Breinlia (Breinlia) presidentei, Spratt, 2011
|
publication ID |
https://doi.org/10.11646/zootaxa.2860.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/4C7B87C3-FF81-FF94-FF44-59F2FA1B7348 |
|
treatment provided by |
Felipe |
|
scientific name |
Breinlia (Breinlia) presidentei |
| status |
sp. nov. |
Breinlia (Breinlia) presidentei sp. nov.
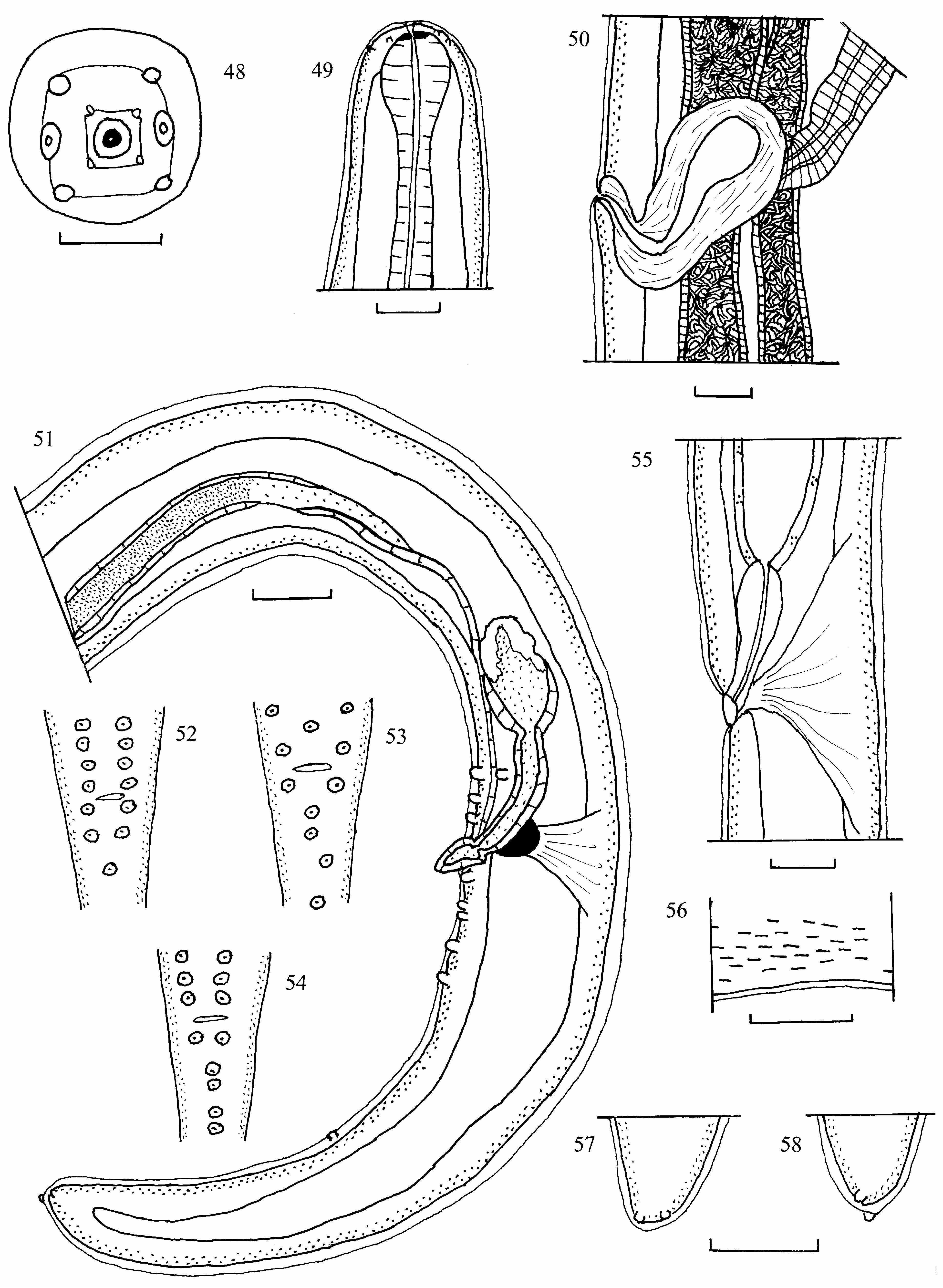
( Figs 48–58 View FIGURES 48–58 )
Type host. Mesembriomys macrurus (Peters) (Eutheria: Muridae )
Other hosts. Mesembriomys gouldii (Gray) , Conilurus penicillatus (Gould) (Eutheria: Muridae ).
Site in hosts. Peritoneal and pleural cavities.
Material examined. Holotype: ♂, from Mesembriomys macrurus, Mitchell Plateau , Western Australia (14 0 46’S, 125 0 47’E), coll. P.J.A. Presidente, 21.vi.1982, holotype ♂ AHC 45865; allotype: ♀, AHC 45866; paratype: 1♂, AHC 45867, paratype 6♀, AHC 45868. GoogleMaps
Other material examined. From M. macrurus: WA : 1♂, 3♀, Mitchell Plateau ( AHC 45869) .
From Mesembriomys gouldii: WA : 5♂, 20♀, Mitchell Plateau, ( QM G232522 ); 1♂, same locality, ( N1846 ) .
From pleural and peritoneal cavities, Conilurus penicillatus: WA : 1♀ posterior fragment, ( N1857 ) , 3♀ and anterior fragment, ( N1958 ) Mitchell Plateau ; 3♂, 3♀, ( AHC 17791) 9km SW Warrender Hill.
Etymology. The species is named after my colleague, the late Dr. Paul J.A. Presidente who collected the material from the Mitchell Plateau and who made a significant contribution to knowledge of wildlife parasitology and pathology in Australia.
Differential diagnosis. Breinlia (Breinlia) presidentei is most similar to B. (B.) mackerrasae from the northern brown bandicoot, B. (B.) oweni from the striped possum in Papua New Guinea, B. (B.) mundayi from a broad spectrum of kangaroos, wallabies, potoroos, and the koala and B. (B.) boltoni from the agile wallaby. It is distinguished from B. (B.) mackerrasae by much shorter males and females, much shorter muscular and particularly glandular portions of oesophagus and tails in both sexes, much longer filament of left spicule (0.20 vs 0.13 as measured from Fig. 2 View FIGURES 1–6 of Walker and McMillan, 1974), number and distribution of cloacal papillae and more anterior position of the vulva. It is distinguished from B. (B.) oweni by slightly longer males and much longer females, presence of square rather than rectangular peribuccal fields in apical view, longer right spicule and muscular oesophagus in males and much longer tail in females, the tail tip in both sexes terminating in only a pair of latero–ventral papillae. It is distinguished from B. (B.) mundayi and B. (B.) boltoni by a shorter left spicule, especially the calomus and lamina, shorter tails in males and females, the absence of internolateral papillae within the inner peribuccal field in apical view, the occurrence of cuticular bosses only on the posterior ventral surface of the male in the 3–4 mm leading to the cloacal aperture, shorter microfilaria with fewer single nuclei terminating the nuclear column and a shorter distance between the last nucleus and the tail tip.
Description. General: Long nematodes with attenuated anterior and posterior extremities. Oral opening small, round, bounded by delicate membrane. Cephalic extremity oval rather than elongated. Four pairs of submedian papillae arranged in outer circle of four large and inner circle of four smaller papillae. Square, cuticular, peribiccal field present, joining bases of papillae of inner circle. Second, less conspicuous peribuccal field present, roughly square, formed by slight elevation of cuticle joining bases of papillae of outer circle. Internolateral papillae absent. Amphids large, lateral, opening at level of outer circle of papillae. Buccal capsule small, narrow, with small, refractile ring at its base. Oesophagus divided into thin muscular and thicker glandular regions. Intestine broad. Cuticle with fine transverse striations, longitudinal, refractile cuticular bosses present only on 3–4 mm of posterior ventral surface of male anterior to cloaca, increasing in density posteriorly. Lateral cords with 3 columns of nuclei, a narrow, central column of widely–spaced, elliptical nuclei with prominent nucleoli and wider, peripheral columns of closely–spaced, elliptical nuclei with prominent nucleoli. Spicules unequal, dissimilar, sclerotised. Gubernaculum present. Lateral and caudal alae absent. Phasmids and deirids not observed.
Male: ( Holotype measurements presented first in italics, followed by paratype and one other). BL 53, 49, 47 mm. MW 249, 239, 249. NR 239, 265, 230. EP not observed. MO 461, 434, 318. GO 980, 875, 875. LS 510, 485, 509; Cal 198, 187, 208, Lam 94, 94, 83, Fil 218, 204, 218. RS 198, 187, 187 with spatulate distal extremity. Posterior end generally tightly coiled. Gub 35, 42, 42. Cloacal papillae 13 in number, four pairs pre– and two pairs and a median single post–cloacal papilla. T 556, 493, 449, terminating in pair of latero-ventral papillae.
Measurements of male B. (B.) presidentei from 6 M. gouldii 3 C. penicillatus are presented in Table 1. Males from M. gouldii with 10 or 11 cloacal papillae, two pairs and a single median papilla pre– and one pair and 4 single median or two pairs and two single median post–cloacal. Some specimens with a single median papilla 229 from tail tip. Tail terminating in pair of latero-ventral papillae. Males from C. penicillatus with 11 or 12 cloacal papillae, three pairs pre–cloacal and one pair and 4 single median post-cloacal papillae. Tail terminating in pair of lateroventral papillae.
Female: ( Allotype measurements presented first, in italics, followed by 6 paratypes and 2 others). BL 140, 148 (136–155). MW 435, 421 (398–451). NR 198, 251 (239–265). EP not observed. MO 461, 458 (413–530); GO 1086, 1118 (1000–1246) long. V 3223, 3786 (2528–4740) from cephalic extremity. A 853, 859 (689–1140) from posterior extremity. Tail terminating in pair of latero-ventral papillae.
Measurements of female B. (B.) presidentei from 5 M. gouldii and 6 C. penicillatus are given in Table 1.
Microfilariae: ( 10 specimens from vagina uterina). BL (185) 181–187). MW 5 (5–6). Tail long, filamentous, nuclear column terminating in at least 4–5 single, elongate nuclei. LNT 28 (27–29). Microfilaria unsheathed. Site in host unknown.
Distribution and hosts. Breinlia (B.) presidentei is known only from these three murid hosts in the Mitchell Plateau region of northern Western Australia and represents the first species of Breinlia from eutherians and the family Muridae in Australia.
Remarks. Breinlia (Breinlia) presidentei is most similar to B. (B.) mackerrasae , B. (B.) oweni , B. (B.) mundayi and B. (B.) boltoni and is distinguished from them under the differential diagnosis provided. Although there are morphometric differences between worms from the two species of Mesembriomys and Conilurus pencillatus measurements in general fall within a similar range and although the size of worms from M. gouldii are somewhat smaller than those from M. macrurus and C. penicillatus , the latter material is not in an optimal state of preservation. In addition, the number and distribution of pre– and post–cloacal papillae in males differ among the three host species. However, this is a common occurrence in this subgenus and insufficient to consider warranting specific recognition. I have been unable to detect any reliable morphological differences which would warrant specific distinction. Consequently, I consider them to represent a single species.
Watts et al. (1992) using complement fixation to assess albumin evolution in the history of Australian rodents recognised a clade containing Conilurus , Leporillus , Mesembriomys , Zyzomys , Uromys and Melomys and noted that within this clade Conilurus , Mesembriomys and Leporillus were particularly close, agreeing with previous electrophoretic and morphological results and the more recent phylogenetic studies of Rowe et al. (2008). No helminth parasites have yet been reported from stick–nest rats belonging to the genus Leporillus .
During the Quaternary glaciations Australia and New Guinea were united to form the island continent known as Sahul. Murine rodents, alone among nonvolant mammals, succeeded in crossing the multiple, deep ocean channels separating Asia from Sahul ( Rowe et al. 2011). Australian rodents are thought to belong to two separate lineages, the “old endemic” group and the “new endemics” (Watts & Aslin 1981; Watts & Baverstock 1994b). These authors suggested that the Australian clade of rodents, represented by Mesembriomys , may have been part of a South–east Asian murine radiation, arising early in its history and that the ancestor of the Australian murines reached the Australian–New Guinean region without leaving any surviving descendants in South– east Asia. Subsequent evolution is thought to have occurred exclusively in the Australian–New Guinean region. This “old endemic” lineage is considered to have invaded Australia around 5–8 million years ago (Watts & Aslin 1981; Watts & Baverstock 1994b; Rowe et al., 2008). Tectonic reconstructions suggest the most plausible configuration of land masses for terrestrial migrations in this region occurred about 7 mya ( Hall 1998). In addition, from this time onwards ocean currents ( Kennett et al. 1985) became favourable for short water dispersals by rafting or swimming, previous currents running into the Indian Ocean rather than towards land.
In contrast, the “new endemics” including the genus Melomys , are thought to have evolved recently in the more eastern regions of South– east Asia with radiations in Sulawesi, New Guinea and arriving in Australia during the Pleistocene about 40,000 years ago. The genus Rattus is thought to have originated in Southeast Asia approximately 3 mya and spread to New Guinea and subsequently Australia about 1 mya ( Watts & Baverstock 1994 b, 1995; Robins et al. 2010).
Comparison of the helminth fauna in the “old endemic”, “new endemic” and the recently arrived cosmopolitan species of Rattus indicates that few species occur in all three groups or even in two of the three groups ( Smales 1997, 2005, 2008, 2009; Smales & Spratt 2008; Smales et al. 2004) offering further support for these propositions.
| QM |
Queensland Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |