Myotis septentrionalis (Trouessart, 1897)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6397752 |
|
DOI |
https://doi.org/10.5281/zenodo.6577852 |
|
persistent identifier |
https://treatment.plazi.org/id/4C3D87E8-FF59-6AE7-FA4F-97FE18FFBA9D |
|
treatment provided by |
Conny |
|
scientific name |
Myotis septentrionalis |
| status |
|
370. View Plate 70: Vespertilionidae
Northern Myotis
Myotis septentrionalis View in CoL
French: Murin nordique / German: Nordamerika-Mausohr / Spanish: Ratonero nérdico
Other common names: Northern Bat, Northern Long-eared Bat, Northern Long-eared Myotis
Taxonomy. Vespertilio gryphus var. septentrionalis Trouessart, 1897 View in CoL ,
Halifax, Nova Scota, Canada.
Subgenus Pizonyx (50 species); lucifugus species group (16 species). See Submyotodon latirostris . Myotis 1s the most speciose genus of bats with 127 species recognized here and new species being recognized regularly. Taxonomic history of subgenera under Myotis is complex, and separate subgenera have been recognized based solely on morphology, notably Myotis (typical mouse-eared myotis ), Leuconoe (large-footed myotis ), and Selysius (whiskered myotis ). Other subgenera include Chrysopteron (Asiatic orange and yellow and African endemic myotis ), Pizonyx (only M. vivesi ), and Rickettia (only M. ricketti , now known as M. pilosus ), although these were often included under different subgenera or as distinct genera. Following several genetic studies, the three traditionally recognized subgenera are not monophyletic. Instead, genetic data suggest there are two major clades in the genus Myotis , one in the New World (with two species from the Old World) and the other in the Old World. These can be broadly grouped into three subgenera, with Pizonyx being the oldest name available for the New World clade. In the Old World, two sister subgenera are recognized here: Chrysopteron (Asiatic orange and yellow and endemically African species) and Myotis (all other Old World forms). Traditionally recognized subgenera Leuconoe and Selysius are included under the subgenus Myotis as synonyms for now. Within subgenera, species groups have been defined here primarily based on genetic data, but exact species composition of these groups is debatable. The New World lucifugus , ruber , vivesi , and albescens species groups along with the Old World brandtii species group are included in Pizonyx, and in the Old World, alcathoe , dasycneme , mystacinus , muricola , montivagus , capaccinu, siligorensis , horsfieldii , macrodactylus , daubentonii , and myotis species groups are recognized under the subgenus Myotis ; subgenus Chrysopteron does not have any species groups. Genus Cistugo has often been included as a synonym of Myotis or as a genus within Myotinae , but following recent genetic and morphological studies, it has been moved to its own family sister to Vespertilionidae , Cistugidae . In 1958, the International Commission of Zoological Nomenclature fixed the gender of Myotis to masculine. Myotis septentrionalis appears to be most closely related to M. auriculus . It has been included under M. keenii as a subspecies, but morphology and genetic data support their distinct specific status. Monotypic.
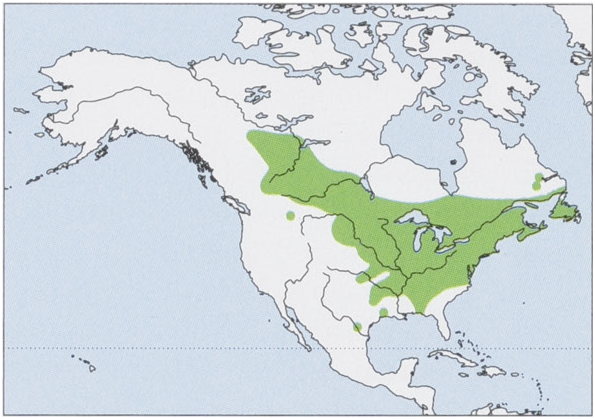
Distribution. Canada from SW Yukon and S Northwest Territories E to S Quebec, including Nova Scotia and Anticosti and Prince Edwards Is, and C & E USA from E Montana to New England and S to NW Florida, with single records from W Montana, NC Louisiana, and S Texas. View Figure
Descriptive notes. Head-body 40-46 mm, tail 36-43 mm, ear 14-19 mm, hindfoot 7-10 mm, forearm 35-39 mm; weight 4-11 g. Females are generally larger and heavier than males. Dorsal pelage of the Northern Myotis is variably dark brown, yellowish brown, or blond (hairs with dark bases); venter is whitish or creamy. Bare face is pinkish, and ears and membranes are dark blackish, except for lighter bases of ears. Ears are long, extending past nose when laid forward; tragus is long and narrow, with pointed tip. Calcar is either unkeeled or with indistinct keel. Baculum is short, with knobbed tip and notched and hollowed base, curving upward laterally at base and tip. Wings attach to bases of toes, and feet are relatively large (shorter than in the Gray Myotis , M. grisescens ). Skull is narrow, with relatively long rostrum; skull can be distinguished from that of Keen’s Myotis ( M. keenii ) by greater canine width at cingulum and generally narrower skull. Dental formula for all species of Myotis except M. ridleyi , M. yosseti, and M.. annectans , 1s 12/3, C 1/1. P 3/3, M 3/3 (x2) = 38. Chromosomal complement has 2n = 44 and FN = 50.
Habitat. Forested habitats, particularly boreal forests. Northern Myotis tend to prefer foraging in areas with closed canopies and avoid open spaces, including areas with substantial deforestation.
Food and Feeding. Northern Myotis feed primarily by aerial hawking but can glean prey from substrates, unlike most related species. Its longertail and greater wing area compared with other aerial-hawking myotis species allows it to glean, with more maneuverability during slow flight in cluttered areas. Foraging occurs under forest canopies, above standing water or streams, along paths and roads, and along forest edges. It eats various species of Lepidoptera , Coleoptera , Neuroptera , Diptera , Hemiptera, Homoptera , Hymenoptera , Orthoptera , and Araneae . In some places in its distribution, it primarily eats Lepidoptera and Coleoptera . In Indiana and Missouri, diets contained Lepidoptera (10-4-96% by volume), with smaller amounts of Coleoptera (0-4-64%), Trichoptera (0-54:-5%), Diptera (0-15-3%), and non-flying prey including Araneae and Lepidoptera larvae. Similarly, Lepidoptera (48:8% by volume) and Coleoptera (38:2%) dominated diets in Kentucky, Ohio, and Tennessee, with smaller amounts of Diptera , Hemiptera, Trichoptera , and other insects. Northern Myotis appear to feed opportunistically. They reportedly use calls of katydids to home in on individual insects, but if the katydids are able to identify that a bat is near, they stop calling which causes the bat to give up on finding the katydid. This suggests that bats rely on listening for katydid calls rather than using echolocation.
Breeding. Northern Myotis mate from late July until September or early October, with more restricted breeding seasons in northern regions. When mating, the male mounts the female from behind and occasionally grabs her neck with his teeth. Females store sperm in their uteri through winter until ovulation occurs in spring. Gestation lasts c.50-60 days; young are born from mid-May to mid-June in the south-eastern part of the distribution and in mid-July in northern part. Females give birth to one young. Lactating females have been caught in mid-June in Missouri, and female with a young was captured in mid-June in Ohio and late June in Missouri. Pregnant females were collected in British Columbia, New York, and Iowa in late June and July. Females appear to wean young after about one month, and young start flying by c.18-21 days old. Volant young have been caught in early August in Missouri and Ohio and as early as July in Iowa and New Hampshire. Oldest wild-banded individual lived to be 18-5 years.
Activity patterns. Northern Myotis roost mainly in trees but can be found in or on buildings and caves. Maternity colonies in New Hampshire, Michigan, and British Columbia roosted in tall, old, and early decaying trees, where they were found in crevices, hollows, or under bark. Adult males and non-reproductive females occasionally use caves and buildings as well as typical tree roosts. In autumn (August-September in Ontario) after breeding, they move from summer roosts to hibernacula, commonly in caves and abandoned mines. They are easily overlooked in hibernacula because they prefer to hibernate deep within caves in crevices. Length of hibernation varies depending on latitude and other environmental factors and can begin in September to early November and last until March, April, or May. Leading up to hibernation in August—-October, weights increase 45% for males and 41% for females. There are two peaks in foraging activity throughout the night, one during first two hours after sunset and anotherjust before sunrise, both of which correspond with peaks in insect activity but there is no significant difference in diet composition between these two foraging bouts. Calls are steep FM sweeps, with high frequency of 126-60 kHz that has shorter duration (c.1 millisecond), broader bandwidth, and lower intensity (78 dB) than other species of Myotis that only forage by aerial hawking. Moths cannot easily detect calls of Northern Myotis , making them easier prey. In Ontario, average start frequency was 126-2 kHz, end frequency was 60-7 kHz, peak frequency was 97-4 kHz, and duration was c.1 millisecond.
Movements, Home range and Social organization. Male and female Northern Myotis roost separately, with reproductive females forming small maternity colonies of less than 60 individuals in spring and summer. Males and non-breeding females roost alone or in small groups ofless than ten individuals. They do not move long distances except when foraging and transitioning from summer roosts to hibernacula. When transitioning, they can move as far as 56 km in large swarms. In Ontario, they were the second most common swarming species at Renfrew Mine, making up more than 10% of swarming bats captured. They will return to the same hibernacula in subsequent years but not always sequentially. Northern Myotis generally hibernate with other bat species, particularly the Little Brown Myotis ( M. lucifugus ), the Big Brown Bat ( Eptesicus fuscus ), and the Tricolored Bat ( Perimyotis subflavus ). One of the largest recorded hibernating populations included ¢.300 Northern Myotis and c.1000 Little Brown Myotis in an abandoned mine in Quebec. Northern Myotis occasionally move among hibernacula through winter. In summer, they frequently switch roosts, and individuals will switch among various trees in a cluster of trees. In Michigan, majority of the trees roosts used by females were in 20-ha, females traveled an average of 191 m between roosts, and they switched roosts about every two days. Roost trees in New Hampshire were an average of 602 m from foraging areas. While foraging, they will use night roosts to rest, frequently caves and tree hollows. Home ranges in West Virginia were 65 ha.
Status and Conservation. Classified as Near Threatened on The IUCN Red List. The Northern Myotis is listed as Threatened under the Endangered Species Act in the USA. It is considerably less common than other species of Nearctic vespertilionids. Its largest threat is White-nose Syndrome caused by the fungus Pseudogymnoascus destructans, causing drastic declines in North American bats in eastern parts of the continent. Currently, Northern Myotis species has not been affected as much by the disease as other species of Myotis , but it is expected thatit will experience significant population decline in the future. White-nose Syndrome has been found in 25 of the 37 US states that Northern Myotis occur in. Currently, the best conservation action is stopping the spread of the disease by regulating cavers that can spread the disease on their clothing when working in multiple caves. Prescribed fires do not appear to represent a major threat to Northern Myotis because they seem to do well after fires have occurred. Nevertheless, deforestation is likely a threat in some regions because they rely heavily on old hardwood trees for roosting and foraging. In West Virginia, they were more commonly associated with areas with partial timber harvest and avoided areas that were severely damaged by excessive timber harvesting. Use of chemical and biological insecticides might also be a source of concern becauseit affects their food supply. Disturbances in cave and mine hibernacula and tree roosts are additional threats.
Bibliography. Alves et al. (2014), Arnold (2007), Baker & Patton (1967), Brack & Whitaker (2001), Brandon (1961), Broders et al. (2013), Brown et al. (2007), Caceres & Barclay (2000), Crnkovic (2003), Faure et al. (1993), Findley (1972), Foster & Kurta (1999), Garroway & Broders (2008), Geluso et al. (2015), Henderson et al. (2008), Hendricks (2012), Hitchcock (1949, 1965), ter Hofstede et al. (2008), ICZN (1958), Johnson, Edwards & Ford (2011), Johnson, Edwards, Ford & Gates (2009), Johnson, Ford & Edwards (2012), Jung et al. (2006), Krochmal & Sparks (2007), Krynak (2010), Lacki, Cox, Dodd & Dickinson (2009), Lausen etal. (2008), Lee Yafu & McCracken (2004), Lowe (2012), Manning (1993), Menzel et al. (2002), Miller, G.S. & Allen (1928), Miller, L.A. & Treat (1993), Mills (1971), Owen et al. (2003), Park & Broders (2012), Patriquin et al. (2013), Platt et al. (2018), Ratcliffe & Dawson (2003), Reynolds et al. (2016), Ruedi et al. (2013), Sasse & Pekins (1996), Sasse & Saugey (2008), Silvis, Ford, Britzke, Beane & Johnson (2012), Silvis, Ford, Britzke & Johnson (2014), Solari (2018p), Stadelmann et al. (2007), Stein & White (2016), Timpone et al. (2010), Whitaker (1972, 1973), Whitaker & Rissler (1992a, 1992b), White et al. (2016), Woodman (1993), Wund (2006), van Zyll de Jong (1979, 1984), van Zyll de Jong & Nagorsen (1994).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Myotis septentrionalis
| Don E. Wilson & Russell A. Mittermeier 2019 |
Vespertilio gryphus var. septentrionalis
| Trouessart 1897 |