Coryphophylax brevicaudus, Vasudevan, Karthikeyan, Dutta, Sushil Kumar & Das, Indraneil, 2012
|
publication ID |
https://doi.org/ 10.5281/zenodo.212150 |
|
DOI |
https://doi.org/10.5281/zenodo.5611899 |
|
persistent identifier |
https://treatment.plazi.org/id/4E76360C-6A28-A71E-E5AF-F8B6FB73FCF5 |
|
treatment provided by |
Plazi |
|
scientific name |
Coryphophylax brevicaudus |
| status |
sp. nov. |
Coryphophylax brevicaudus sp. nov.
( Fig. 2–6 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 , Table 1 View TABLE 1 –3)
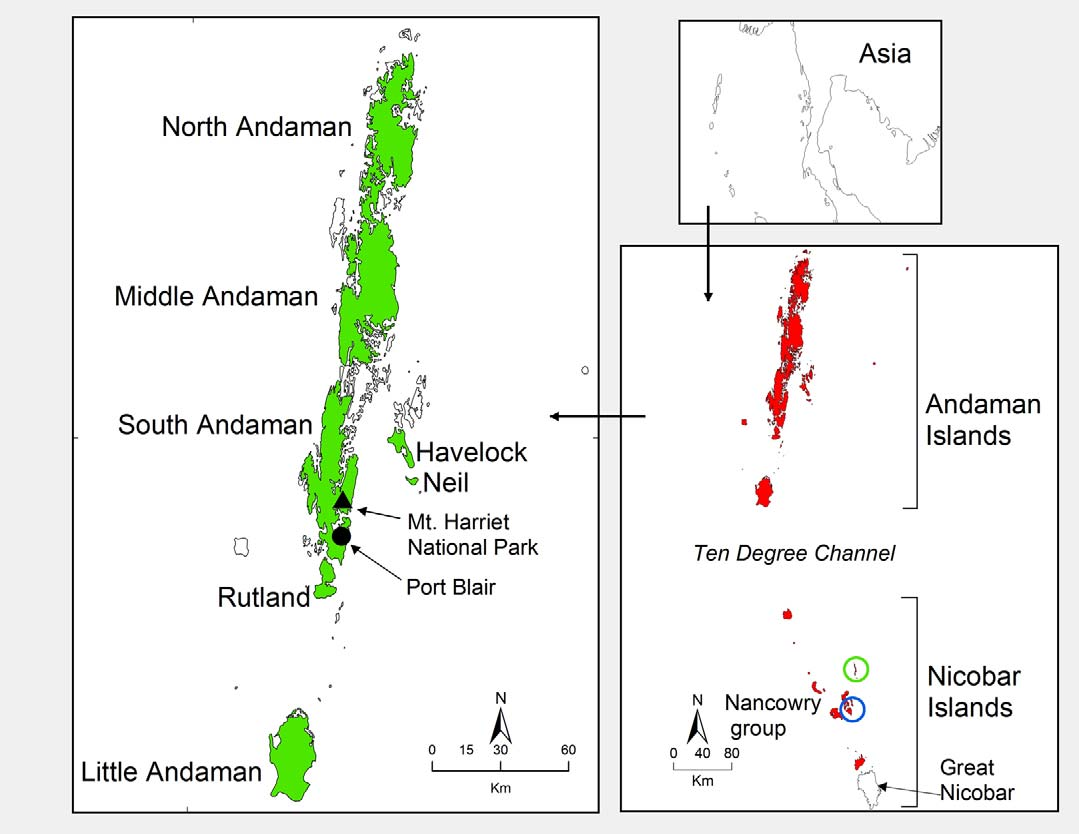
Holotype: ZSIC 25962, Adult male, Mount Harriet National Park, georeferenced latitude E 11.710579°, longitude N 92.735944°, South Andaman Island, Bay of Bengal, India, ca. 290 m asl. ( Fig. 1 View FIGURE 1 ). Collected by Harikrishnan S. and S. R. Chandramouli on 21 December 2010.
Paratypes: ZSIC 25963 (female), ZSIC 25964 (male), paratopotypes. Collected by S. Harikrishnan and S. R. Chandramouli on 25 June 2010.
Diagnosis: We allocate the new species to the genus Coryphophylax for showing the following suite of morphological characters: exposed tympanum, femoral pores absent, no lateral dermal expansions on body, absence of postorbital spines and lack of cephalic or nuchal spines.
The new species bears resemblance to several Asian genera of agamids, including the Malayan Aphaniotis Peters, 1864 and Gonocephalus Kaup, 1825 and the south Asian Otocryptis Wagler, 1830 , but whether this is a result of phylogeny or convergent evolution warrants a phylogenetic study of the Southeast Asian agamids. Aphaniotis can be distinguished morphologically from Coryphophylax in having a concealed tympanum, while Gonocephalus includes species with distinct cephalic and/or nuchal spines. Otocryptis can be separated from Coryphophylax in showing a concealed tympanum.
Coryphophylax brevicaudus sp. nov. is diagnosed by: small adult size (mean SVL 57.97 mm); a relatively short tail (mean TaL/SVL = 1.93); tail narrows abruptly from base after cloacal opening; mid-body scale count range 110–121; males and females show a nearly uninterrupted flap of skin with small conical spines forming nuchal and dorsal crests; adult colouration reddish-brown or greyish-brown, with or without dark brown markings; presence of a thin yellowish white subocular stripe.
Description of holotype: adult male, SVL 63.60 mm. Morphometric data are summarized in Table 1 View TABLE 1 . Head elongate (HW/HL ratio 0.61), maximum height less than maximum width; snout pointed; rostral broader than high; nostrils in upper half of single nasal shield, which is separated from rostral by a single scale; nasal in contact with first supralabial; supralabials 11/11; infralabials 11/11; mental shield narrower than rostral; two postmentals; anterior infralabials bordered on the inside by a row of enlarged scales; genials weakly keeled; scales on gular pouch strongly keeled, slightly smaller than genials; scales on top of snout small and smooth except median row, where there are three keeled scales; two enlarged, keeled scales, separated from each other by two small scales follow this row, each of ca. x 4 as large as adjacent snout scales; supraorbital scales keeled; six canthals along sharp canthus-rostralis, followed by eight compressed supraciliaries; orbit diameter 74% of distance between anterior border of orbit and snout tip; tympanum exposed, its greatest diameter 44% horizontal diameter of orbit; enlarged keeled scale between tympanum and orbit; one row of enlarged scales along dorsal inner border of orbit; a cluster of enlarged, elongated and keeled scales present just anterior to occiput, among which middle scale is largest; three enlarged spinous scales arranged in a line on both sides of occiput, separated from each other by 5–6 scales; nuchal crest begins at a point at the same level as the scale in the middle of this series; posterior region of jaws swollen, with three small spinous scales near angle of jaws.
Nuchal and dorsal crests low but well developed, composed of an erect flap of skin upon which are numerous conical compressed scales; skin flap continuous from nuchal to dorsal region, with a small region above shoulder, where conical compressed scales are lacking; dorsal crest continues to tail base; a weak antehumeral fold extending across throat; body scales minute, strongly keeled and intermixed with numerous enlarged spine-like scales; two parallel rows of enlarged spinous scales, separated from each other by a few scales on either side of vertebral region, the first of these separated from dorsal crest by ca. three scale rows, the second separated from first by 5–6 scale rows; 121 rows of scales around middle of body; scales on dorsum oriented postero-dorsally, while lateral ones oriented postero-ventrally; ventral scales with sharp keels and larger than laterals, genials and gular scales.
Limbs slender and covered with strongly keeled scales; scales under thighs weakly keeled; length of hindlimb ca. 92% SVL; relative length of fingers 4=3>5>2>1; relative lengths of toes 4>3>5>2>1; fifth toe longer than fourth finger; 19 subdigital lamellae under third finger; 26 subdigital lamellae under fourth toe; subdigital lamellae with sharp keels, bicarinate; tail slender, tapering abruptly posterior to cloaca; scales on dorsal and ventral surface of tail with sharp keels; tail length 124 mm, or 195% SVL.
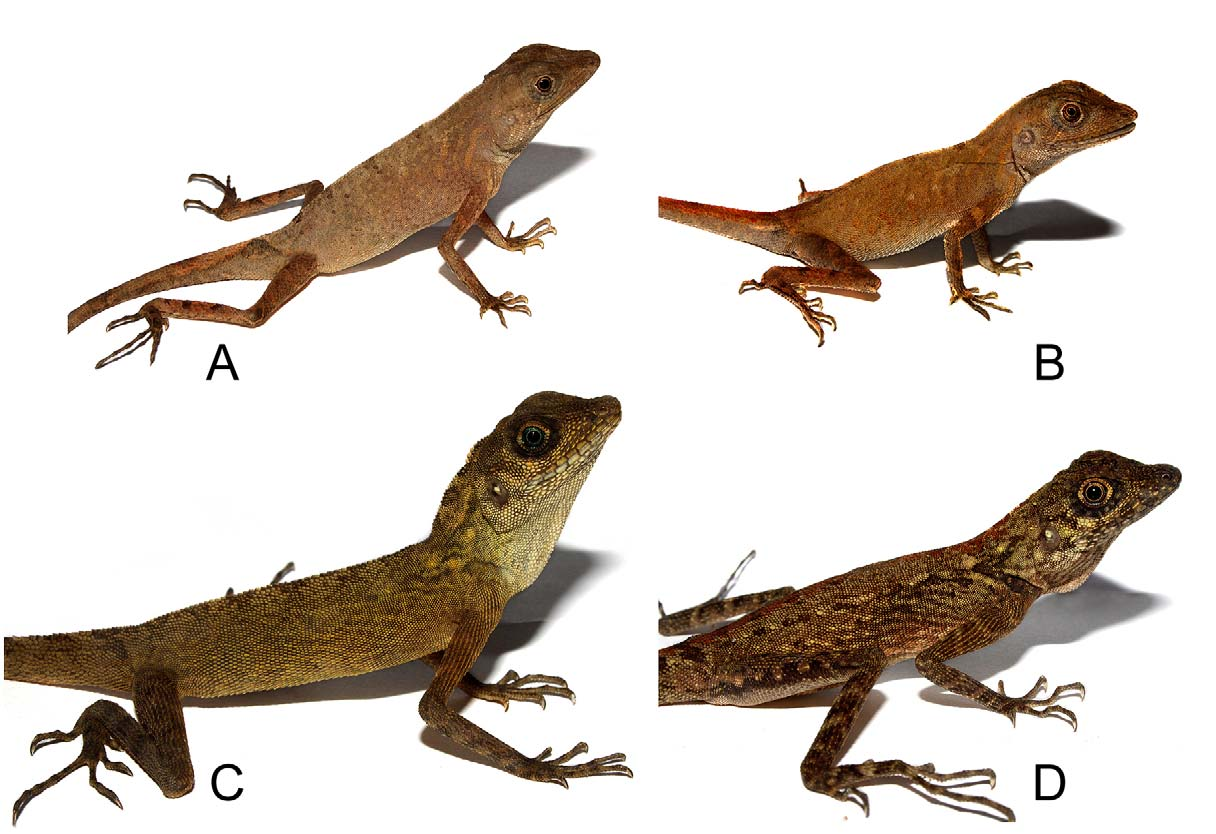
In life ( Fig. 3 View FIGURE 3 A), dorsum greyish-brown, lighter on vertebral region, with four dark brown vertebral spots; flanks darker brown; head greyish-brown, with a cream-coloured line from behind and below orbit to angle of mouth; infralabials grey; underside of chin greyish-white; gular pouch grey; light orange reticulations on sides of neck and anterior body; ventrals light yellowish-brown; limbs dark brown, banded with lighter brown; posterior part of fourth toe and foot dark brown, contrasting sharply with light brown of rest of foot; tail dark brown on sides basally, dark colour of both sides meeting dorsally at tail base of the tail, and separate lighter brown colour of tail dorsum from light brown of body dorsum; distally, tail banded alternatively with dark and light brown.
Description of paratypes: The two paratypes agree with the holotype in general morphology, scalation and colour, except for differences in mid-body scale count, which are 117 and 110, respectively. ZSIC 25963 also shows two enlarged scales on temporal region. ZSIC 25963 shows 11/10 (left/right) supralabials and 9/9 infralabials, while ZSIC 25964 shows 10/11 supralabials and 11/11 infralabials. ZSIC 25963 has 17 lamellae under the third finger and 28 lamellae under the fourth toe. ZSIC 25964 has 18 lamellae under the third finger and 27 lamellae under the fourth toe. In life, ZSIC 25963 had orange-red gular pouch, and body dorsum was a more intense shade of reddish-brown ( Fig. 3 View FIGURE 3 B). Morphometric data on holotype and the paratypes are summarized in Table 1 View TABLE 1 .
millimeters. The abbreviations are: SVL—snout-vent length, TaL—tail length, TaH—tail height, TaW—tail width, HL—head
length, HW—head width, HD—head depth, SL—snout length, JL—jaw length, OD—orbit diameter, TD—tympanum
diameter, TrL—torso length, TrW—torso width, TrH—torso height, HuL—upper arm length, Rad—forearm length,
F1–F5—length of fingers 1–5, Fem—thigh length, Tib—shin length, T1–5—length of toes 1–5.
Comparison with congener: For morphometric comparison using Discriminant Function Analysis, only live specimens from the Andaman Islands (type locality of both named species under the genus) have been used (see Appendix 2). For comparison of SVL, TaL, and meristic data, 158 live individuals from across the range of C. subcristatus in Andaman and Nicobar Islands are also used. Meristic characters are compared with museum specimens from both Andaman Islands and Nicobar Islands ( Car Nicobar, Nancowry and Tillanchong) (See Appendix 3).
The most apparent difference between C. brevicaudus sp. nov. and its congener is the difference in adult body size. C. brevicaudus sp. nov. adults are smaller than C. subcristatus (mean SVL 57.97± 5.92 mm [n = 18] vs. 77.05± 12.63 mm [n = 158], respectively). C. brevicaudus sp. nov. has a relatively shorter tail than C. subcristatus (mean TaL/SVL = 1.93 ±0.10 vs. 2.42±0.17, respectively). In C. brevicaudus sp. nov., tail narrows abruptly from base after cloacal opening, while in C. subcristatus tail is relatively muscular at base and gradually narrows to tip.
This difference is assumed to be due to the relatively greater arboreal habits of C. subcristatus , which uses tail as a support while perching vertically on tree trunks. The known mid-body scale count in C. subcristatus is 85–100, while the mid-body scale count in C. brevicaudus sp. nov. is 110–121. Sexual size dimorphism (SSD) scores are different for the two species, with C. subcristatus having a higher average SSD score (0.21) compared to C. brevicaudus sp. nov. (0.14). In C. brevicaudus sp. nov., both male and female have an uninterrupted flap of skin with small conical spines forming nuchal and dorsal crests. In C. subcristatus , nuchal crest is considerably higher than dorsal crest in males, with a diastema above shoulder. Additionally, there is considerable variation in crest structure in C. subcristatus , primarily in relative length of spines on nuchal and dorsal crests, with some populations exhibiting elongated backward curving spines on nuchal crest. Our observations indicate that the degree of development of the nuchal and dorsal crest in C. subcristatus is correlated with body size and larger individuals tend to have better developed crest. Coryphophylax maximiliani Fitzinger in Steindachner, 1867 and Tiaris humei Stoliczka, 1873 were described based on specimens with such enlarged nuchal and dorsal crests ( Steindachner, 1867, Plate II-6; Annandale, 1904). The new species is not conspecific with either of these, as indicated by its much smaller size, greater number of scales around the body and lack of backward curving conical spines on nuchal and dorsal crests. Females of C. subcristatus have a low nuchal crest, while dorsal crest is absent or barely indicated in large females.
Adults of C. brevicaudus sp. nov. are reddish-brown or greyish-brown, while in C. subcristatus the adult body dorsum vary from greenish-brown, to reddish-brown to dark brown ( Fig. 4 View FIGURE 4 ). Adult males of C. brevicaudus sp. nov. are reddish-brown or greyish-brown, with faint dark reticulations. Adult males of C. subcristatus are greenishbrown or brown, sometimes with black and yellow reticulations, especially on the anterior body. Many individuals of C. subcristatus have a thin pale line running parallel to the dorsal crest on flanks of body, a pattern unknown in C. brevicaudus sp. nov. A white or yellowish-white line starts at the lower posterior portion of the orbit and passes to the corner of the mouth in almost all individuals of C. brevicaudus sp. nov., while this pale line is absent in C. subcristatus . Regardless of the colour of the rest of the tail, the basal dorsal region of the tail in C. brevicaudus sp. nov. is light brown, yellowish-brown, or orange, bordered by dark brown or black, while this marking is absent in C. subcristatus . Female C. brevicaudus sp. nov. are similar in appearance to the males, while female C. subcristatus have a different colour pattern from that of male C. subcristatus , being greyish-brown or yellowishbrown with black blotches and reticulations on the body ( Fig. 4 View FIGURE 4 ). In female C. subcristatus , the vertebral region is typically spotted with dark brown, and in some individuals, a dark reddish-brown vertebral stripe is present. Sub adult and juvenile C. subcristatus resemble the females in colour and pattern. Juveniles of C. brevicaudus sp. nov. are similar in colour to the adults except for the presence of a distinct light vertebral stripe, with five dark brown or black diamond-shaped vertebral spots, which fade in adults. The gular pouch is well developed in both males and females of C. brevicaudus sp. nov., and becomes orange-red during the breeding season. Though previous authors have described the gular pouch of the male C. subcristatus as “reticulated with yellow, red and black” (e.g., Stoliczka, 1873), all males we have seen in the Andaman Islands had gular sacs that were primarily yellow or white, with little black or red reticulations. Many populations of this species from the Nicobar Islands show black and yellow reticulations or spots on the gular pouch. The gular pouch in the female is less developed in C. subcristatus .
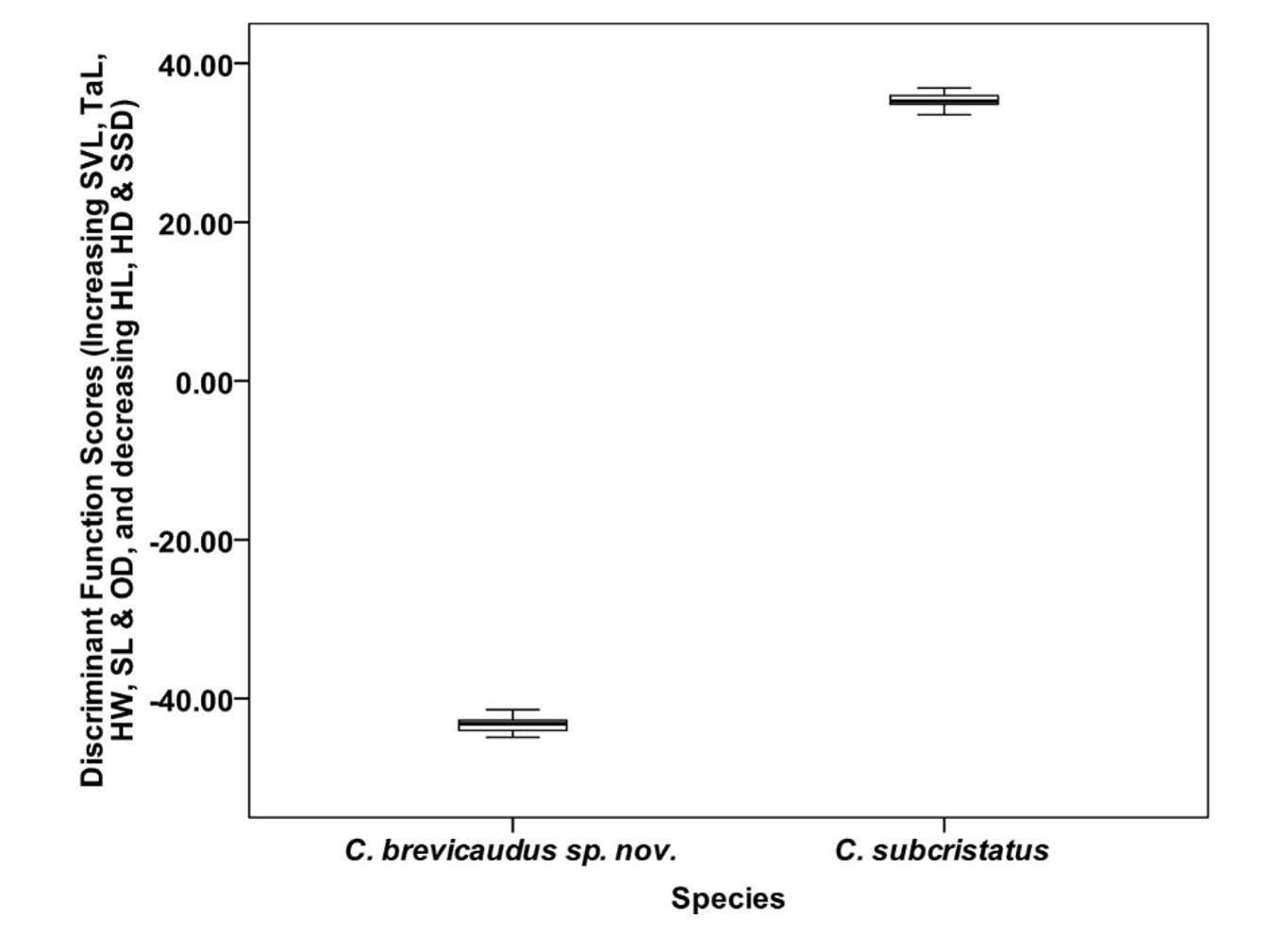
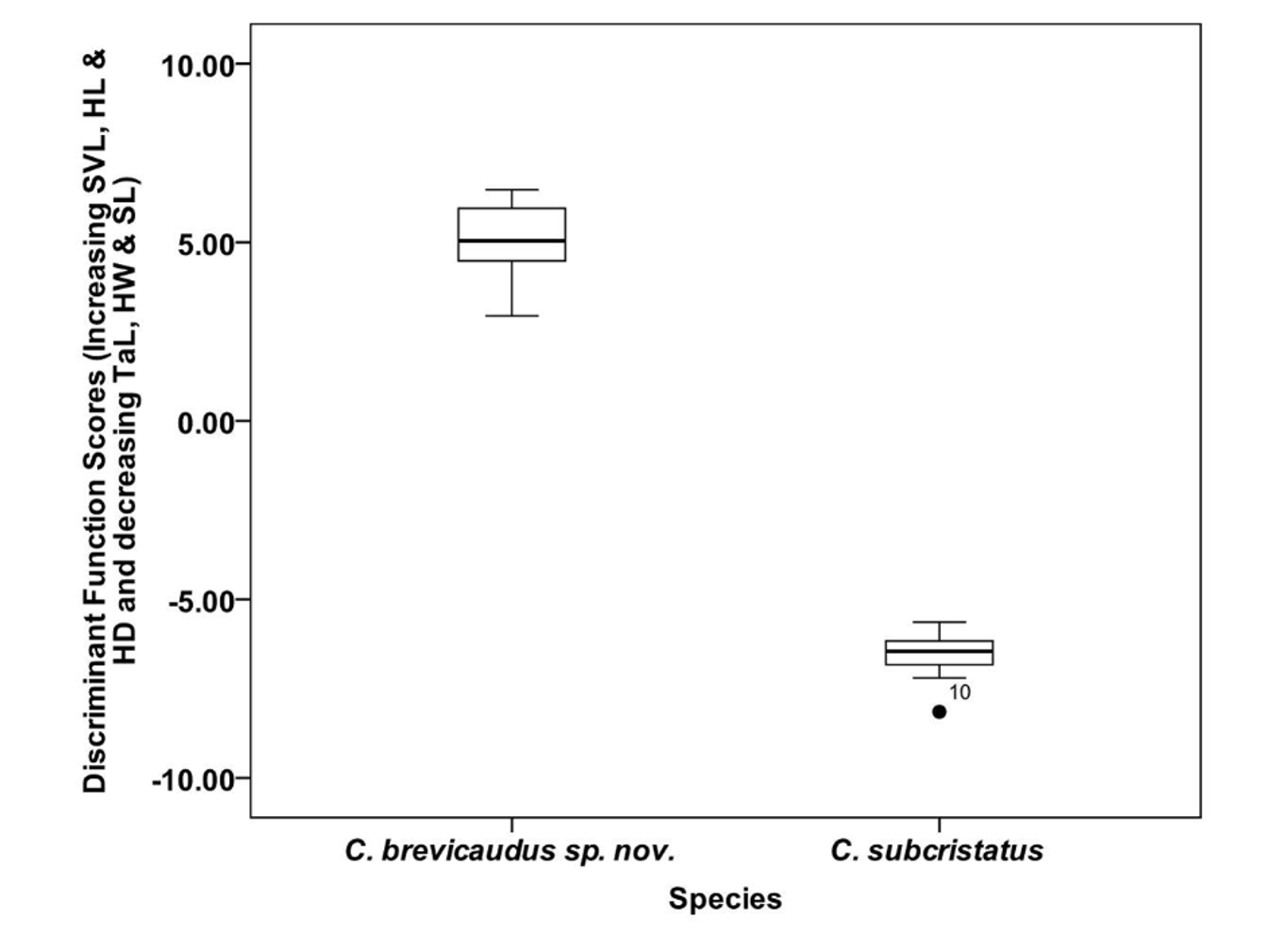
Morphometric data from 36 live individuals (after removal of juveniles and sub adults) were used in a Discriminant Function Analysis, grouped into two ( C. brevicaudus sp. nov. and C. subcristatus ). Twelve variables were used in the analysis. For males, the group means were significantly different for SVL, TaL/SVL, HL/SVL, HW/SVL, HD/SVL, SL/SVL, OD/SVL and SSD ( Tables 2a–2b View TABLE 2 a View TABLE 2 b ). In the case of females SVL, TaL/SVL, HL/SVL, HW/SVL, HD/SVL and SL/SVL were significantly different. For both males and females, among the significant variables, maximum difference in mean value was in TaL/SVL while OD/SVL had the least difference ( Table 2a & 2b View TABLE 2 a View TABLE 2 b ). For both males and females, only one canonical discriminant function was used in the analysis. In the case of males, for the first canonical discriminant function, the eigenvalue was 1696.07, canonical correlation was 1 and Wilk’s lambda was 0.001, and significant at P <0.001. In the case of females, for the first canonical discriminant function, the eigenvalue was 38.77, canonical correlation was 0.99 and Wilk’s lambda was 0.025, and significant (P <0.001) The first canonical discriminant function explained 100% of variance in both cases. The Chi-square value for Wilk’s lambda was significant in both cases (Chi-square = 89.24, df = 12 and Chi-square = 31.31, df = 12, for males and females respectively, P <0.001). In the classification, prior probabilities were set at 0.5 for each group. All individuals (100% of original group cases) were correctly classified in their respective groups. The discriminant function scores for the two species are shown in Figure 5 View FIGURE 5 & 6 View FIGURE 6 .
* Represents significance at α = 0.05 * Represents significance at α = 0.05 Etymology: The specific epithet refers to the short tail in the new species.
Suggested Common Name: We suggest “Short-tailed Bay Island forest lizard” as a common English name for this new species. We also suggest the use of the name “Short-crested Bay Island forest lizard” for Coryphophylax subcristatus , a translation of its species nomen.
Natural History: The natural vegetation of the type locality is composed of four major types: Andaman tropical evergreen forest, giant evergreen forest, southern hilltop tropical evergreen forest and semi-evergreen forest ( Champion & Seth, 1968). Coryphophylax brevicaudus sp. nov. is semi-arboreal to terrestrial in habits, and so far has been recorded only in evergreen and semi-evergreen forests. Individuals were seen perching on low bushes and twigs or on leaf-litter. They occur syntopically in the same habitat as C. subcristatus , but tend to prefer lower and thinner perches than C. subcristatus , and are not as abundant as C. subcristatus . Additionally, C. subcristatus shows caudal autotomy and regeneration ( Smith, 1935; personal observations) but we have not recorded this in C. brevicaudus sp. nov.
Distribution: Coryphophylax brevicaudus sp. nov. is known only from the Andaman group of islands, specifically from the islands of South Andaman, Rutland, Alexandria, Little Andaman, Middle Andaman, North Andaman, Tarmugli, Havelock and Neil ( Fig. 1 View FIGURE 1 ). The altitudinal distribution is from 20–350 m asl. In all islands surveyed, it was only recorded from primary evergreen and semi-evergreen forests. It is likely that the species occurs in more islands in the Andaman archipelago. We have conducted surveys in fifteen Nicobar Islands, but this species was not recorded in any of the islands in this group. As with several other endemic herpetofauna, the Ten Degree Channel appears to be a barrier for dispersal of this species into the Nicobar Islands.
TABLE 1. Morphometrics of the type series of Coryphophylax brevicaudus sp. nov. All measurements are expressed in
| ZSIC 25962 (Holotype) | ZSIC 25963 (Paratype) | ZSIC 25964 (Paratype) | |
|---|---|---|---|
| Male/Female | Male | Female | Male |
| SVL | 63.6 | 55.45 | 63.72 |
| TaL | 124 | 117 | 119 |
| TaH | 6.7 | 4.86 | 7.3 |
| TaW | 5. 98 | 4.4 | 5 |
| HL | 19.3 | 16.19 | 18.33 |
| HW | 11.67 | 9.5 | 10.86 |
| HD | 9.91 | 8.93 | 9.57 |
| SL | 8.58 | 6.62 | 8.01 |
| JL | 20.74 | 17.3 | 19.67 |
| OD | 6.31 | 6.09 | 6.44 |
| TD | 2.78 | 2.19 | 2.75 |
| TrL | 26 | 25 | 29.19 |
| TrW | 13 | 12.35 | 11.26 |
| TrH | 12.95 | 11.14 | 10.59 |
| Hum | 15.18 | 12.63 | 14.39 |
| Rad | 13.2 | 11.2 | 12.9 |
| F 1 | 2.46 | 2.72 | 2.4 |
| F 2 | 4.48 | 4.61 | 3.35 |
| F 3 | 7.6 | 6.69 | 6.91 |
| F 4 | 7.76 | 6.95 | 6.91 |
| F 5 | 4.8 | 3.57 | 4.87 |
| Fem | 19.15 | 14.93 | 18.67 |
| Tib | 20.65 | 17.79 | 19.71 |
| T 1 | 3.48 | 2.14 | 2.78 |
| T 2 | 4.67 | 4.17 | 4.0 7 |
| T 3 | 9.05 | 7.1 | 8.17 |
| T 4 | 13.98 | 13.83 | 12.8 |
| T 5 | 7.88 | 6.55 | 7.15 |
| ZSIC |
Zoological Survey of India |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.