Euastacus clydensis Riek, 1969
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5222.3.6 |
|
publication LSID |
lsid:zoobank.org:pub:21C3E606-A79C-4E4B-90B8-7DC7371508CA |
|
DOI |
https://doi.org/10.5281/zenodo.7467336 |
|
persistent identifier |
https://treatment.plazi.org/id/4A5D1F77-784B-F216-A2EB-CA15C1A0F8CA |
|
treatment provided by |
Plazi |
|
scientific name |
Euastacus clydensis Riek, 1969 |
| status |
stat. nov. |
Euastacus clydensis Riek, 1969 View in CoL stat. rev.
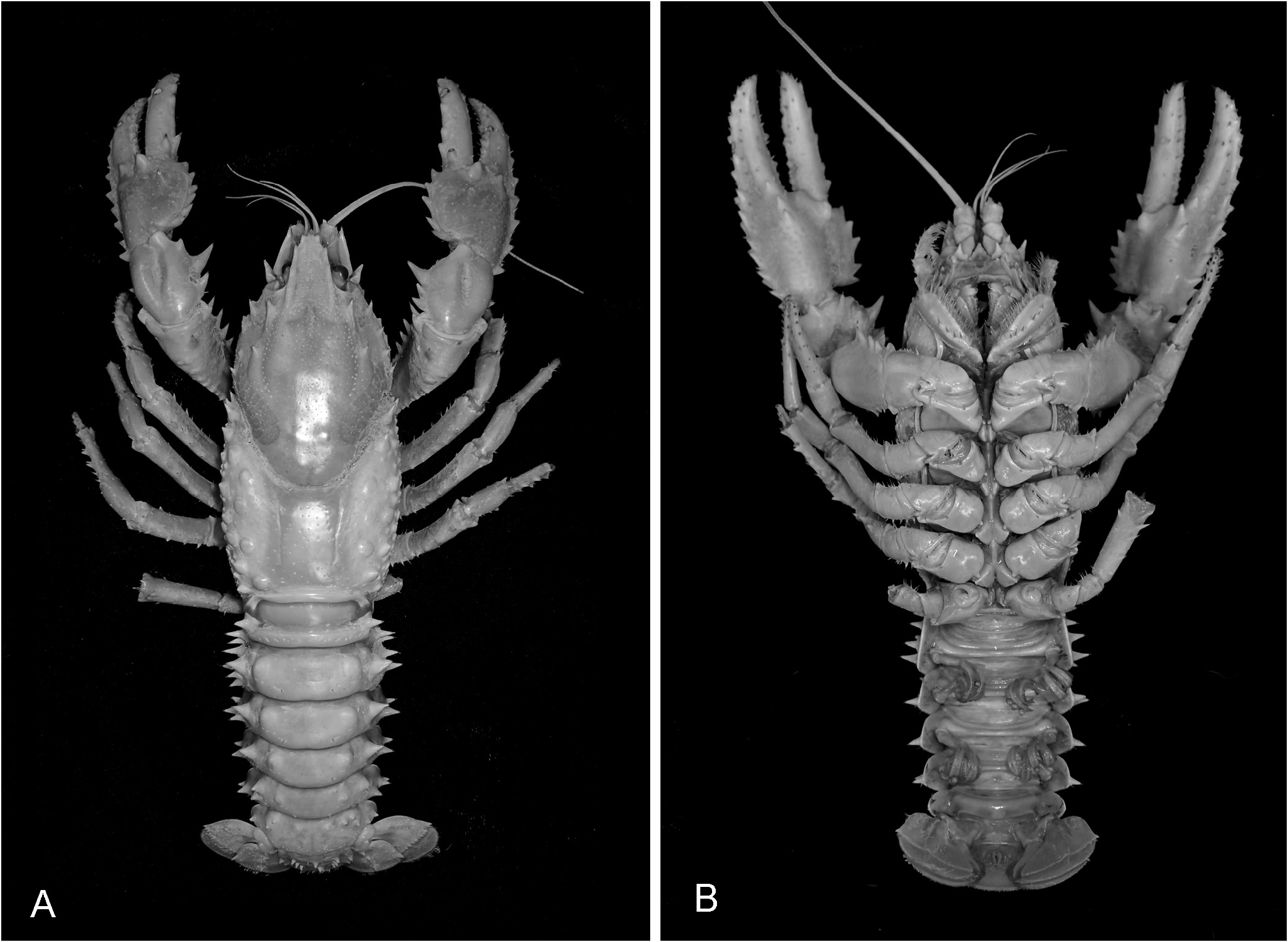
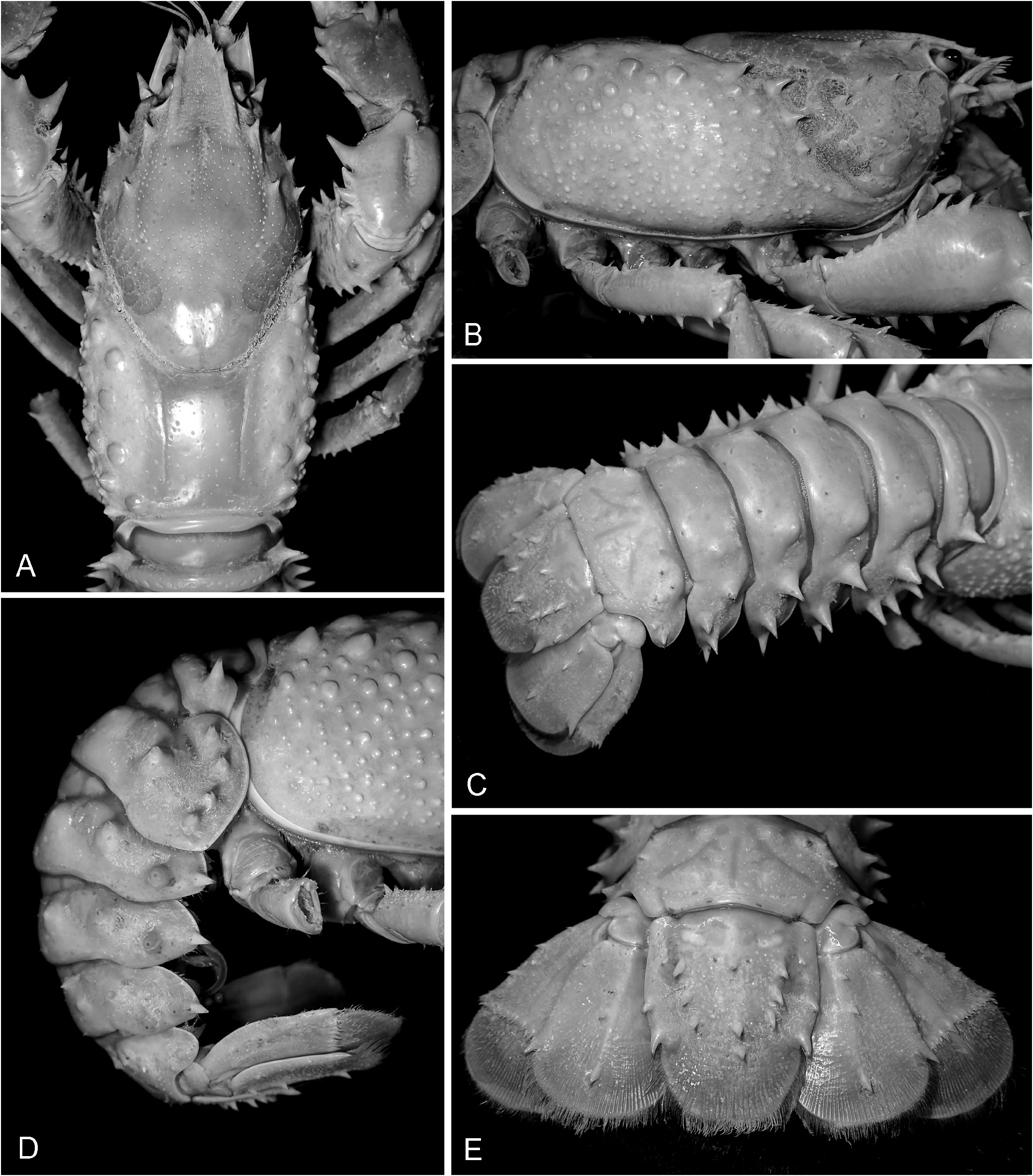
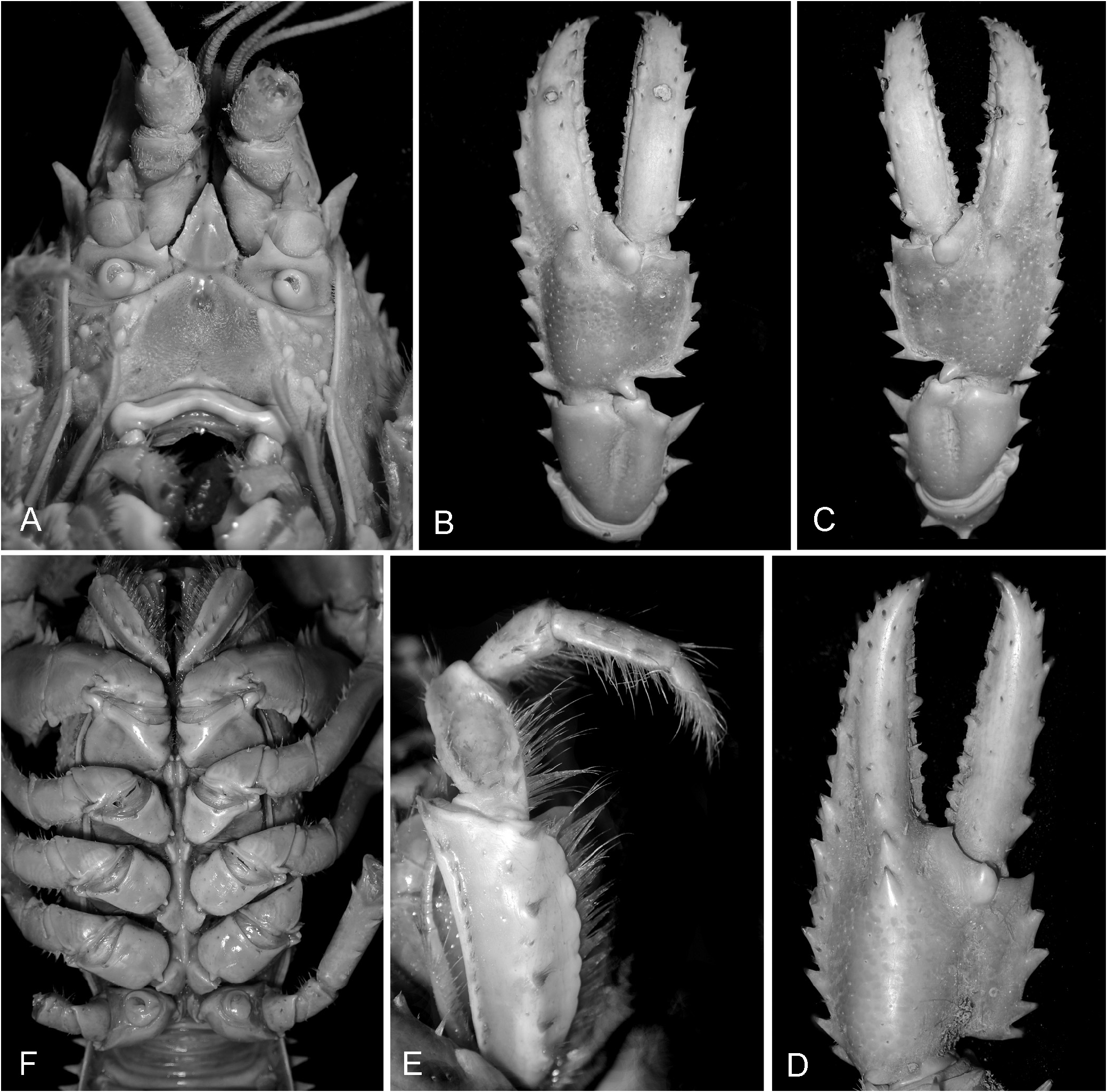
( Figs 1–4A, B View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 , 5 View FIGURE 5 )
Euastacus clydensis Riek, 1969: 911 View in CoL , 912.— Merrick 1993: 66, fig. 25— Springthorpe & Lowry 1994: 82.
Euastacus spinifer View in CoL .— Morgan 1997: 93 [far southern specimens only: Clyde River, Clyde Mountain, Conjola, Boyne Creek, Brooman] [Not E. spinifer (Heller, 1865) View in CoL ]
Type material. HOLOTYPE: AM P15491 , male (CL 88.08 mm, OCL 73.37 mm), Clyde River, New South Wales, coll. N. Williams, 25 May 1959 . PARATYPES: AM P15489 , female (CL 102.62 mm, OCL 86.04 mm) ; AM P15488 , female (CL 120.91 mm, OCL 102.67 mm), 19 July 1959 ; AM P15490 , female (CL 86.74 mm, OCL 73.18 mm), stream at Conjola, near Ulladulla, New South Wales, coll. D.D. Francois & J. Harvie, 19 June 1959 .
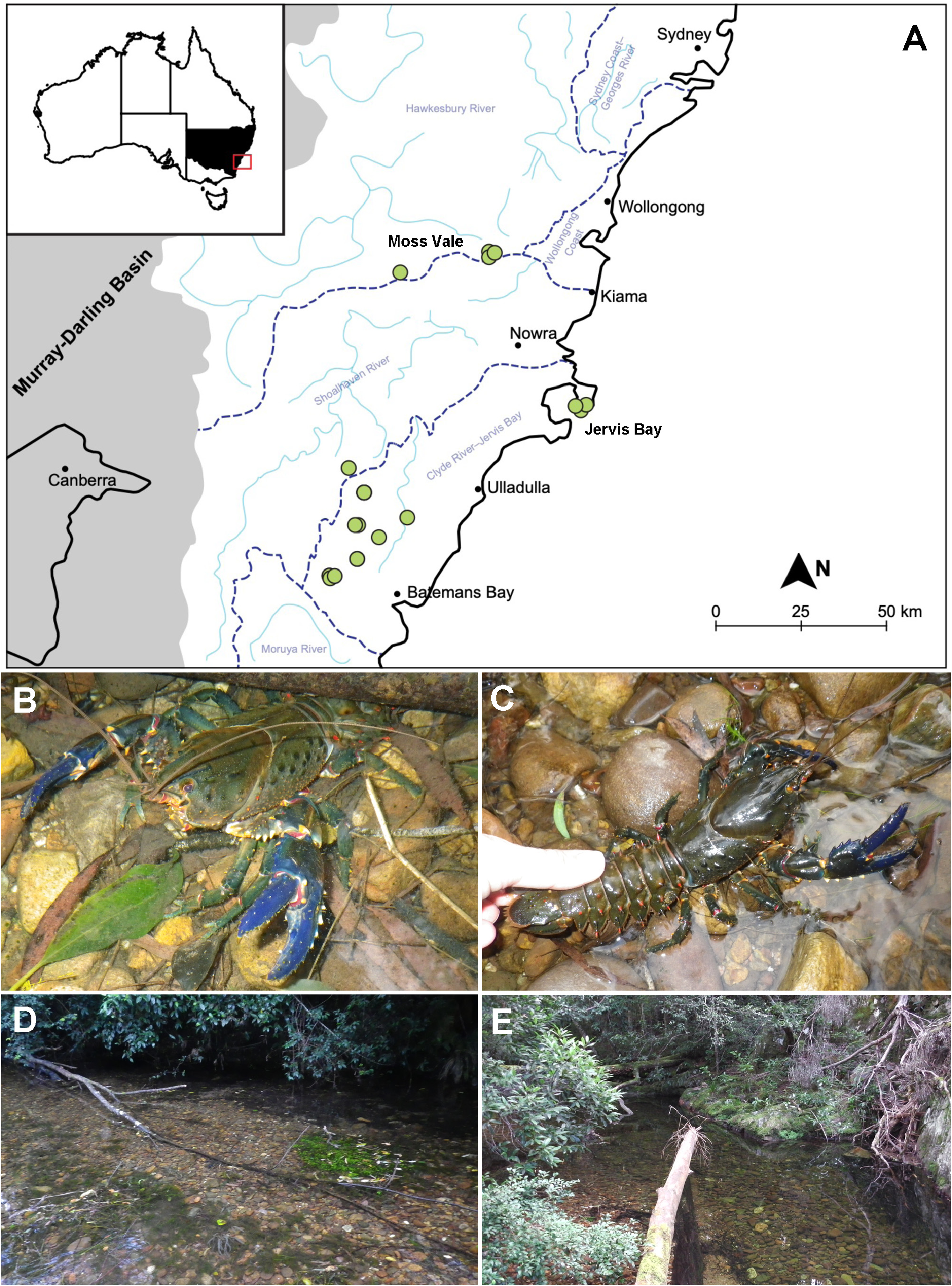
Other material examined. Joadja Creek, New South Wales: AM P99455 , 2 juvenile males (CL 16.47 mm, OCL 12.22 mm; CL 28.23 mm, OCL 22.18 mm), 2 juvenile females (CL 21.74 mm, OCL 15.80 mm; CL 24.62 mm, OCL 18.86 mm), 34°24’46”S, 150°12’20”E, 29 October 2010 GoogleMaps .
Illawarra Highway, Wingecarribee River, Moss Vale, New South Wales: ACP3626 , male (CL 38.16 mm, OCL 34.93 mm), 34°34.870’S, 150°30.792’E, coll GoogleMaps . R.B. McCormack, 28 April 2011 ; ACP3627 , male (CL 18.43 mm, OCL 13.84 mm), 34°34.870’S, 150°30.792’E, coll GoogleMaps . R.B. McCormack, 28 April 2011 ; ACP3628 , male (CL 60.66 mm, OCL 51.60 mm), 34°34.870’S, 150°30.792’E, coll GoogleMaps . R.B. McCormack, 28 April 2011 ; ACP3629 , male (CL 56.61 mm, OCL 47.74 mm), 34°34.870’S, 150°30.792’E, coll GoogleMaps . R.B. McCormack, 28 April 2011 ; ACP3624 , female (CL 40.12 mm, OCL 33.88 mm), 34°34.88’S, 150°30.667’E, coll GoogleMaps . R.B. McCormack, 28 April 2011 ; ACP3625 , male (CL 74.18 mm, OCL 64.71 mm), 34°34.88’S, 150°30.667’E, coll GoogleMaps . R.B. McCormack, 28 April 2011 .
Beecroft Peninsula, Jervis Bay, New South Wales: ACP 6009 , male (CL 85.14 mm. OCL 71.97 mm), 29 m asl (above sea level), Cat Creek , 35°04.730’S, 150°48.179’E, coll GoogleMaps . R.B. McCormack, 2 July 2017 ; ACP 6011 , female (CL 34.92 mm, OCL 27.21 mm), 33 m asl, Cat Creek , 35°04.748’S, 150°48.227’E, coll GoogleMaps . R.B. McCormack, 2 July 2017 ; ACP 6008 , male (CL 43.22 mm, OCL 34.48 mm), 24 m asl, Cat Creek , 35°04.749’S, 150°48.052’E, coll GoogleMaps . R.B. McCormack, 2 July 2017 .
Clyde River Catchment , New South Wales: AM P99431 , female (CL 80.12 mm, OCL 66.09 mm), Clyde River, Buckenbowra , 35°01.05’S, 149°59.232’E, coll. NSW GoogleMaps DPI Fisheries , 17 November 2011 ; ACP 4757 , male (CL 85.84 mm, OCL 70.80 mm), 197 g, 69 m asl, Currowan Creek, Currowan State Forest , 35°20.727’S, 150°04.664’E, coll GoogleMaps . R.B. McCormack , 24 May 2013 ; AM P33995 , 1 female (CL 70.93 mm, OCL 57.36 mm), Boyne Creek on Yadboro Road, west of Ulladulla , 35°23’S, 150°16’E, coll. G.J. Morgan & S.J. Harders, 29 October 1981 GoogleMaps ; AM P99442 , juvenile (CL 21.19 mm, OCL 17.27 mm), Clyde River, Tumble Bar Farm , 35°26.483’S, 150°14.421’E, coll. NSW GoogleMaps DPI Fisheries , 21 November 2011 ; ACP 4941 , female (CL 45.91 mm, OCL 36.3 mm), 25 g, 209 m asl, Bimberamala River , 35°27.940’S, 150°04.830’E, coll GoogleMaps . R.B. McCormack , 8 August 2013 ; ACP 4945 , male (CL 36.08 mm, OCL 27.86 mm), 219 m asl, tributary Bimberamala River , 35°27.940’S, 150°04.670’E, coll GoogleMaps . R.B. McCormack , 8 August 2013 ; AM P34111 , juvenile (CL 20.53 mm, OCL 14.57 mm), Stoney Creek, Yadboro State Forest , 35°28’S, 150°05’E, coll. G.J. Morgan & S.J. Harders, 29 October 1981 GoogleMaps ; ACP 5101 , female (CL 18.54 mm, OCL 13.45 mm), 132 m asl, Bimberamala River, Browns Gully Road , 35°30.313’S, 150°08.894’E, coll GoogleMaps . R.B. McCormack , 9 November 2013 ; AM P15544 , female (CL 96.36 mm, OCL 80.09 mm), creek on eastern side of Clyde River between Brooman and Shallow Crossing, near Batemans Bay , 35°36’S, 150°10’E, coll. J.C. Yaldwyn & F.J Beeman, 20 February 1966 GoogleMaps ; AM P15545 , 1 male (CL 35.96 mm, OCL 27.95 mm), 1 female (CL 50.83 mm, OCL 39.9 mm), Clyde River, Batemans Bay , 35°36’S, 150°10’E, coll. J.C. Yaldwyn & F.J. Beeman, 20 February 1966 GoogleMaps ; AM P99430 , male (CL 26.22 mm, OCL 19.11 mm), Clyde Catchment, Buckenbowra River , 35°37.8’S, 149°59.232’E, coll. NSW GoogleMaps DPI Fisheries , 17 November 2011 ; AM P13052 , female (CL 37.25 mm, OCL 28.52 mm), Cabbage Tree Creek , east foot Clyde Mountain , coll. E.F. Riek, August 1954 ; ACP 4783 , female (CL 31.52 mm, OCL 26.16 mm), 130 m asl, Buckenbowra River, No Name Mountain Road , 35°38.292’S, 149°59.404’E, coll GoogleMaps . R.B. McCormack , 24 May 2013 ; ACP 4784 , female (CL 20.43 mm, OCL 16.78 mm), 139 m asl, Buckenbowra River, No Name Mountain Road , 35°38.292’S, 149°50.404’E, coll GoogleMaps . R.B. McCormack , 24 May 2013 .
Diagnosis. Male cuticle partition absent. Thoracic spines present. Carapace with first postorbital ridge armed in juveniles, usually armed in adults; second postorbital ridges unarmed in adults; 2–4 medium sized and sharp cervical spines in adults; 4–17 large sized and pointed dorsal thoracic spines; general tubercles medium sized and moderately distributed. Antennular basipodite with 2 lobes (usually 1 sharp in juveniles); antennular coxopodite with 1–3 spines. Interantennal spine broad, triangular, margins irregularly tuberculate, apex acute. Scaphocerite widest at midlength. Cheliped lateral propodal spine rows well developed; 4–6 mesial propodal spines; 0 or 1 dorsal apical propodal spine; 1–4 spines above propodal cutting edge; 0 or 1 small blunt tubercle lateral to dactylar base dorsally; 0–6 blunt tubercles lateral to dactylar base ventrally; dorsal apical dactylar spine absent; 0–5 spines above dactylar cutting edge; 1 or 2 apical mesial dactylar spines; 0–2 dorsal mesial dactylar base spines; 0 or 1 marginal mesial dactylar basal spine; dactylar groove distinct; 2 or 3 mesial carpal spines; 1 large ventral carpal spine; 0–3 small ventromesial carpal spines; 2 medium lateral carpal spines.
Description. Cephalothorax. Rostrum reaching midlength or end of antennal article 3 ( Fig. 1A View FIGURE 1 , 2A View FIGURE 2 ). Rostral margins convergent or parallel, rostral bases parallel, carinae long. Rostrum with 2 or 3 sharp marginal spines along distal half; apical spine similar size to first marginal spine, other marginal spines reducing in size posteriorly. Rostrum width 0.12–0.14 OCL.
Anterior cephalothorax moderately spinose; specimens <25 mm OCL with 0–3 small to medium spines or protrusions ventral to postorbital ridges; large specimens> 25 mm OCL with 3–10 large spines or tubercles ( Fig. 2B View FIGURE 2 ). First postorbital ridge generally well-defined, anterior spine sharp in juveniles (<30 mm OCL), blunt to sharp in adults ( Fig. 2A View FIGURE 2 ). Second postorbital ridge spines variable, with sharp anterior spine in juveniles <30 mm OCL, blunt to sharp in adults. Suborbital spine large. Antenna with scaphocerite lateral margin straight to slightly concave distally, unarmed laterally widest proximal to midlength ( Fig. 4A, B View FIGURE 4 ); 1 or 2 basipodite lobes, blunt or sharp (usually 1 sharp in juveniles); coxa with 1–3 medium or sharp spines, outer spine (coxapodite spine) prominent. Epistome pilose, anterior produced to form inter-antennal spine; interantennal spine broad, slightly longer than wide in adults, surface glabrous, margins irregularly tuberculate, apex acute ( Fig. 2A View FIGURE 2 ).
Branchial surfaces of specimens from Batemans Bay and Moss Vale with 8–17 medium to large, prominent dorsal thoracic spines, arranged in 2 irregular rows in specimens> 47 mm OCL) and 2 irregular rows in juveniles (<40 mm OCL), with dorsal row consisting of large spines, central large and ventral row consisting of smaller spines, spines blunter, more rounded in smaller specimens; numerous small spines; general tubercles medium sized, moderately distributed ( Fig. 1 View FIGURE 1 , 2 View FIGURE 2 ). Branchial surface of individuals from Beecroft Peninsula with 4–9 small to medium, dorsal thoracic spines, arranged in 1 or 2 irregular rows in specimens 27–72 mm OCL, with dorsal row consisting of medium spines, ventral row consisting of smaller spines, spines blunter, more rounded in smaller specimens; numerous small spines; general tubercles medium sized, moderately distributed. 2–4 sharp, medium sized, cervical spines (in specimens> 34 mm OCL), dorsalmost largest, sharpest; 0 or 1 small cervical spine in small juveniles (<20 mm OCL). Areola length 0.33–0.39 mm OCL. Areola width 0.15–0.27 mm OCL. Carapace width 0.54–0.65 mm OCL. Carapace depth 0.45–0.57 mm OCL.
Thoracic sternal keel. Pereopod 1 sternal processes with posterior margins oblique, unarmed; ventral edges cristate; processes close to apart, parallel; median keel following pereopod 1 slightly concave. Pereopods 2–4 sternal processes triangular, increasing in size posteriorly, posterior margins oblique, unarmed; processes widely apart, open, anterior margins straight, scoops absent; median keel following pereopods 2 slightly concave, that following pereopod 4 slightly convex ( Fig. 3F View FIGURE 3 ).
Abdomen. Spines developing> 20 mm OCL. Small to medium D spine on somite 1, spine variable from small and sharp to medium and blunt; 1 small to large D spine on somite 2–5, spine varying from rounded to sharp. Large, sharp D-L spine on somite 1; 1 medium to large sharp D-L spine on somites 2–5 ( Fig. 2D View FIGURE 2 ). Some individuals> 70 mm OCL with array of 2–7 small to medium sharp D and D-L spines covering the dorsal surface of somite 5. Somite 6 with array of 6–12 small sharp D and D-L spines covering dorsal surface (specimens> 40 mm OCL). Somite 2 with 2–7 large sharp Li spines (usually 3–5), 0–2 spines in specimens <27 mm OCL. Somites 3–5 with 1 large sharp Li spine. Somite 6 with 0–1 small sharp Li spine. Lii spines generally absent on somite 2 (four specimens with 1–2 spines on one side); somite 3–6 with 1–2 Lii spines (specimens> 40 mm OCL), medium sized, sharp. Dorsal boss absent or weakly developed in specimens <35 mm OCL. Abdomen width 0.45–0.55 mm OCL (male), 0.49–0.59 mm OCL (female).
Tailfan. Spination moderate, standard spines medium to large in size. Telson with 6–14 surface spines (specimens> 27 mm OCL); lateral margin with 1–3 spines, proximal spines present in specimen> 47 mm OCL ( Fig. 2E View FIGURE 2 ). Uropod endopod surface with 1–3 lateral spines (in specimens> 40 mm OCL), 1–5 (usually 3) spines along margin or just above (in specimens> 40 mm OCL), absent in specimens from Moss Vale. Uropodal exopod with 3–5 marginal spines in adults (> 65 mm OCL), absent in specimens from Moss Vale. Telson length 0.22–0.44 OCL.
Maxilliped 3. Laterodistal corner of ischium produced to a distinct point, mesial margin broadly rounded; exopod distal article reaching to about midlength of ischium, flagellum overreaching ischium ( Fig. 3E View FIGURE 3 ).
Pereopod 1 (First Cheliped). Chelae usually elongated 0.35–0.49 mm OCL, with propodal teeth becoming medium to well developed in specimens> 30 mm OCL.
Dactylus ( Fig. 3B–D View FIGURE 3 ) with dorsal apical spine absent; 1–5 small spines or tubercles above dactylar cutting edge. Spines above cutting edge absent on ventral surface; 1 or 2 (usually 2) apical mesial dactylar spines. Basal mesial margin with 0–2 dorsal and 0 or 1 marginal mesial dactylar basal spine. Dactylus length/propodus length: 0.49–0.61 (male), 0.51–0.61 (female).
Propodus ( Fig. 3B–D View FIGURE 3 ) lateral spine row well developed. Dorsal lateral propodal spine row extending from apex to base of propodus. Ventral lateral propodal spine row slightly less developed, occurring up to approximately the length of propodus; 4–6 mesial propodal spines, 0 or 1 small dorsal apical propodal spine on specimens> 66 mm OCL, absent on juvenile specimens; 1–4 small distal spines or tubercles above dorsal cutting edge developing> 36 mm; 1 rounded tubercle lateral to dactylar base dorsally (in specimens> 36 mm OCL); 1–4 tubercles or blunt spines lateral to dactylar base ventrally (1 specimen with 6); 1–3 blunt spines at ventral dactylar articulation. Precarpal spine absent. Angular tubercles posterior to dactylar articulation sometimes present on specimens> 70 mm OCL (typically 1 side only). Propodus length 0.41–0.99 OCL (male), 0.67–0.90 OCL (female). Propodus width/propodus length: 0.35–0.48 OCL (male), 0.37–0.49 (female). Propodus depth/propodus length: 0.22–0.29 OCL (male), 0.22– 0.32 OCL (female).
Carpus ( Fig. 2A View FIGURE 2 , 3B, C View FIGURE 3 ) with 1–3 (usually 2) large mesial spines, distalmost spine distinctly larger and sharper than proximal; 2 short, small–medium sized lateral carpal spines. Ventral carpal spine usually large sharp, group of 0–3 small rounded ventromesial spines. Articular condyle without spine. Carpal groove deep; dorsal spines absent.
Merus ( Fig. 1 View FIGURE 1 , 2A, B View FIGURE 2 ) with 1–10 medium to large dorsal spines; first 2 distal spines medium, most others small on specimens <60 mm OCL, all medium sized> 70 mm OCL. Outer meral spine small to absent.
Pereopods 2–3: chelate, dactylus, propodus, carpus smooth, usually unarmed (some individuals with 0–2 spines); merus extensor margin with 1–6 spines, flexor margin with 1–3 spines; ischium extensor margin with 0–4 spines (usually 2 or 3), flexor margin unarmed.
Pereopod 4: simple. Propodus flexor margin multispinose; anterior surface smooth, posterior surface multispinose. Carpus distal and flexor margins with scattered spines. Merus extensor margins with 3–7 spines; flexor margin with 2–6 spines. Ischium with 2–4 extensor spines.
Pereopod 5: simple. Propodus flexor margin multispinose; posterior surface smooth, anterior surface multispinose. Carpus distal and flexor margins with scattered spines. Merus extensor margins with 1–5 spines; flexor margin with 1–8 spines. Ischium unarmed. Male gonopore without cuticle partition ( Fig. 3F View FIGURE 3 ).
Gastric mill (holotype and paratypes, OCL 73.18–102.67 mm): TAA count 0.5. TAP count 9–11 (usually 9). Spread 8.5–9.5. Secondary zygocardiac ossicle ear absent. Urocardiac ossicle with 10 or 11 ridges.
Colouration. Overall dark brownish-green; underside, thoracic spines dark green; abdominal spines, cheliped propodal spines and general carapace tubercles orange, cheliped fingers deep blue ( Merrick 1993: fig. 25; Fig. 5B, C View FIGURE 5 ).
Size. Male (n = 12) OCL 13.84–73.37 mm; female (n = 13) OCL 13.45–102.67 mm.
Common name. We propose the common name, Clyde Giant Spiny Crayfish.
Distribution. Presently known from southern New South Wales, occurring in the Shoalhaven, and Jervis Bay– Clyde River catchments from Moss Vale south to the vicinity of Clyde Mountain; 24–219 m asl ( Fig. 5A View FIGURE 5 ).
Ectocommensals. Two unidentified temnocephalan species were recorded from E. clydensis : Temnosewellia sp. and Temnohaswellia sp.
Habitat. As in many other giant spiny crayfish, E. clydensis favours large, generally permanent, clear flowing streams with bedrock, loose boulders, cobbles and sandy vegetated substrate, and vegetated margins forming a moderate to dense canopy ( Fig 5D, E View FIGURE 5 ). Adults occupy Type I burrows ( Horwitz & Richardson 1986), with burrows often commencing under large rocks or sunken logs along or close to the stream margins suitable for deep excavation. In the Clyde River drainage, burrow entrances were observed from near the water’s edge to over 1.2 m water depth (as measured under low flow conditions) with largest burrows at 0.6–1.2 m depth. Juveniles usually opportunistically shelter under rocks or logs in shallow excavations in the shallow parts of the main stream bed.
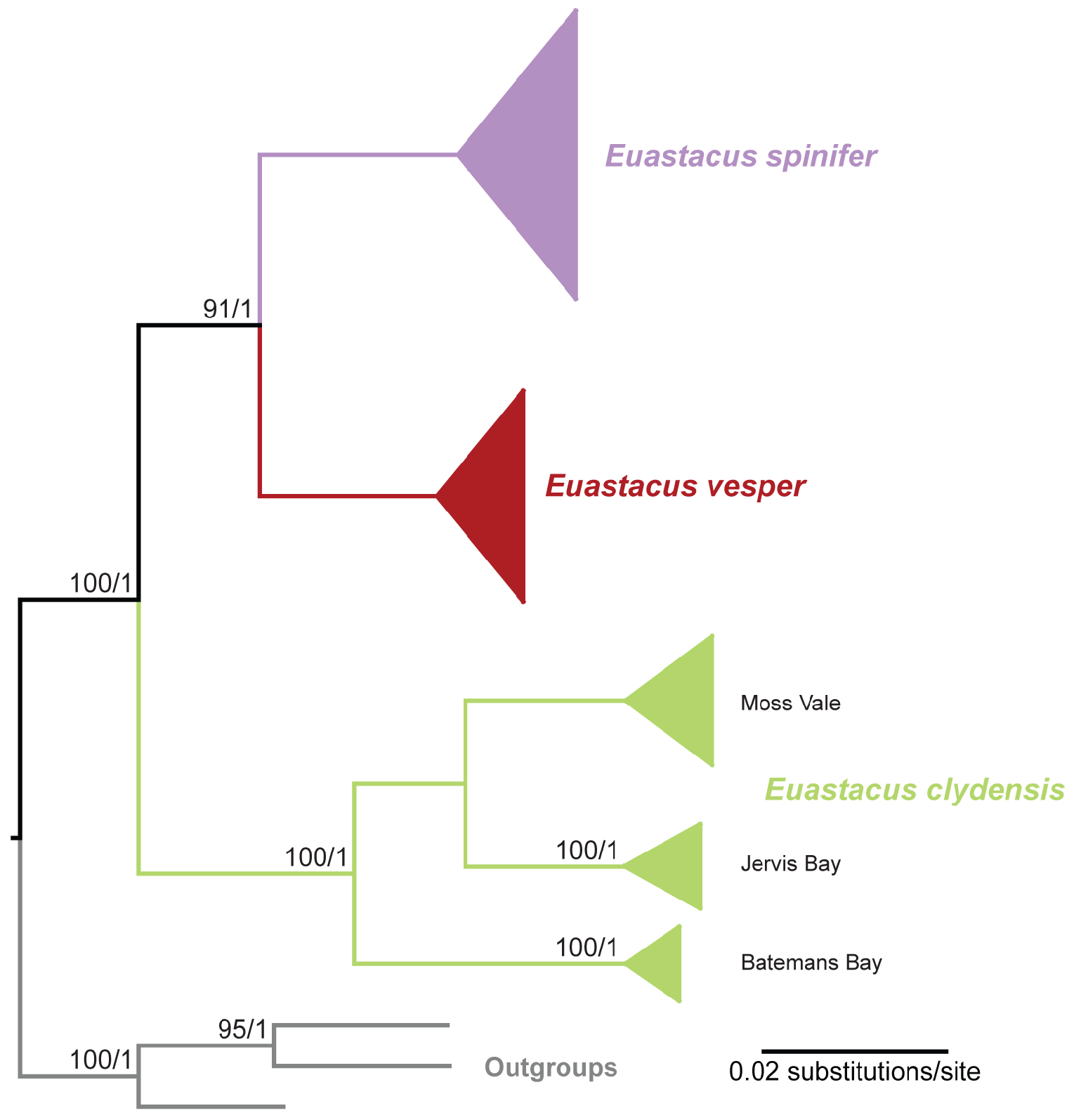
Phylogenetic relationships and pairwise differences. The phylogenetic analysis of mitochondrial COI with additional sampling for E. clydensis supports monophyly of E. clydensis as the sister group of the E. spinifer + E. vesper clade as previously recovered by Van Der Wal et al. (2022) ( Fig. 6 View FIGURE 6 ). The mean pairwise sequence divergence in COI for E. clydensis compared to E. spinifer and E. vesper are 5.44% and 5.67%, respectively. These differences are within the range of interspecific divergence observed between other species of Euastacus (Furse at al. 2013). See Van Der Wal et al. (2022) for detailed evaluation of inter- and intraspecific divergences and population structure within E. spinifer and allies.
Remarks. With formal recognition of E. clydensis as a valid species separate from E. spinifer , the revised distribution of the latter is from the catchments of the upper Nepean and Georges River just south of Sydney (near Cataract; ~ 34°S), northwards through the Sydney Basin and Blue Mountains, and to the vicinity of Kempsey (30– 31°S). Euastacus clydensis ranges from the Southern Highlands in the vicinity of Moss Vale (~ 35°S), south to the vicinity of Batemans Bay (~ 35.6°S) in the southern Shoalhaven and Clyde River drainages. Euastacus clydensis has not yet been recorded from localities west of Jervis Bay between the Clyde River catchment and Moss Vale; it is not clear if the distribution is continuous or disjunct. Other species of Euastacus , such as E. guwinus Morgan, 1997 , and E. yanga Morgan, 1997 , have been sampled in this region, so the apparent absence of E. clydensis is conspicuous, indicating that further sampling is required. The population on Beecroft Peninsula, however, appears to be isolated given the geography of the locality. Euastacus clydensis exhibits strong cheliped and abdominal spination throughout its range ( Figs. 1 View FIGURE 1 , 2 View FIGURE 2 , 3B–D View FIGURE 3 ), although the northernmost specimens (Moss Vale area) lack marginal spines on the uropodal exopod and endopod, present in the specimens from further south. Available specimens from Beecroft Peninsula have fewer dorsal thoracic spines than those from other localites (4–9 vs 8–17), but this may be related to their smaller body size. Additional, larger specimens from the Beecroft Peninsula are required. One specimen from the Beecroft Peninsula (ACP 6009, male, OCL 71.97 mm) has a single mesial carpal spine on each cheliped, rather than the typical two (or occasional three spines) in other specimens. This unusual Beecroft Peninsula specimen also has a medially incised, slightly truncated telson that appears to be the result of a moulting abnormality.
Females of E. clydensis are mature by 70–80 mm OCL, with the smallest ovigerous female recorded from Bimberamala River and Currowan Creek in 2013 at 73.1 mm OCL (specimen released). Here, E. clydensis commences breeding in late May to early June in water temperatures of 10–10.8°C. Hatchlings are released in mid to late December in water temperatures of 18–18.6°C. Eleven ovigerous females (73.1–106.2 mm OCL) captured and re-released in the area in May–August 2013 carried 336– 640 eggs (mean 466).
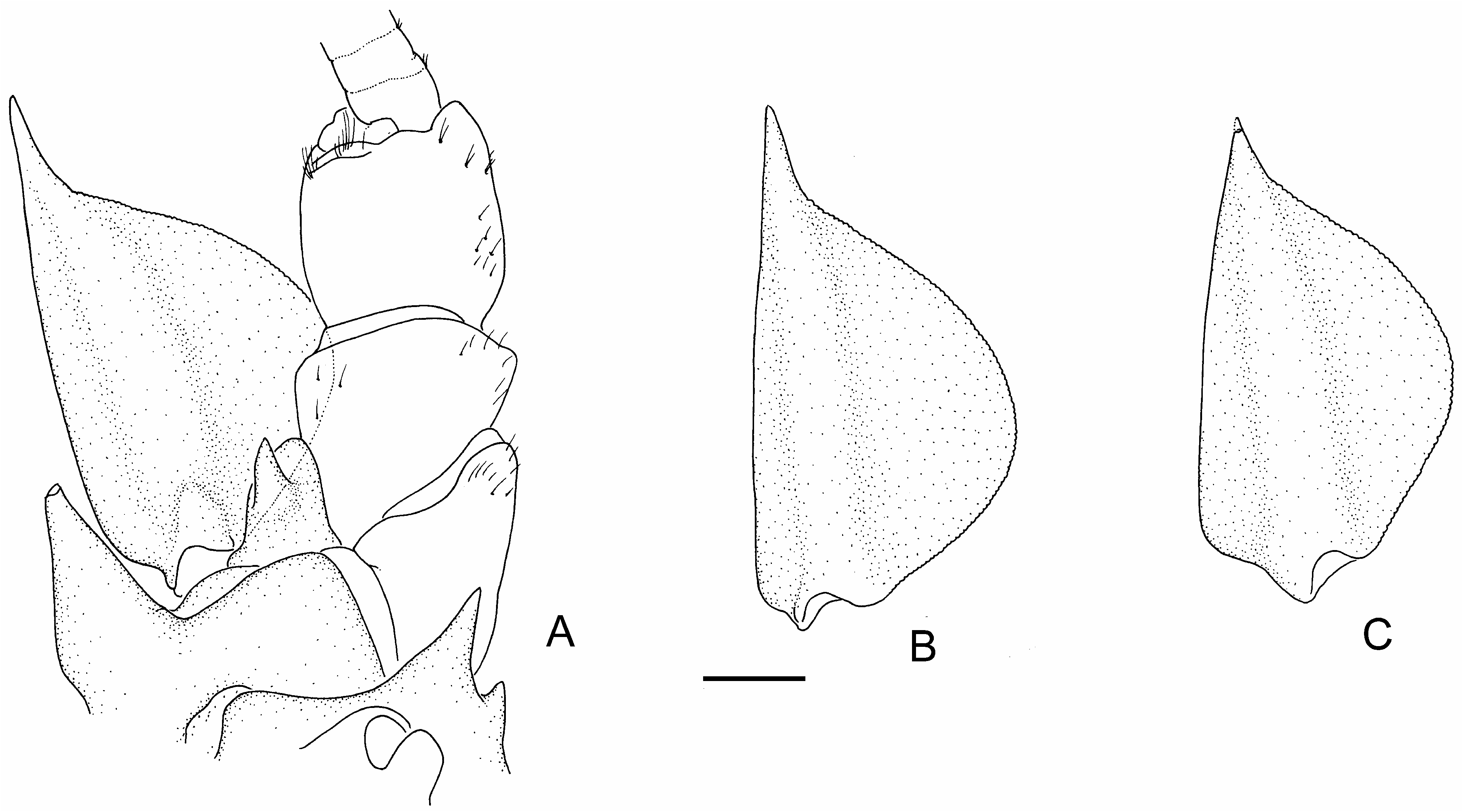
Like E. spinifer , E. clydensis can be readily separated from E. vesper by the presence of two instead of three irregular rows of thoracic spines (though usually blunt) and the presence of anterior basipodite spines (absent in E. vesper ) ( McCormack & Ahyong 2017) ( Fig. 3A View FIGURE 3 ). Morphological distinctions between E. clydensis and E. spinifer , however, are much more subtle. Euastacus clydensis has more pronounced cheliped and abdominal spination than E. spinifer (apart from populations of E. spinifer north of the Sydney Basin) ( Figs. 1 View FIGURE 1 , 2 View FIGURE 2 ). Abdominal spines are well developed in E. clydensis , particularly the D spines ( Fig. 2C, D View FIGURE 2 ), which are sharper than those on most sizematched individuals of E. spinifer . Body spination, however, can be quite variable in E. spinifer between localities, even after allometric effects are considered (i.e., spination generally becomes more pronounced with increasing body size), so the general degree of spination must be used with caution and in association with other features. The primary diagnostic differences between E. clydensis and E. spinifer are in the shape of the antennal scaphocerite and the degree of cheliped dactylar spination. The scaphocerite in specimens exceeding 60 mm OCL is widest at the midlength in E. clydensis , but widest proximal to the midlength in E. spinifer and E. vesper ( Fig. 4 View FIGURE 4 ). Unfortunately, at smaller sizes, the scaphocerite is widest proximally in all three species, so subadults will be difficult to distinguish using this feature. As Morgan (1997) observed in his account of E. spinifer , strong spination of the extensor margin of the cheliped dactylus is a feature of southern populations (= E. clydensis ) and northern populations, with minimal cheliped dactyl spination in intermediate populations (Sydney Basin and the Blue Mountains). Individuals of E. clydensis typically have one dorsal mesial dactylar base spine and one marginal mesial dactylar base spine, both of which are usually absent in E. spinifer . Additionally, the mesial dactylar edge in E. clydensis typically has more spines between the marginal apical dactylar spines and the marginal mesial dactylar base spine than E. spinifer (usually 3–4 in E. clydensis and 0–2 in E. spinifer ). Euastacus clydensis and northern E. spinifer , both of which have similarly spinous cheliped dactyli, will be difficult to distinguish based on cheliped features but can nevertheless be separated based on the antennal scaphocerite. Finally, the uropodal endopod margin of E. clydensis is usually more spinous (1–5 spines, usually 3) than in E. spinifer (usually 0–2 spines).
| AM |
Australian Museum |
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Euastacus clydensis Riek, 1969
| Wal, Cara Van Der, Ahyong, Shane T., Lo, Nathan, Ho, Simon Y. W. & Mccormack, Robert B. 2022 |
Euastacus spinifer
| Morgan, G. J. 1997: 93 |
Euastacus clydensis
| Springthorpe, R. T. & Lowry, J. K. 1994: 82 |
| Merrick, J. R. 1993: 66 |