Nyctalus noctula, Schreber, 1774
|
publication ID |
https://doi.org/ 10.5281/zenodo.6397752 |
|
DOI |
https://doi.org/10.5281/zenodo.6403392 |
|
persistent identifier |
https://treatment.plazi.org/id/4C3D87E8-FFF3-6A4D-FA50-9DD2198ABD15 |
|
treatment provided by |
Conny |
|
scientific name |
Nyctalus noctula |
| status |
|
15. View Plate 55: Vespertilionidae
Common Noctule
French: Noctule commune / German: Grof 3er Abendsegler / Spanish: Néctulo mediano
Other common names: Noctule, Noctule Bat
Taxonomy. Vespertilio noctula Schreber, 1774 View in CoL ,
France.
Nyctalus noctula is sister to the clade including N. lasiopterus and N. aviator . N. plancyi has sometimes been included within this species, but is here recognized as a full species. The name labiatus has been moved to N. plancy: because of the clear morphological differences between N. noctula and labiatus, although labiatus has not been properly compared with N. plancyi ; further research is required. Three subspecies recognized.
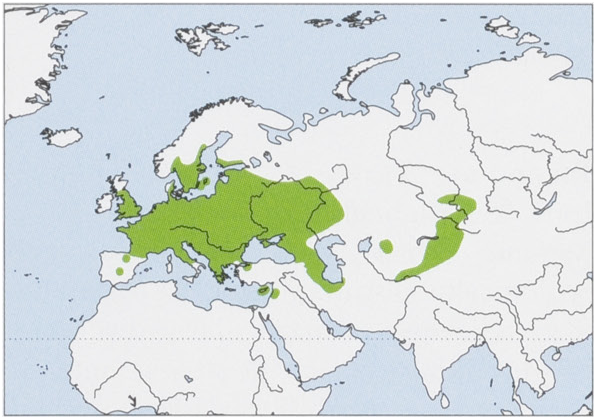
Subspecies and Distribution.
N. n. noctula Schreber, 1774 — throughout Europe from Great Britain, France, and Spain E to W Russia, W Kazakhstan, and SW Turkmenistan, including S Scandinavia, Gotland and Oland Is, and Cyprus ( Cyprus records somewhat tentatively regarded as this subspecies). Absent throughout much of Iberia and is locally extinct in Portugal.
N. n. lebanoticus D. L.. Harrison, 1962 — WC & SW Syria, Lebanon, and NE Israel.
N. n. mecklenburzevi Kuzyakin, 1934 — SC & E Kazakhstan, SC Russia, W Uzbekistan, Tajikistan, Kyrgyzstan, and NW China (Xinjiang).
The species may be present in N Africa, with two records claimed from Algeria in 1858, but these may represent N. lasiopterus ; further sampling is needed. View Figure
Descriptive notes. Head-body 60-89 mm, tail 40-66 mm, ear 16-21 mm, hindfoot 12-14 mm, forearm 47-60 mm; weight 17-44 g. Dorsal pelage of the Common Noctule is a distinctive reddish brown (individual hairs unicolored), while ventral pelage is slightly paler. Juveniles and freshly molted adults are duller brown throughout. Ventral pelage extends onto wings and interfemoral membrane, as in other noctules. Face, ears, and membranes are dark brown, and tail extends a few millimeters past the uropatagium. Muzzle is short, with large glands between nostrils and eyes, and ears are short and triangular, with 4-5 folds on outer edge. Tragus is very short and rounded, mushroom-shaped, as is characteristic of the genus. Wings attach at ankle, and calcar reaches halfway to tail. Postcalcarial lobe is wide, with a visible T-shaped piece of cartilage. Common Noctules give off a distinctive musky odor. Skull is relatively high and wide; lambdoidal crest is undeveloped, and zygomatic arch is thin; I is larger than I*; crown of P* is one-half the size of or nearly equal to I’; lower molars are nyctalodont. Chromosomal complement has 2n = 42 and FN = 54.
Habitat. Primarily found in temperate deciduous forests, wetlands, and agricultural fields and pastures; found at elevations from sea level up to ¢. 1900 m, preferring lowland regions but in Switzerland can be found at ¢. 1900 m. Common Noctules are well adapted to surviving in urban settings throughout their distribution, flying and foraging throughout cities and suburban areas.
Food and Feeding. Insectivorous. Common Noctules are fast-flying aerial hawkers that forage primarily at higher altitudes above the canopy of forests or over open areas. Their diet consists mainly of large flying insects such as larger moths, beetles (e.g. cockchaters Melolontha spp.), and crickets, and smaller swarming insects, such as chironomids, anisopodids, and tipulids ( Diptera ), as well as some Trichoptera. When feeding on swarming species, the noctules can catch many more of them at once, which can be seen as a form of aerial filterfeeding. In Switzerland, harder coleopteran prey (Melolontha spp. in spring and Geotrupes spp. in autumn) were preferred over swarming insects during spring and autumn, while swarming insects were preferred during summer. This suggests a rather opportunistic hunting strategy for the species. Although they hibernate throughout winter, they will also forage as much as possible and have been recorded flying throughout winter and at temperatures below 0°C. During winter in central Europe, two groups of arachnids (Araneida, Acari) and nine orders of insects (Homoptera, Heteroptera, Psocoptera, Neuroptera , Coleoptera , Hymenoptera , Lepidoptera , Diptera , Siphonaptera ) were identified in fecal samples from roosting bats. Lepidoptera (moths in particular making up the most important component throughout), Diptera , Coleoptera , and Araneida were the most prolific portions of their diet throughout winter but this changed markedly through the season. The presence of arachnids indicates that the species may also be foraging by gleaning, although this has not been reported.
Breeding. Like other noctule species, the Common Noctule exhibits delayed fertilization, mating in late summer and early autumn before entering hibernation. During late summer, single males establish mating roosts, emitting shrill mating calls at the roost entrance or during flight, as well as having a strong odor, in order to attract a small harem of up to 20 females (4-5 is more common). Females stay with the male for c.1-2 days, in which time they copulate. Young are generally born in late June or July after just over two months of gestation. Litter size is 1-2 young, although two is commonest. Maternal colonies generally occur in the northern portion of their range, as this is where females migrate to in spring. Weaning occurs after c.3—4 weeks and young are able to forage for themselves by six weeks old, by which time they have become completely volant. Young are left in groups within roosts while females forage. Females will often switch roosts throughout the breeding season. Males generally begin mating after their first year.
Activity patterns. Nocturnal, only foraging for a total of around one hour, spread over two equal bouts after sunset and before sunrise. They are fast fliers and often perform deep dives during flight. Roosting generally occurs in hollow portions of old trees, commonly in woodpecker holes, as well as in crevices in buildings and rock structures. During winter, they create larger roosts in tree holes, rock crevices, crevices in buildings, and underground in some cases. They are most active from March or April until around October, hibernating throughout winter (October-March/April). Common Noctules do still forage throughout winter (as do other noctule species), although little research has been performed into the winter habits of this species or its congeners. They remain in a torpid (hibernation) state throughout the day and even much of the night, but this is interrupted for foraging bouts during the night. They are regularly active at temperatures between 0°C and —-7°C but if temperatures drop below —-10°C, they will not fly. Calls are very loud (maximum energy at c¢.25 kHz on average) and are usually audible to the human ear, unlike most bat calls. Common Noctules exhibit two call types, both of which have an FM/QCF call shape; call type one have start frequencies of 23-8-52-2 kHz, end frequencies of 21-4-26-2 kHz, maximum energy at 22-4-27 kHz, middle frequencies of 21-4-28-7 kHz, call durations of 8-8-23.4 milliseconds, and interpulse intervals of 120-3—413-1 milliseconds; call type two has a start frequencies of 18:2-30-4 kHz, end frequencies of 17-3-23 kHz, maximum energy of 17-5-23-6 kHz, middle frequency of 17-4-24-6 kHz, call durations of 13-2-29-9 milliseconds, and interpulse intervals of 120-2-807-5 milliseconds. The bats regularly switch between the two call types while foraging. Predators include diurnal avian raptors (Accipiter, Circus, Falco), herring gulls (Larus argentatus ), corvids, and great grey shrikes (Lanius excubitor).
Movements, Home range and Social organization. Common Noctules are highly migratory throughout their range, traveling between breeding and hibernating areas. They breed throughout their range but hibernate only in the southern portion, usually traveling north from their winter quarters. Females generally migrate further and more often than males. Males are often sedentary and in northern parts of their range they will remain year-round whereas females will migrate south during the winter. Most individuals will not travel more than 1000 km, although the longest known migration within Europe for this species was 1546 km. They may not migrate as far or at all in southern and western portions of their range, where they are primarily sedentary. In early spring after leaving hibernation, they will create mixed-sex colonies that eventually disperse during late spring and develop into summer colonies. During summer, females create large maternity colonies, with an average of 20-50 individuals, occasionally ¢.100 individuals, while males and juveniles will roost alone or in smaller groups. Roosts can be much larger and have a mix of males and females during winter, including as many as ¢.1000 individuals in a single roost.
Status and Conservation. Classified as Least Concern on The IUCN Red List. The Common Noctule is widespread and common throughout much of its distribution. There are no major threats to the species, although it may be threatened by the destruction of roosting sites in older trees. It has become locally extinct in much of the Iberian Peninsula, including all of Portugal.
Bibliography. Avery (1986), Benda et al. (2007), Bihari (2004), Celuch & Kafuch (2005), Csorba & Hutson (2016), Fedyk & Fedyk (1970), Gloor et al. (1995), Gorfol et al. (2009), Jones (1995), Kanuch, Janetkové & Kristin (2005), Kleiman (1969), Mackie & Racey (2007), Mikula et al. (2016), Petit & Mayer (1999), Racey (1974a), Rachwald (1992), Ruczynski et al. (2007), Samiya et al. (1993), Schober & Grimmberger (1998), Spitzenberger (2002), Strelkov (1969, 1997), Vogler & Neuweiler (1983).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Nyctalus noctula
| Don E. Wilson & Russell A. Mittermeier 2019 |
Vespertilio noctula
| Schreber 1774 |