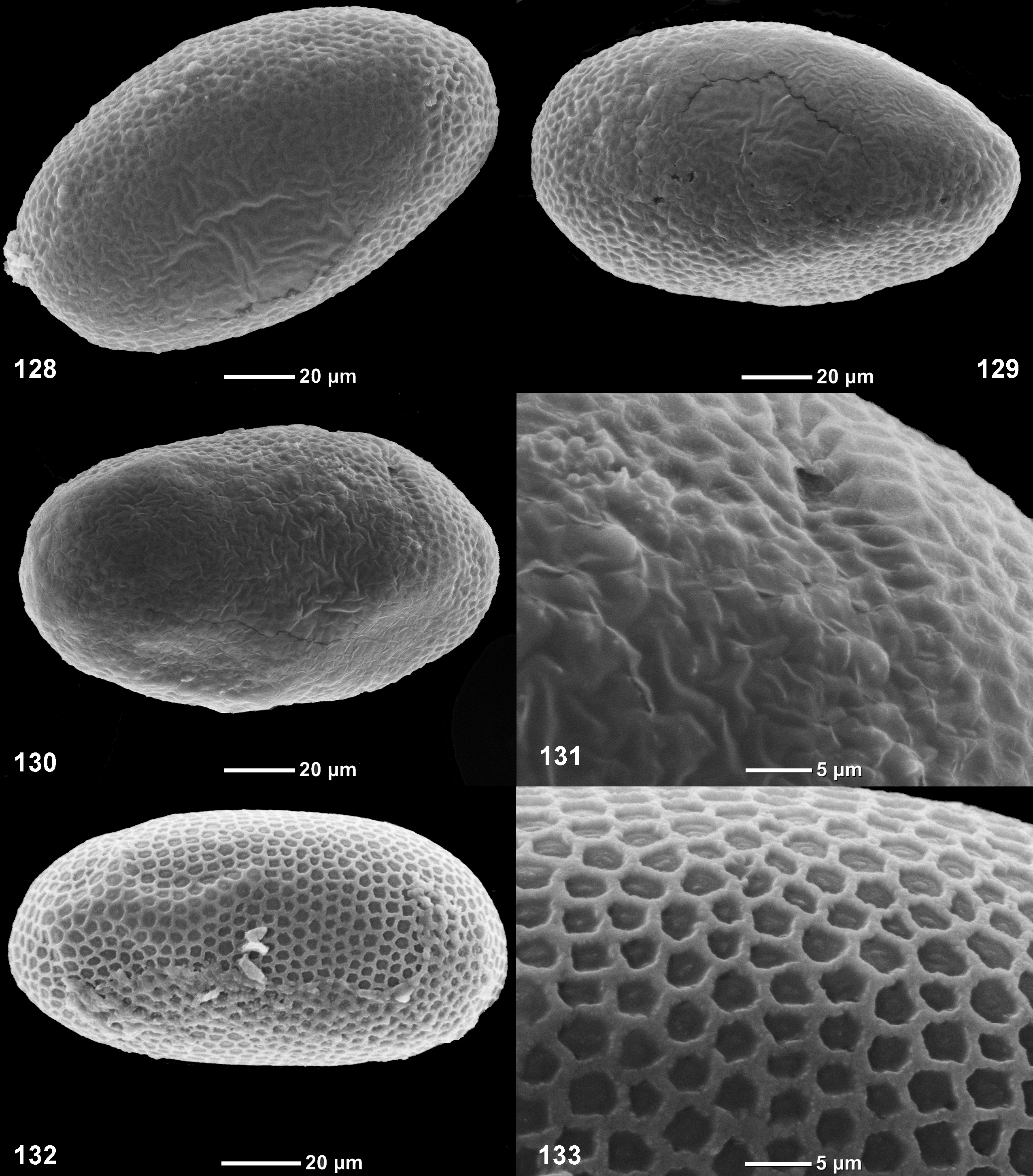
(24) Eggs ( Figs 122–131 View FIGURES 122 – 127 View FIGURES 128 – 133 ). Eggs are oval, with more or less expressed convex net-like relief, somewhat different in different species.
Distribution. Whole of Africa, Madagascar and Arabian Peninsula. Such area of distribution is unknown for other mayfly taxa, which are either Afrotropical (often including Madagascar), or Palaearctic (including northern Africa), or widespread, including both Afrotropical and Palaearctic regions, but not limited to Africa and Arabia.
Composition. Together with three species described below, Cheleocloeon contains 10 formally described species, which can be presumably arranged into three groups:
A. Group yolandae (hind wings absent; claws without large denticles); includes 2 species— Ch. yolandae Wuillot (in Wuillot & Gillies) 1993 and Ch. carinatum Wuillot (in Wuillot & Gillies) 1993.
B. Group clavifolium (hind wings present in male; claws without large denticles); includes 5 species:— Ch. clavifolium sp. n., Ch. lancetofolium sp. n., Ch. truncifolium sp. n., Ch. madagascariense Gattolliat & Monaghan (in Gattolliat & Barber-James & Monaghan) 2009 and Ch. dimorphicum ( Soldán & Tomas 1985) .
C. Group excisum (hind wings present in male; claws with large pointed denticles); includes 3 described species— Ch. soldani Gattolliat & Sartori 2008 , Ch. excisum ( Barnard 1932) and Ch. littorale McCafferty 2001 .
Species erroneously placed to Cheleocloeon . Lugo-Ortiz & McCafferty (1997b) described the species mirandei [ Cheleocloeon ] based on larvae from Madagascar; now attributed to Bugilliesia ( Gattolliat et al. 2009) .
Lugo-Ortiz & McCafferty (1998) attributed to Cheleocloeon the species falcatum Crass 1947 [ Centroptilum ], described as male imagoes only; actually, this species cannot belong to Cheleocloeon , because its unistyligers are widely separated ( Crass 1947: Fig. 20 View FIGURES 19 – 29. 19 – 23 b) [see character (23) of Cheleocloeon ]; most probably, these imagoes belong to Cloeonini and possibly are conspecific with larvae described as Securiops macafertiorum ( Lugo-Ortiz 1996) (unpublished data).
Gillies (2001) described the species sigiense [ Cheleocloeon ] based on imagoes and larvae of both sexes associated by rearing. This species differs from all Cheleocloeon by the following characters: labrum without setae on outer surface ( Gillies 2001: Fig. 4 View FIGURES 1 – 6. 1 – 5 ), while labrum of Cheleocloeon has several setae [see character (3) and Fig. 1 View FIGURES 1 – 6. 1 – 5 ]; labial palp with 2nd segment moderately projected ( Gillies 2001: Fig. 7 View FIGURES 7 – 18. 7 – 15 ), while in Cheleocloeon its projection is larger [see character (8) and Fig. 5 View FIGURES 1 – 6. 1 – 5 ]; patella-tibial suture is present on middle and hind legs only, being absent on fore legs ( Gillies 2001: Figs 9, 10 View FIGURES 7 – 18. 7 – 15 ), while in Cheleocloeon it is equally developed on all legs [see character (11) and Fig. 21 View FIGURES 19 – 29. 19 – 23 ]; unistyligers are widely separated ( Gillies 2001: Fig. 3 View FIGURES 1 – 6. 1 – 5 ), while in Cheleocloeon they are brought together [see character (23) and Figs 98 View FIGURES 95 – 98. 95 – 96 , 104–106 View FIGURES 103 – 106 , 108, 110 View FIGURES 107 – 112. 107 – 109 ]. The species sigiense [ Cheleocloeon ] should be moved to some taxon within the plesiomorphon Protopatellata.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Cheleocloeon Wuillot & Gillies 1993
| Kluge, Nikita J. 2016 |
dimorphicum
| Wuillot 1993: 213 |
| Gillies 1990: 99 |