Oecomys matogrossensis, Saldanha & Rossi, 2021
|
publication ID |
https://doi.org/10.1093/jmammal/gyaa145 |
|
publication LSID |
lsid:zoobank.org:pub:1A4491B9-A4D5-490A-9238-732C2B95473E |
|
persistent identifier |
https://treatment.plazi.org/id/4A62951A-291F-FFB1-BD9C-FE80FDA2FEE5 |
|
treatment provided by |
Felipe |
|
scientific name |
Oecomys matogrossensis |
| status |
sp. nov. |
Oecomys matogrossensis sp. nov.
Figures 5–8 View Fig View Fig View Fig View Fig ; Tables 2 and 3
Oecomys sp. 1 : Di-Nizo et al. 2017:843.
Oecomys catherinae View in CoL western clade: Suárez-Villota et al. 2018:190.
Oecomys aff. catherinae: Saldanha et al. 2019:44 View in CoL .
Oecomys catherinae View in CoL western clade: Brandão et al. 2019:266.
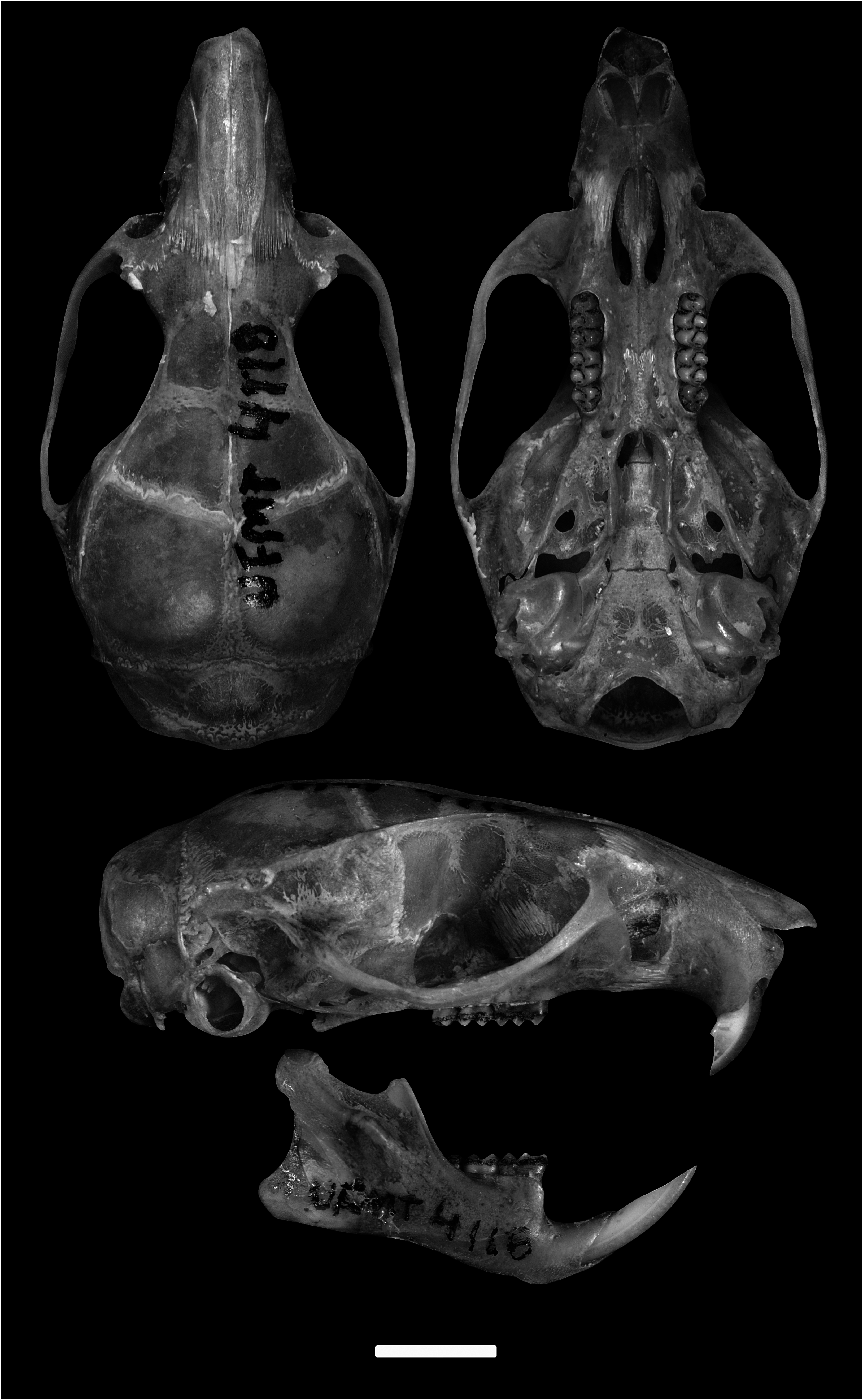
Holotype.— UFMT 4118, adult, male, preserved in dry skin, skull, and complete postcranial skeleton. Collected by Rogério Vieira Rossi (field number RVR 89) on 12 May 2014. Hologenotypes Cytb and i7-fgb are deposited in GenBank (accession number MK874365 View Materials and MT978188 View Materials , respectively).
Measurements (in mm) and body mass of the holotype.— TL = 226, HBL = 106, T = 120, E = 17, HFL = 26, ONL = 28.90, CIL = 25.86, BH = 8.36, CZL = 20.51, ZL = 13.35, LD = 6.71, BZP = 2.96, BN = 3.74, LN = 11.15, LR = 9.08, BR = 4.44, LIB = 5.20, OL = 10.39, ZB = 14.98, LIP = 3.81, BIP = 9.58, BIF = 2.12, LIF = 3.94, BRP = 4.58, LPB = 5.95, MB = 10.32, BOC = 5.99, BB = 4.38, BI = 1.51, BM 1 = 1.32, CLM = 4.77, Bm1 = 1.22, CLLM = 4.97, LLD = 2.89, LCIB = 14.64, MH = 6.67, body mass 33 g.
Paratypes.— UFMT 1773, adult, female, preserved as skin and skull. Collected by T. S. Santos-Júnior on 29 October 2009 (field number UTP 159 ) from the Teles Pires Hydroelectric Power Plant, Paranaíta municipality, Mato Grosso state, coordinates: 9°29′06″S, 56°28′19″W, left bank of the Teles Pires River, tributary of the Tapajós River. Measurements: HBL = 105.82 mm, T = 122.05 mm, E = 15.72 mm, HFL = 25.52 mm, weight 34 g. UFMT 1680, adult, male, preserved in skin and skull. Collected by T. S. Santos-Júnior on 18 April 2009 (field number UTP 02 ) at the Teles Pires Hydroelectric Power Plant , Paranaíta municipality, Mato Grosso state, coordinates: 9°29′10″S, 56°28′19″W, left bank of the Teles Pires River , tributary of the Tapajós River . Measurements: HBL = 125 mm, T = 132 mm, E = 17 mm, HFL = 24 mm, weight 22 g GoogleMaps .
Type locality.— Estância Santa Clara, Alta Floresta municipality, left bank of the Teles Pires River , tributary of the Tapajós River , Mato Grosso state, coordinates: 10°00′25″S, 56°02′17″W ( GPS coordinates taken at the trap site) GoogleMaps .
Additional material.— Twenty specimens from Mato Grosso state, Brazil: skin of MSF 1878 View Materials , female ( 9°46′12″S, 56°10′15.6″W, Alta Floresta); skin and skull of MZUSP 29531 View Materials , female ( 10°10′01″S, 59°27′W, Aripuanã); skins of MSF 1406 View Materials , female; MSF 1407 View Materials , 1411 View Materials , males ( 11°34′58″S, 55°10′01″W, Cáceres); skin and skull of MZUSP 35543 View Materials , male ( 11°34′58″S, 55°10′01″W, Cláudia); skin and skull of MZUSP 29516 View Materials , female ( 13°04′58″S, 53°16′58″W, Gaúcha do Norte); skin and skull of MZUSP 35537 View Materials , female ( 10°19′01″S, 58°28′58″W, Juruena); skin and skull of UFMT 931, female ( 12°51′S, 58°56′W, Juruena); skins and skulls of UFMT 1772, 1774, 1806 GoogleMaps ,
a External measurements were transcribed from original skin labels.
females ( 9°29′06″S, 56°28′19″W, Paranaíta); skins and skulls of AC 207 , 230 , males; AC 255 , 266 , females ( 15°04′45.9″S, 56°33′15.7″W, Rosário Oeste); skins of RFO 013, female; RFO 027, 029, 040, males ( 15°10′33.6″S, 59°59′38.4″W, Vila Bela da Santíssima Trindade ). Four specimens from Pará state, Brazil: skins and skulls of UFMT 1775, 1776, males; UFMT 1686, UFMT 1690, females ( 9°18′40″S, 56°46′05″W, Jacareacanga). One specimen from Rondônia state, Brazil: skin and skull of MPEG 34224 View Materials ( 12°43′S, 60°7′W, Vilhena) GoogleMaps .
Diagnosis.— Soft, long dark reddish-brown dorsal fur (length: 7–11 mm); ventral fur dark cream, composed of gray-based hairs throughout the venter and self-dark cream hairs on areas of the throat, neck, forelimbs, and genitalia; caudal tuft absent, but hairs exceed tail tip (< 1 mm). Rostrum narrow and moderately long; posterior margin of the nasals V- or U-shaped, surpassing or aligned to the posterior portion of the maxillary– frontal suture, and premaxillaries terminating anterior to the nasals; supraorbital crest moderately developed, similar to the temporal crest; parietal slightly expanded below the lateral edges of the dorsum of the braincase; hamular process narrow; subsquamosal fenestra present; alisphenoid strut usually absent; complete stapedial circulation (pattern 1— Voss 1988); and anterior cingulum present on the first upper molar.
Morphological description.— Oecomys matogrossensis is characterized by medium size ( HBL: 85–135 mm), tail longer (TL: 106–160 mm) than combined head and body (i.e., tail from 104% to 148% of HBL). Soft and abundant pelage, with hairs varying from 7 to 11 mm in length. Dorsal hairs gray-based with terminal tips dark reddish-brown, mixed with some gray-based and dark brown-tipped hairs ( Fig. 5A View Fig ). Flanks lighter than the dorsum, with poorly marked lateral/ventral transition. Venter dark cream with gray-based hairs throughout the belly, except on the throat, neck, forelimbs, and genitalia, where the hairs are self-dark cream ( Fig. 5A View Fig ). Hairs shortest and lightest in the rostrum. Mystacial vibrissae abundant and dark brown, with the longest surpassing the posterior edge of the pinnae. Ears light brown at the base and dark brown at the end, with orange and some brown hairs on the outer and inner pinnae surfaces. Manus and pes dorsally covered with white hairs, mixed with brown hairs in the median portion; on the manus, the brown fur covers the arms toward the body. Ungual hairs white, abundant and long, surpassing the claws, except for digit I. Distal end of digit I surpassing the second interdigital pad. Plantar surface with small scales between pads, four developed interdigital pads equal in size and closely positioned, a hypothenar pad smaller than the interdigital pads, and a developed and long thenar pad that extends proximally. Tail completely brown, but slightly bicolor on the proximal portion. Caudal scales with three hairs, with the longest hair exceeding two scale rows in length. Caudal tuft absent, but hairs exceed tail tip about 1 mm in length. Four mammary pairs distributed as follows: one pectoral, one postaxial, one abdominal, and one inguinal (Voss and Carleton 1993).
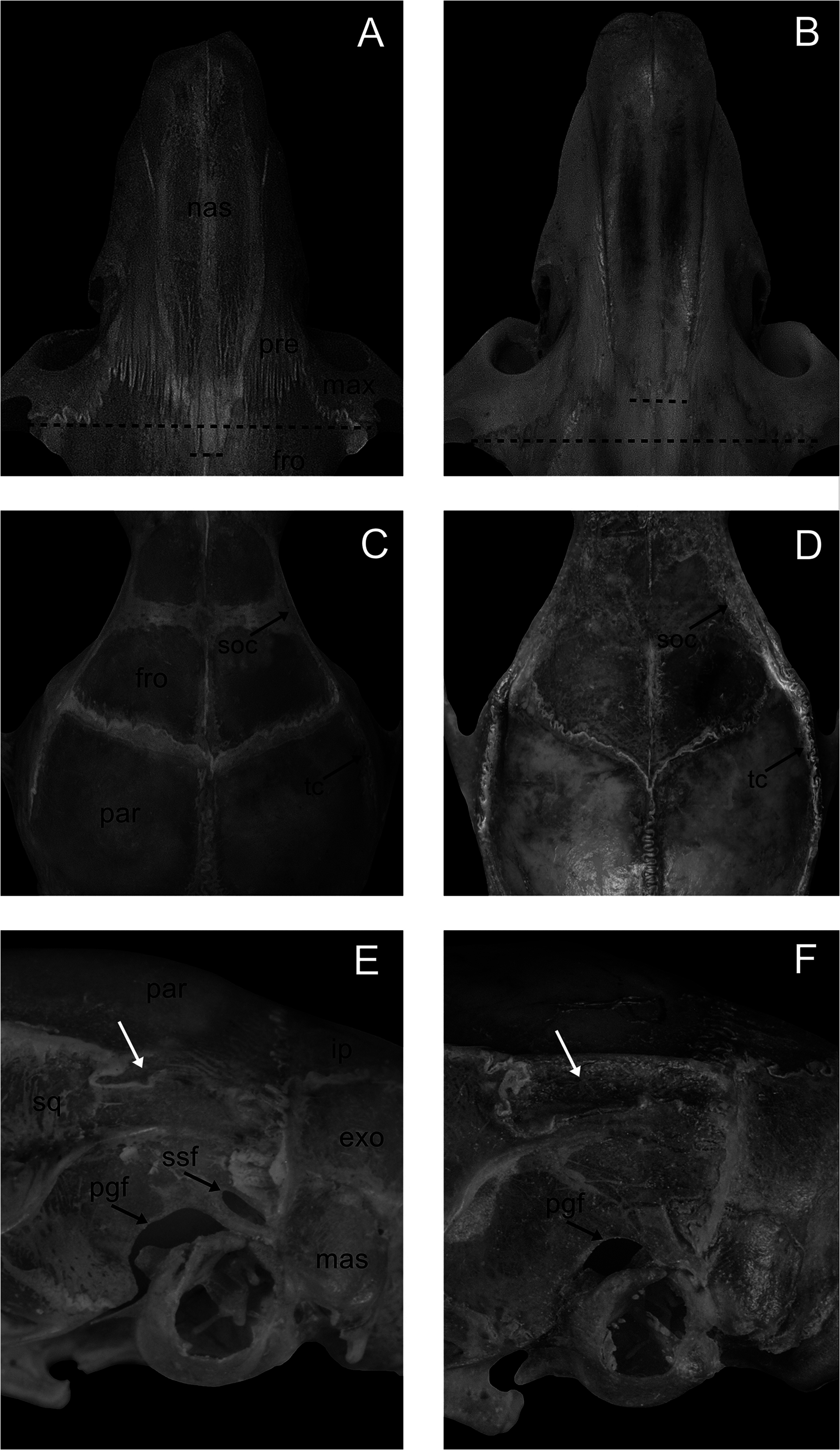
Skull small when compared to the other lineages of the O. catherinae complex s.s. ( Table 2), and medium compared to other congeners. Rostrum narrow and moderately long. Anterior margins of the nasal bones rounded, surpassing the anterior portion of premaxillary bones; posterior margin of nasals V- or U-shaped, aligned or extending beyond the posterior portion of the maxillary–frontal sutures ( Figs. 6 View Fig and 7A View Fig ), and premaxillaries terminating anterior to the nasals. Poorly developed zygomatic notch, but dorsally visible. Lacrimal in contact with maxillary and frontal bones. Interorbital region convergent anteriorly, with narrow median region; supraorbital ridges moderately developed, as well as the temporal ridges ( Figs. 6 View Fig and 7C View Fig ). Rounded cranial vault. Interparietal wider than posterior border of frontals, but not contacting the squamosal. Exoccipital crests absent or poorly developed in dorsal view ( Fig. 6 View Fig ).
Zygomatic plate broad, slightly projected anteriorly to the maxillary root of the zygomatic, and posterior margin positioned anteriorly to M1. Jugals small, their maxillary and squamosal processes not overlapping. Squamosal root of the zygomatic arch expanded laterally. Parietal slightly expanded below the lateral edges of the dorsum of the braincase (greater expansion in UFMT 1680; Fig. 7E View Fig ). Hamular process narrow; subsquamosal fenestra small or medium, never larger than the postglenoid foramen ( Fig. 7E View Fig ). Lambdoidal crests poorly developed. Mastoid with small fenestra that does not contact the edge of the exoccipital, or with a larger fenestra that reaches the exoccipital edge.
Incisive foramina small, drop- or oval-shaped, with posterior margin not reaching the first molars. Palate long and wide (sensu Hershkovitz 1962) with conspicuous posterolateral palatal pits located in deep palatal depressions. Mesopterygoid fossa U-shaped, aligned with the posterior margin of the maxillary; roof of mesopterygoid fossa fully ossified or with small perforations. Parapterygoid fossa dorsally excavated, but not reaching the mesopterygoid level. Alisphenoid strut absent (except in MPEG 34224 and UNEMAT – AC 266). Primitive pattern of stapedial circulation (pattern 1 of Voss 1988), with stapedial foramen and posterior opening of the alisphenoid canal developed, and presence of squamosal-alisphenoid groove and sphenofrontal foramen. Tegmen tympani not contacting the squamosal, or contacting the squamosal punctually. Small ectotympanic bulla; periotic slightly exposed and usually reaching the carotid canal. Eustachian tube narrow and short, with edge parallel to the basioccipital suture ( Fig. 6 View Fig ).
Mandible robust, with mental foramina dorsally located in the diastema, anterior to m1. Upper and lower masseteric ridges converge and fuse only in the anterior portion, below the anterior portion of m1. Capsular process lower incisor small and conspicuous. Coronoid process small and pointed. Angular process short. Condyloid process elongated ( Fig. 6 View Fig ).
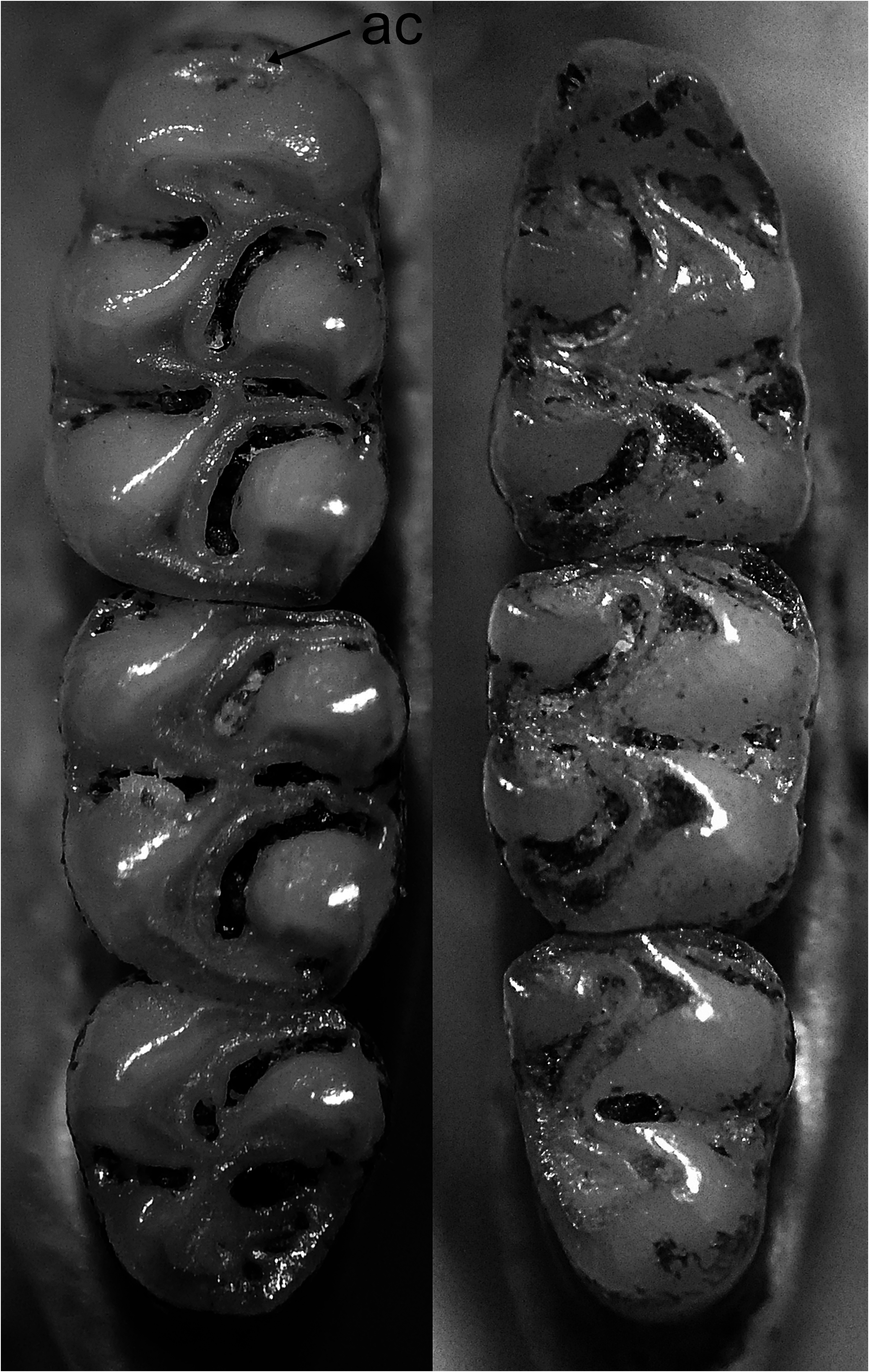
Upper incisors opisthodont ungrooved, with yellow-orange anterior enamel surfaces; upper molar rows parallel arranged in opposite labial-lingual pairs. Molars are pentalophodont and brachyodont. Upper first molar (M1): anterior cingulum (i.e., stylar shelf on the anterior border of the procingulum, positioned anteriorly to the anterocone) present (refer to Pires et al. 2016 for description and comments on this structure); procingulum with a central fossete; anterocone not divided by anteromedian flexus (anteromedian flexus present in UFMT 1773, dividing the procingulum into anterolabial and anterolingual conules); anteroloph present and connected to the anterocone and paracone by parastyle; paracone connected to protocone by median mure, with long paraflex; protocone connected to the anterior mure and separated from the anterocone by protoflexus; paralophule present in paracone and connected to the mesoloph; posteroloph fused to metacone, with presence of fossetus derived from the posteroflexus; labial and lingual accessory roots absent; labial and lingual cingula absent ( Fig. 8 View Fig ). Upper second molar (M2): anteroloph present; anterolingual cingulum present, but poorly developed in the anterior portion of the protocone; lingual cingulum present; paralophule connecting paracone to mesoloph; posteroloph present ( Fig. 8 View Fig ). Upper third molar (M3): smaller than the half of M1 and larger than half of M2; mesoloph and short posteroloph present; hypocone slightly reduced with respect to protocone and separated from the protocone by hypoflexus; reduced metacone ( Fig. 8 View Fig ).
Lower first molar (m1): anteromedian flexid present, dividing procingulum into anterolabial and anterolingual conulids; procingulum with anteromedian fossetid well-developed. Anterolabial cingulum connected to a well-developed protostylid; ectolophid present; metaconid with metalophulid connected to mesolophid that is fused in its distal portion to the entoconid; posterolophid long and fused by wear to the entoconid; hypolophulid present ( Fig. 8 View Fig ). Lower second molar (m2): anterolabial cingulum present and well-developed; entoconid small; small ectolophid and ectostylid present; posterolophid connected to the entoconid ( Fig. 8 View Fig ). Lower third molar (m3): smaller than half of m1 and same length as m2; anterolabial cingulum present; small ectolophid and ectostylid present; posterolophid probably present and connected to the entoconid, that is reduced ( Fig. 8 View Fig ).
Karyotype.— Specimens of the western clade exhibited 2n = 54, FN = 54, with a small submetacentric pair (pair 1) and all the other autosomes acrocentric (pairs 2–26). The X chromosome is large and submetacentric, with a short heterochromatic arm, and the Y chromosome is medium sized, metacentric and entirely heterochromatic (see Suárez-Villota et al. 2018).
Etymology.— The specific name is a geographical reference to the Brazilian state of Mato Grosso, where the holotype was collected and where most of the species distribution is located.
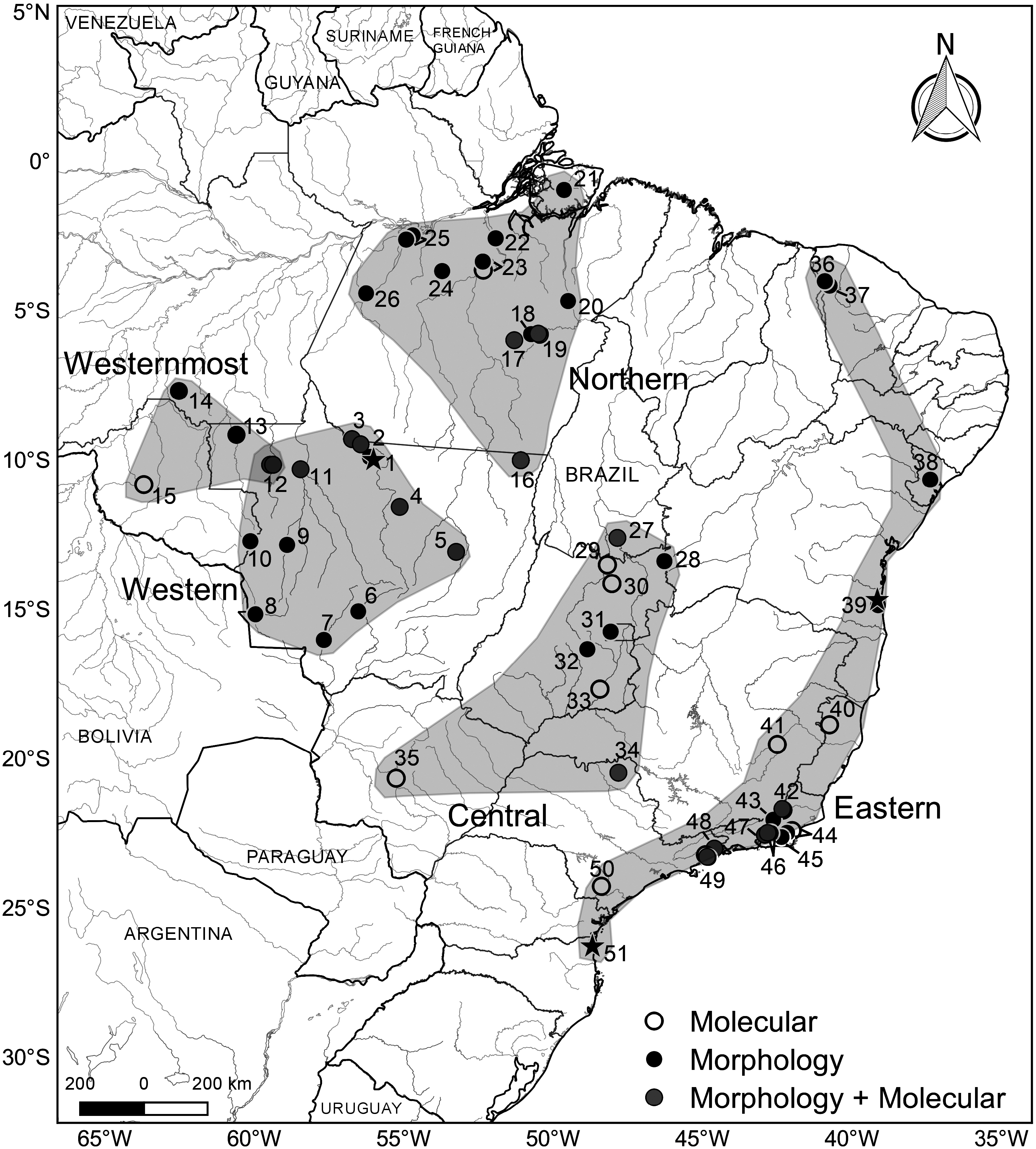
Distribution and sympatry.— Oecomys matogrossensis occurs in southern Amazonia; the species’ distributional area extends from Jacareacanga (on the right bank of the Teles Pires River in the extreme southwest of Pará state) southward to Cáceres (in the extreme southwest of Mato Grosso state),
and the premaxillaries aligned to the posterior margin of the nasals in O. catherinae ( BNMH 9.11.19.24); (C) supraorbital and temporal crests poorly developed in O. matogrossensis ; (D) supraorbital crest dorsolaterally expanded in O. catherinae northern lineage ( MPEG 39899), being more developed than temporal crest; (E) subsquamosal fenestra present, always smaller than postglenoid foramen, and parietal slightly expanded below the lateral edges of the dorsum of the braincase (white arrow) in O. matogrossensis ; (F) subsquamosal fenestra ossified and parietal deeply expanded onto the lateral surface of the braincase (white arrow) in O. catherinae northern lineage ( MPEG 39899). exo, exoccipital; fro, frontal; ip, interparietal; mas, mastoid; max, maxillary; nas, nasal; par, parietal; pgf, postglenoid foramen; pre, premaxillary; soc, supraorbital crest; sq, squamosal; ssf, subsquamosal fenestra; tc, temporal crest.
and from Vilhena ( Rondônia state) and Aripuanã (between the Juruena and Aripuanã rivers, in Mato Grosso state) eastward to Gaúcha do Norte (on the left bank of the Xingu River, Mato Grosso state). The species occurs in sympatry with O. cleberi and the westernmost lineage of O. catherinae complex s.s. on the west margin of the Juruena River, Mato Grosso state; and with O. bicolor , O. paricola , and O. roberti , in the municipality of Cláudia, Mato Grosso state ( Suárez-Villota et al. 2018; Saldanha et al. 2019).
Habitat information.— Oecomys matogrossensis was collected in forested habitats from the southern Amazonia, particularly from the Amazonia–Cerrado transition zones, and the edge of the Pantanal biome. Also, two collecting points are located in the Cerrado biome (Localities 6 and 9; Fig. 1 View Fig ), but habitat information was not available on the vouchers’ labels.
Comparisons.— Oecomys matogrossensis is distinguished from O. catherinae complex s.s. lineages by exhibiting smaller body size ( Tables 2 and 3; Fig. 5 View Fig ) and short dorsal pelage ( 7–11 mm) with dark reddish-brown coloration, versus long dorsal pelage ( 9–15 mm) with reddish-brown color. In addition, the new species has a darker cream ventral pelage when compared to the central and eastern lineages ( Fig. 5 View Fig ; Table 3).
The skull is smaller in O. matogrossensis than in O. catherinae complex s.s. lineages ( Table 2). The rostrum is narrow and moderately long in the new species, but is wide and long in eastern and westernmost lineages of O. catherinae complex s.s., wide and short in the central lineage, and narrow and short in northern lineage ( Table 3). The posterior margin of the nasals extends beyond the posterior portion of the maxillary–frontal suture in O. matogrossensis ( Fig. 7A View Fig ), contrasting with the O. catherinae complex s.s. lineages, whose posterior margin of the nasals does not reach or is aligned to the posterior part of the maxillary–frontal suture ( Fig. 7B View Fig ). In O. matogrossensis , the interorbital region is narrower compared to the eastern, westernmost, and northern lineages of O. catherinae complex s.s. Moreover, the supraorbital and temporal crests are moderately developed in the new species ( Fig. 7C View Fig ), whereas the supraorbital crest is dorsolaterally and distinctly more developed than the temporal crest in the O. catherinae complex s.s. lineages ( Fig. 7D View Fig ). The subsquamosal fenestra always is present in O. matogrossensis ( Fig. 7E View Fig ), while it is reduced in the eastern and westernmost lineages of O. catherinae complex s.s., and fully ossified in the central and northern lineages ( Fig. 7F View Fig ). The parietal is slightly expanded below the lateral edges of the dorsum of the braincase in O. matogrossensis ( Fig. 7E View Fig ), while it is deeply expanded onto the lateral surface of the braincase in the O. catherinae complex s.s. lineages ( Fig. 7F View Fig ).
Because O. catherinae is similar externally to O. rex in pelage color and texture, we provide comparisons of O. matogrossensis with the latter species. Externally, the new species differs from O. rex by exhibiting shorter dorsal fur ( 7–11 mm versus 9–14 mm in O. rex ) and lacking a caudal tuft (versus a small tuft present in O. rex ). Cranially, the skull is smaller in O. matogrossensis (mean 28.9 mm) than in O. rex (mean 31.05 ± 3.10 mm; n = 8); the supraorbital and temporal crests are moderately developed in the former species (versus strongly developed supraorbital crests, joining in a prominent temporal crest in O. rex ); and the subsquamosal fenestra always is open in O. matogrossensis , while it is reduced or absent in O. rex .
Oecomys matogrossensis can be distinguished from other congeneric sympatric species, such as O. bicolor View in CoL , O. cleberi View in CoL , O. roberti View in CoL , and O. paricola View in CoL , by exhibiting medium body and cranial size (versus smaller body and cranial size in O. bicolor View in CoL and O. cleberi View in CoL ); dark reddish-brown dorsal coloration (versus orangish-brown in O. bicolor View in CoL and O. cleberi View in CoL ; reddish- to orangish-brown in O. roberti View in CoL ; and reddish-brown in O. paricola View in CoL ); dark cream ventral coloration (versus totally white or cream venter in O. bicolor View in CoL and O. cleberi View in CoL ; fully white or a white ventral midline with gray-based lateral bands in O. roberti View in CoL ); and absence of caudal tuft (versus presence of caudal tuft in O. bicolor View in CoL , O. cleberi View in CoL , and O. paricola View in CoL ). Detailed descriptions of the mentioned species can be found in Rocha et al. (2012), Carleton and Musser (2015), and Rocha et al. (2018).
| T |
Tavera, Department of Geology and Geophysics |
| CZL |
Centro de Zoologia |
| BR |
Embrapa Agrobiology Diazothrophic Microbial Culture Collection |
| MB |
Universidade de Lisboa, Museu Bocage |
| BOC |
Bingham Oceanographic Collection |
| BB |
Buffalo Bill Museum |
| BM |
Bristol Museum |
| MH |
Naturhistorisches Museum, Basel |
| MPEG |
Museu Paraense Emilio Goeldi |
| AC |
Amherst College, Beneski Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Oecomys matogrossensis
| Saldanha, Juliane & Rossi, Rogério Vieira 2021 |
Oecomys aff. catherinae :
| SALDANHA, J. & D. C. FERREIRA & V. F. DA SILVA & M. SANTOS-FILHO & A. C. MENDES-OLIVEIRA & R. V. ROSSI 2019: 44 |
Oecomys catherinae
| BRANDAO, M. V. 2019: 266 |
Oecomys catherinae
| SUAREZ-VILLOTA, E. Y. & A. P. CARMIGNOTTO & M. V. BRANDAO & A. R. PERCEQUILLO & M. J. J. SILVA 2018: 190 |
Oecomys sp. 1
| DI-NIZO, C. B. & K. R. D. S. BANCI & Y. SATO-KUWABARA & M. J. J. SILVA 2017: 843 |