Myotis horsfieldii (Temminck, 1840)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6397752 |
|
DOI |
https://doi.org/10.5281/zenodo.6577946 |
|
persistent identifier |
https://treatment.plazi.org/id/4C3D87E8-FF36-6A8A-FA79-9C2F1687BA9D |
|
treatment provided by |
Conny |
|
scientific name |
Myotis horsfieldii |
| status |
|
468. View Plate 73: Vespertilionidae
Horsfield’s Myotis
Myotis horsfieldii View in CoL
French: Murin de Horsfield / German: Horsfield-LangfuRfledermaus / Spanish: Ratonero de Horsfield
Other common names: Common Asiatic Myotis, Horsfield's Bat, Lesser Large-tooth Bat
Taxonomy. Vespertilio horsfieldii Temminck, 1840 View in CoL ,
Mount Gede, Java, Indonesia .
Subgenus Myotis ; horsfieldii species group. See M. ridleyi . Myotis horsfieldit was previously included under M. adversus and later found closely related to M. macrotarsus and M. hasseltii , with M. browni in a more basal position. Myotis horsfieldii represents a species complex in need of deep revision. Five subspecies recognized.
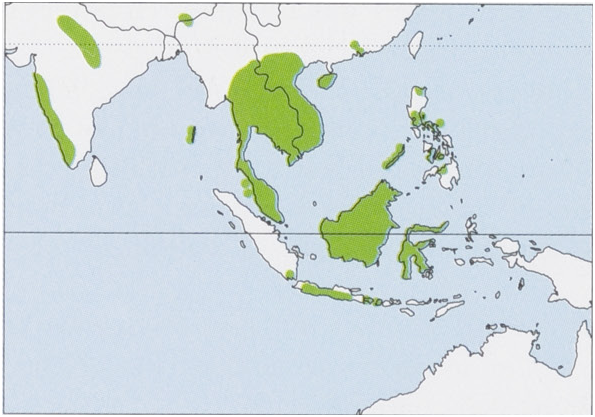
Subspecies and Distribution.
M.h.deignaniShamel,1942—SEChina(Guangdong,Hongkong,andHainan)andmainlandSEAsia.
M.h.dryasK.Andersen,1907—AndamanandNicobarIs.
M.h.jeanne:E.H.Taylor,1934—Philippines.
M. h. peshwa Thomas, 1915 — C, W & NE India (Madhya Pradesh, Assam, Meghalaya, Maharashtra, Goa, Karnataka, Kerala, and Tamil Nadu). View Figure
Descriptive notes. Head-body 44-51 mm,tail 33-43 mm, ear 13-5—- 17 mm, hindfoot 7-11 mm, forearm 33-8-41- 5 mm; weight 5-7- 5 g. Specimens from India (peshwa) are slightly larger (forearm 36-5—41- 5 mm) and have browner pelage. Subspecies dryas from the Andaman Islands is on average smaller (forearm 34-8-37- 1 mm). Fur is soft and dense,feet large, and ears long. Upperparts are dark brown to almost black, with dark brown hair tips that can be grizzled with gray in some individuals. Underparts are gray to dark gray, with pale gray or buffy hair tips and very dark roots. Fur surrounding anal region is nearly white. Ears are dark brown to black and relatively tall compared with size of head and have smoothly convex anterior borders,slightly concave posterior borders, and bluntly pointed tips. Tragus is narrowly pointed and is less than one-half the height of pinna. Feet are moderately large and exceed one-half the length of tibia. Claws are small. Interfemoral and wing membranes are chocolate-brown. Wing membrane attaches to metatarsus below ankle. Subspecies deignani has tricolored hairs: broad band of dark basal hairs, followed by pale buff band, and then narrow dark brown band, tipped with flaxen. Mingling of these colors gives it a grizzled appearance compared with individuals from Java, which does not have a tricolored appearance and is larger in external and cranial measurements. Baculum is small and saddle-shaped, with rounded tip (specimen from India). Of two penial bones from Vietnam, one (probably immature specimen) was similar to that described from India. The second was larger, c. 0-72 mm, with almost parallel sides, abruptly narrowing to blunt tip; its upper side had abrupt tooth-like elevation on its basal onequarter. Skull is medium-sized, with moderately broad (7-2-7- 7 mm) braincase. Rostrum is robust, with shallow depression in its midline. Sagittal crest is absent or very weak. Supraocciptal is bulbous. Zygomata are well developed and outwardly flared. C! is nearly twice the height of P*. P? is ¢.50% of crown area of P?, usually lies in tooth row oris slightly displaced internally, and is in close contact with P*. Upper molars are typically myotodont. P, is ¢.50% the height of the canine, which is higher than P. P,, which occasionally can be displaced internally, is sometimes in close contact with P,. Condylo-basal lengths are 14-3-14- 8 mm; maxillary tooth row lengths are 5-8- 6 mm. Chromosomal complement has 2n = 44 and FN = 52 ( Thailand, Peninsular Malaysia, and Sabah in Borneo) and 2n = 44 and FN = 50 (Hainan, China). This difference in numbers of chromosomal arms was due to use of different criteria for classification of chromosome types.
Habitat. Wide variety of habitats including wooded areas with local supply of freshwater, mixed and hill forests, and tea estates ( India); lowland forests and agricultural areas, adjacent to limestone karst ( Myanmar); dry dipterocarp and dry evergreen forests ( Thailand); lowland forests,riverine forests, disturbed forests, and open land near villages ( Vietnam); lowland and hill forest ( Malaysia); lowland forests, close to large rivers and streams (Borneo and south-western Sumatra); secondary and primary lowland, montane, and mossy forests and agricultural areas ( Philippines); and secondary forests (Sulawesi) from sea level up to elevations of ¢. 1450 m.
Food and Feeding. Horsfield’s Myotis typically flies in circles c. 10 cm above water, similar to European Daubenton’s Myotis ( M. daubentonii ). It was observed foraging near forest streams, skimming insects from water surface in some instances. On Da Lat plateau, Vietnam, it foraged in the air, similar to the Nepalese Whiskered Myotis ( M. muricola ).
Breeding. In India (Madhya Pradesh), pregnant Horsfield’s Myotis were caught in February-March. In Malaysia, pregnant females were recorded in January-June and October; a few females had twin fetuses.
Activity patterns. Horsfield’s Myotis is nocturnal. On Pulau Langkawi, Malaysia, it has been found roosting in limestone caves. In India, it often roosts in culverts, tunnels, and houses and under bridges. In Madhya Pradesh, it was found in leaves of a palmyra palm. On the Philippines, it roosted beneath a large rock over a stream. It might depend on caves near water sources; it is usually absent where streams are lacking. It disappears when water dries up. Echolocation calls are steeply FM without any CF component; frequencies are 45-100 kHz. Call characteristics imply that it can detect prey close to substrate or over water.
Movements, Home range and Social organization. Horsfield’s Myotis is often the most common insectivorous bat species in some areas. It was the most frequently captured species in tunneltraps, representing 45% and 66% of tunnel trap captures on the west and south slopes of Mount Isarog, Luzon. It normally roosts in small (2-5) to mediumsized groups. One roost had more than 100 individuals. In Assam, it was netted while coming out of a culvert where a group of 4-5 individuals was roosting. Horsfield’s Myotis often share roosts with species of Rhinolophus, Hipposideros, and Miniopterus .
Status and Conservation. Classified as Least Concern on The IUCN Red List. Horsfield’s Myotis is widespread, presumably has a large population, occurs in a number of protected areas, tolerates some habitat modification, and is unlikely to be declining fast enough to qualify for listing in a more threatened category. In South Asia,its habitat is being deforested for timber, firewood, and agriculture. It is also threatened by disturbance of roosting sites.
Bibliography. Abramov et al. (2010), Amador et al. (2018), Bates & Harrison (1997), Bates, Hendrichsen et al. (1999), Bates, Nwe Tin et al. (2005), Boitani et al. (2006), Borisenko & Kruskop (2003), Boro et al. (2018), Corbet & Hill (1992), Ellerman & Morrison-Scott (1951), Francis (2008a), Francis, Borisenko et al. (2010), Francis, Guillén-Servent & Robinson (1999), Harada & Kobayashi (1980), Heaney, Balete, Dolar et al. (1998), Heaney, Balete & Rickart (2016), Hendrichsen, Bates, Hayes & Walston (2001), Hill (1983), Honacki etal. (1982), Huang, J.C.C. etal. (2014), Hughes et al. (2011), Keng etal. (2014), Khajuria (1984), Kingston et al. (2006), Kock & Dobat (2000), Koopman (1994), Kruskop (2013a, 2013b), Lim etal. (2017), Medway (1983), Molur et al. (2002), Payne et al. (1985), Phillipps & Phillipps (2016), Robinson et al. (1995), Rosell-Ambal, Tabaranza, Heaney, Gonzales et al. (2008), Ruedi & Mayer (2001), Ruedi et al. (2013), Sedlock (2001), Sedlock et al. (2008), Shamel (1942), Simmons (2005), Smith & Xie Yan (2008), Srinivasulu & Srinivasulu (2012), Srinivasulu et al. (2017), Stadelmann, Jacobs et al. (2004), Stadelmann, Lin Liangkong et al. (2007), Supanuam etal. (2013), Vanitharani (2006), Volleth & Heller (2012), Wordley et al. (2014), Wu Yi, Motokawa et al. (2009).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Myotis horsfieldii
| Don E. Wilson & Russell A. Mittermeier 2019 |
Vespertilio horsfieldii
| Temminck 1840 |