Liphistius batuensis Abraham, 1923
|
publication ID |
https://doi.org/10.5281/zenodo.893555 |
|
DOI |
https://doi.org/10.5281/zenodo.6042382 |
|
persistent identifier |
https://treatment.plazi.org/id/4C30A452-FFC7-FFD1-B9CA-FC603BB0FE3A |
|
treatment provided by |
Plazi |
|
scientific name |
Liphistius batuensis Abraham, 1923 |
| status |
|
Liphistius batuensis Abraham, 1923 View in CoL
Figs 19-20 View Fig. 19 View Fig. 20
Liphistius batuensis Abraham, 1923a: 15 View in CoL -21, pl. 1, figs 1-9 (description of male and female). Synonymy and taxonomic literature, see World Spider Catalog (2017).
Types: BMNH; male lectotype (designated by Haupt, 1983: 282) and 11 female plus 4 juvenile paralectotypes (all not examined); Malaysia, Selangor, Batu Caves ; XII.1921 - I.1922; leg. H.C. Abraham.
Material examined: MHNG; 1 male; Selangor, Kuala Lumpur, Batu Caves; 24.VII.1969; leg. R. Pilet. – MHNG; 2 females ; Batu Caves, près de Kuala Lumpur;
XI.1976; leg. B. Koepchen. – MHNG, sample SIM- 01/13; 2 males (matured 18.X.2001, 8.II.2002), 2 females (moulted 13.I.2002; 24.I.2002), exuviae of 3rd female (spider not collected); Selangor, Batu Caves, Dark Cave, Caverns B, C, D ( 3°14’12.7’’N, 101°41’00.0’’E), 100 m; 12.VII.2001; leg. P.J. Schwendinger. – SMF-13907, n° 3; 2 females, 1 juv. male; Selangor, Batu Caves ; leg. Clark, det. Roewer, 1962 (identified as 3 females). – SMF-13908, n° 4; macerated remains of 2 specimens (one a juvenile male, the other without opisthosoma); Selangor, Batu Caves ; leg. Clark, det. Roewer, 1962 GoogleMaps . – SMF-21945/1; 1 female; no locality data; 19.XI.1933; det. W. S. Bristowe.
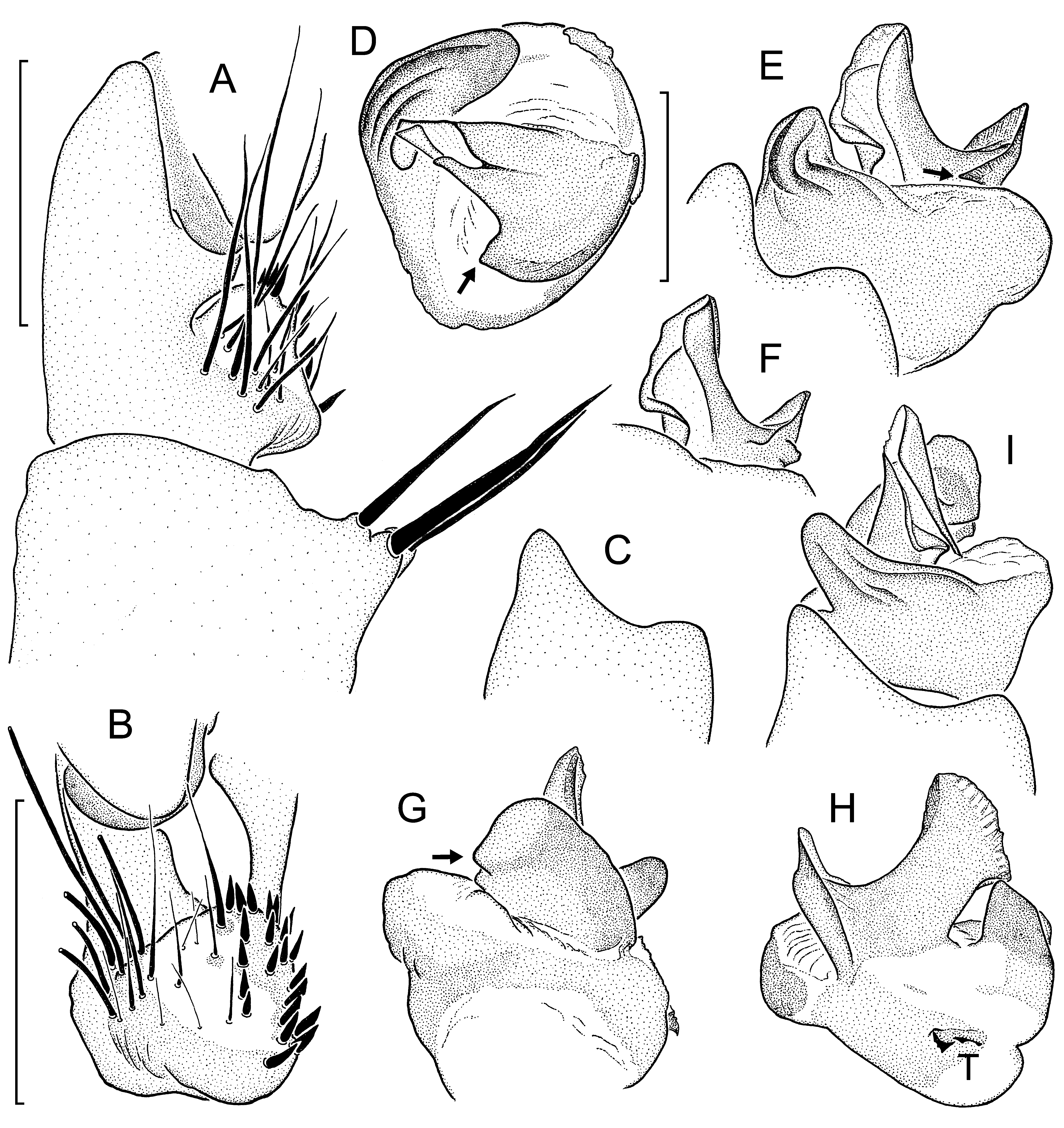
Diagnosis: Medium-sized, light brown-coloured species in both sexes. Males characterized by cumulus carrying a group of very long, thick bristles ( Fig. 19 View Fig. 19 A- B); tegulum with exceptionally small and narrow proximal edge carrying few denticles ( Fig. 19H View Fig. 19 ); distal edge of contrategulum with 2-4 quite long parallel ridges pointing towards dorsal apex, without denticles ( Fig. 19 View Fig. 19 D-E, I); para-embolic plate distinctly elevated ( Fig. 19 View Fig. 19 E-I), with sharp proventral angle at base ( Fig. 19 View Fig. 19 D-E, G; see arrows); apex of embolus proper wide in prolateral and retrolateral view ( Fig. 19 View Fig. 19 E-F, H), its dorsal wall much wider than its ventral wall, end of dorsal wall sharply bent proventrad ( Fig. 19 View Fig. 19 D-F, I). Females characterized by poreplate largely unpigmented in its posterior portion, more or less distinctly separated from short, wide posterior stalk; posterior portion of genital atrium not markedly bent ventrad; no anterolateral lobes on poreplate; CDO very wide, its anterior margin sunken and clearly outlined, its posterior margin at level of poreplate and not clearly outlined; genital atrium in most cases without hairs, its lateral folds weakly developed ( Fig. 20 View Fig. 20 ).
Additions to description of male: Scopula: Tarsus I with thin scopula in distal half of ventral side, divided for its entire length by a narrow pale, glabrous longitudinal median stripe and by some bristles; tarsus II likewise, scopula only distally divided; tarsus III with thin scopula in distal three-fifths, only distally divided; tarsus IV with thin scopula (narrower than on other tarsi) in distal half, only distally divided.
Palp: Tibial apophysis basally wide in ventral view, moderately long, slightly set back from distal margin of tibia ( Fig. 19A View Fig. 19 ), its apical megaspines relatively long and thin (but not as thin as illustrated in Murphy & Platnick, 1981: figs 9, 12, 15, 21 and in Platnick & Sedgwick, 1984: figs 70-72, 74), dorsal ones longer than ventral ones. Prodorsal-apical lobe of cymbium distinctly longer and more pointed than proventral-apical one ( Fig. 19C View Fig. 19 ). Paracymbium of average size and depth ( Fig. 19 View Fig. 19 A-B), carrying very long (longest ones reaching base of embolus complex), thick bristles in a loose group on non-elevated cumulus ( Fig. 19A View Fig. 19 ). Subtegulum without apophysis. Tegulum with very small proximal edge carrying only few denticles ( Fig. 19H View Fig. 19 ). Pigmented bridge between tegulum and contrategulum on retrolateral side of palpal organ disconnected ( Fig. 19H View Fig. 19 ). Contrategulum with indistinct, short and wide ventral process; distal edge with 2-4 parallel ridges pointing towards tongue-shaped dorsal apex ( Fig. 19 View Fig. 19 D-E, I). Para-embolic plate distinctly elevated ( Fig. 19 View Fig. 19 E-I), with sharp proventral angle at its base ( Fig. 19 View Fig. 19 D-G, see arrows). Embolus proper with wide apex ( Fig. 19 View Fig. 19 E-F, H); dorsal wall of sclerotised part much wider than ventral wall, end of former sharply bent ventrad and partly overlapping membranous part of embolus proper ( Fig. 19 View Fig. 19 D-F, I); sclerotised part of embolus proper retrolaterally with a single longitudinal distal keel ( Fig. 19 View Fig. 19 D-F, G); membranous part of embolus proper unpigmented throughout.
Additions to description of female: Posterior margin of genital sternite straight or indistinctly invaginated ( Fig. 20 View Fig. 20 ). Vulval plate ( Fig. 20 View Fig. 20 ) short, wide; posterior portion and lateral margins of poreplate largely unpigmented, more or less distinctly separated from short, wide, well-pigmented posterior stalk; posterior part of genital atrium only slightly and gradually curved ventrad; anterior margin of poreplate slightly and widely invaginated, without anterolateral lobes; CDO very wide, its anterior margin sunken and clearly outlined, its posterior margin level with poreplate and not clearly outlined ( Fig. 20A View Fig. 20 , C-D, F-G); receptacular cluster very large, strongly protruding anteroventrad, reaching beyond anterior margin of poreplate; genital atrium without lateral hairs and in most cases without median hairs, its lateral folds indistinctly developed ( Fig. 20B, E, H View Fig. 20 ).
Remarks: The apical megaspines on the tibial apophysis of the male palp are not as thin as illustrated by Platnick and Sedgwick, and the one situated most dorsally is not as long as shown by these authors ( Platnick & Sedgwick, 1984: figs 70-72, 74).
It is not clear what Platnick & Sedgwick (1984: 25) mean with their diagnostic character “short, erect tegular apophysis”.
Variation: Carapace lengths in males (n=3) 5.24-5.99, in females with fully developed vulval plate (n=7) 4.76-6.67; carapace widths 4.60-5.56 and 4.09-5.56, respectively. The scopula on tarsus I is divided for its entire length in two males, only distally so in the third; the scopula on tarsus IV extends over the distal half in two males, over the distal three-fifths in the third. One mature male retains a proventral “tibial spur” ( sensu Platnick & Goloboff, 1985) on its right leg I. Variation in the shape of the vulval plate of females examined is shown in Fig. 20 View Fig. 20 (see also Murphy & Platnick, 1981: figs 26-27 and Platnick & Sedgwick, 1984: figs 75-76). One female has a single median hair in its genital atrium ( Fig. 20D View Fig. 20 ); in all other females examined the genital atrium is completely devoid of hairs ( Fig. 20 View Fig. 20 A-C, E-H). Most specimens examined have fully developed anterior median eyes; only one small female has a reduced (but cornea present) AME on one side.
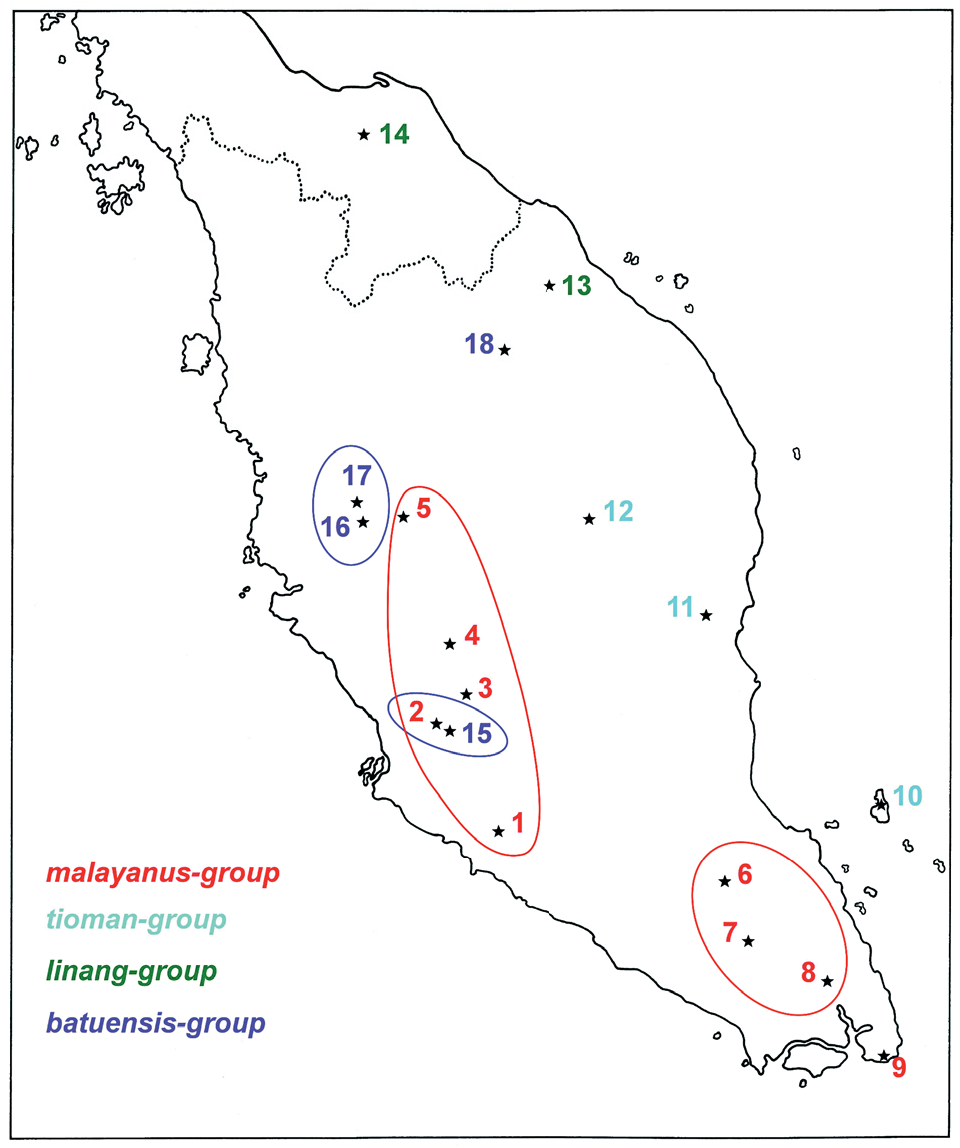
Distribution: First reported from the Batu Caves by Ridley (1899: 580), this specis is only known from the Dark Cave and the Rift Cave which are part of the Batu Cave system ( Lim & Yussof, 2009: 127-128; Fig. 1 View Fig. 1 , locality 15) north of Kuala Lumpur, and from the Anak Takun Cave in the Templer Park which lies about 9 km further north ( Fig. 1 View Fig. 1 , locality 2). The latter record is based only on females and juveniles ( Platnick & Sedgwick, 1984: 26). The Gua Anak Takun population was reported to contain hundreds of burrows in 1961 ( McClure et al., 1967), only one in 1986 ( Yussof, 1987), three in 2006 ( Lim & Yussof, 2009). Steiner (1998: 148) reported that the Gua Anak Takun population had probably become extinct, which fortunately proved to be incorrect. Neverthess the future of this population, which no longer receives legal protection, is bleak ( Lim & Yussof, 2009: 131). Fortunately the Batu Cave populations are doing much better ( Lim & Yussof, 2009: 125-126).
| MHNG |
Museum d'Histoire Naturelle |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Liphistius batuensis Abraham, 1923
| Peter J. Schwendinger 2017 |
Liphistius batuensis
| Abraham H. C. 1923: 15 |