Rallocytus ridiculus, Grischenko & Gordon & Melnik, 2018
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4484.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:D66524CF-9C6D-4DF4-8CA2-B2C9708CF5FD |
|
DOI |
https://doi.org/10.5281/zenodo.5989833 |
|
persistent identifier |
https://treatment.plazi.org/id/521587E4-560D-5544-09EE-FB41884FF9F3 |
|
treatment provided by |
Plazi |
|
scientific name |
Rallocytus ridiculus |
| status |
sp. nov. |
Rallocytus ridiculus n. sp.
( Figs 42–44 View FIGURE 42 View FIGURE 43 View FIGURE 44 , 54 View FIGURE 54 )
Material examined. Holotype: ZIRAS 1/50723, colony detached from nodule, YMG R.V. Yuzhmorgeologiya cruise YMG4–06, Stn 79, 16 August 2006, 13.23973° N, 134.39873° W, 4810 m. Paratype 1: ZIRAS 2/50724, colony detached from nodule, YMG R.V. Yuzhmorgeologiya cruise YMG4–07, Stn 130, 4 August 2007, 13.22832° N, 134.56370° W, 4830 m. Paratype 2: NIWA 127726, colony detached from nodule, YMG R.V. Yuzhmorgeologiya cruise YMG4–14, Stn 359, 19 January 2016, 14.08687° N, 131.78558° W, 5122 m. Additional material: YMG18–01, Stns 6, 31, 34; YMG4–06, Stn 83; YMG4–07, Stn 116; GLD4–08, Stns 155, 159; GLD4–09, Stns 173, 183, 196; GLD4–11, Stns 206, 223; GLD4–12, Stn 262; YMG4–13, Stn 283; YMG4–14, Stns 323, 328, 338, 340. Total specimens examined 23, 18 with dimorphic orifices.
Etymology. Latin, ridiculus , absurd, alluding to the dimorphic zooid(s), which can sometimes include/ comprise the ancestrular zooid.
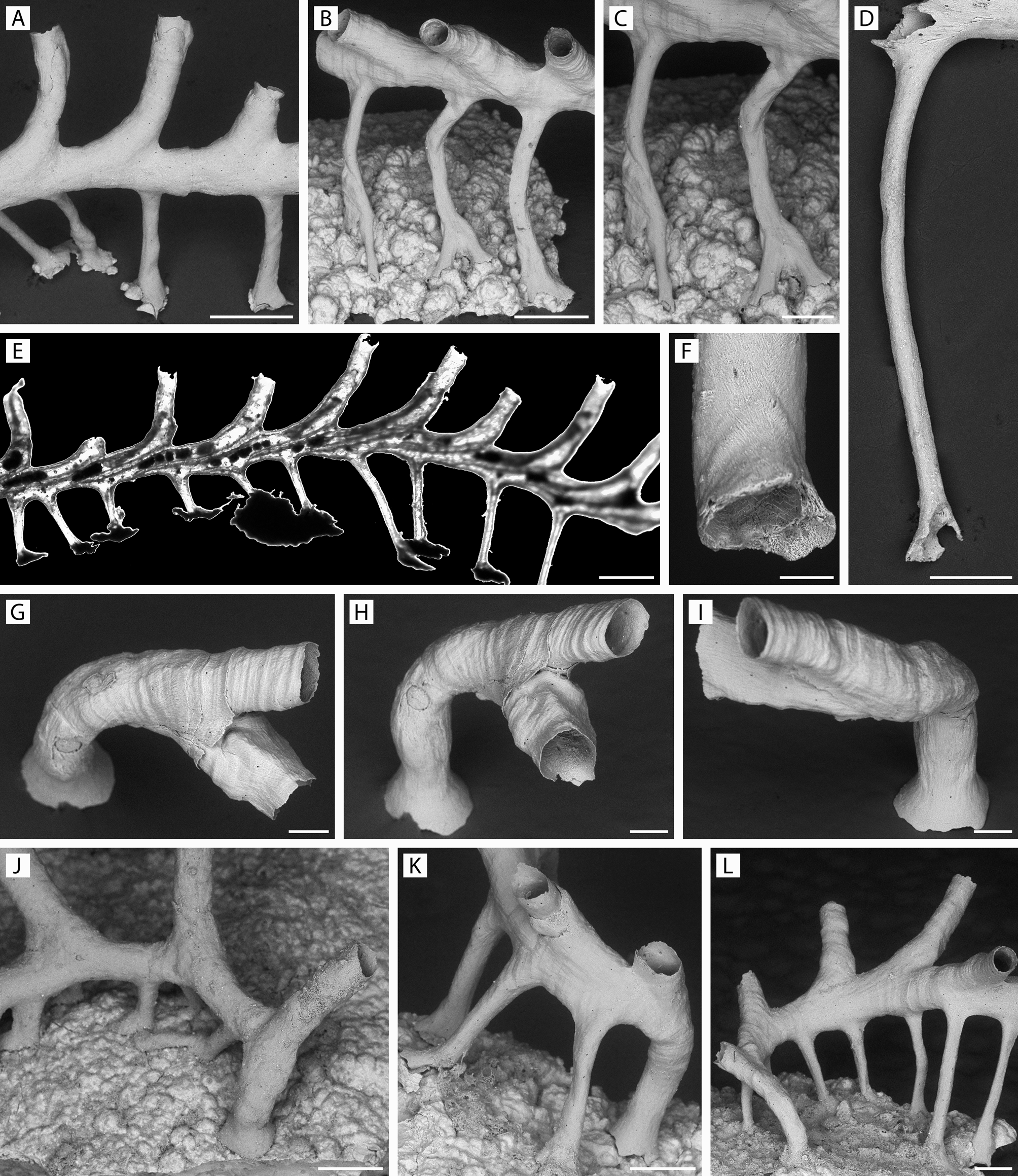
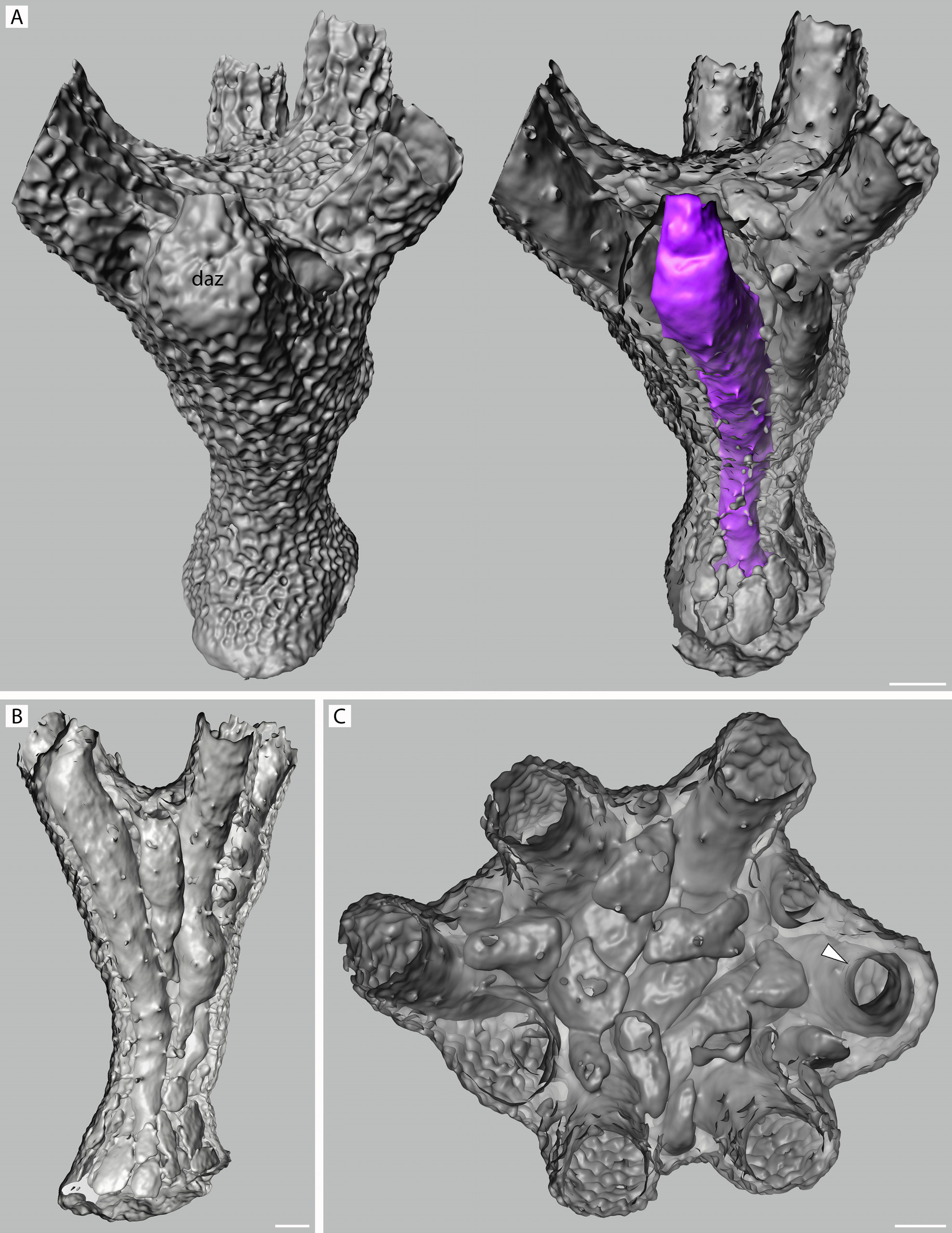
Description. Colony erect, pedunculate, with autozooidal peristomes radiating outwards around periphery of capitulum; maximum of nine autozooidal peristomes and one dimorphic peristome ( Fig. 42A–D View FIGURE 42 ), or seven autozooidal peristomes and two dimorphic peristomes ( Fig. 43A–C View FIGURE 43 ), in single uneven whorl. Column relatively short, flaring proximally to skirt-like base ( Fig. 43C View FIGURE 43 ), distally to wider capitulum. Entire colony surface strongly malleate, each dimple surrounded by distinct angular ridge ( Figs 42F, H View FIGURE 42 , 43D–H View FIGURE 43 , 54A View FIGURE 54 ); pores simple, sparse, more evident in CT scans ( Fig. 54B View FIGURE 54 ). Surface ultrastructure generally of imbricated platy crystallites ( Fig. 43I View FIGURE 43 ), in places irregularly arranged and lacking preferred growth direction. Capitulum broadly and shallowly calyciform, calyx center with few to several shallow alveoli and other smaller such openings at surface ( Figs 42A, B View FIGURE 42 , 43A View FIGURE 43 ); CT scans indicate these appear as flattened chambers in optical section ( Fig. 54C View FIGURE 54 ).
Autozooidal peristomes in single subregular series, skeletal surface like that of column, with very sparse pores. Peristomial apertures mostly slightly irregular ( Figs 42A–D View FIGURE 42 , 43A–G View FIGURE 43 ). Inner surface of peristome tubes typically weakly corrugated, smooth to lightly textured, no pustules or spinules ( Figs 42G View FIGURE 42 , 43K View FIGURE 43 ).
Dimorphic peristomes 1–2 in calyx whorl, evident from their smaller apertures, chamber size as in ordinary zooids; dimorphism sometimes pertaining to ancestrular peristome ( Fig. 54A, B View FIGURE 54 ). Capacious gonozooid(s) not seen. Dimorphic apertures inferred to be female, facing frontalwards ( Fig. 42A, B, D–G View FIGURE 42 ) or inclined towards calyx center ( Fig. 43A–G View FIGURE 43 ; one of the two such apertures). Dimorphic zooids seen as early as four-zooid stage colony ( Fig. 44E–L View FIGURE 44 ) or lacking in young colony ( Fig. 44M–P View FIGURE 44 ).
Ancestrula not seen in isolation, but obviously erect judging from smallest (three-zooid) colony stage seen ( Fig. 44A–D View FIGURE 44 ), which lacks expanded base; CT scans confirm that ancestrular peristome originates from center of dome-like protoecium ( Fig. 54 View FIGURE 54 , right image). First daughter zooid budded from ancestrular peristome soon after development to achieve two-zooid colony; third zooid develops between these ( Fig. 44A View FIGURE 44 ). Medium-sized colonies with central space ( Fig. 44E View FIGURE 44 ) filling with alveoli as capitulum expands radially ( Fig. 44E View FIGURE 44 , 54C View FIGURE 54 ).
Measurements (mm). Holotype, ZIRAS 1/50723 ( Fig. 42 View FIGURE 42 ): Colony height 1.24; capitulum 1.23 × 0.88; ZL 0.440–0.598 (0.524 ± 0.051) (n = 9); PeL 0.085–0.242 (0.167 ± 0.054) (n = 9); PeD 0.163–0.202 (0.185 ± 0.013) (n = 9); ApL 0.118–0.151 (0.136 ± 0.012) (n = 9); ApW 0.113–0.142 (0.126 ± 0.009) (n = 9). Dimorphic zooid (n = 1): ZL 0.553; PeL 0.218; PeD 0.213; OpL 0.083; OpW 0.092.
Remarks. Rallocytus ridiculus n. sp. is striking for its malleated surface texture (seen also in Calyssopora vasiformis n. sp.) and the dimorphic zooids within the calyx whorl.
There are significant implications attached to a potential female reproductive function for the dimorphic zooids. First, a CT scan of one mature colony in which a dimorphic peristome appears in the calyx whorl shows this zooid to be the ancestrula ( Fig. 54A–C View FIGURE 54 ). Can this mean that the dimorphic peristomes seen in several juvenile colonies (e.g. Fig. 44E–H, I–L View FIGURE 44 ) also pertain to the ancestrula? We cannot be sure; another mature colony had two dimorphic peristomes ( Fig. 43A–E View FIGURE 43 ), and either one, or neither, was ancestrular. But, if so, then it would seem to pose an energetic problem for early colony development, unless the dimorphic ancestrular zooid also has a feeding polypide, as seems likely. Alternatively (or additionally), the timing of budding of the first daughter zooid (from the ancestrular peristome, not the protoecium) may be such as to ensure that at least one feeding zooid is contemporaneous with a non-feeding ancestrula. A zooid pair in Fig. 44A View FIGURE 44 indicates simultaneity of development of the first two zooids (apparent autozooids, in this case).
Second, if a dimorphic peristome occurs in a juvenile colony (as in Fig. 44E–H, I–L View FIGURE 44 ), how is this peristome maintained as the zooid grows upwards? Or is the modified peristomial aperture shed and regrown during polypide recycling? Such a thing has never been reported. The fact that at least one cyclostome species in this fauna has multiple brown bodies (i.e. in Pandanipora helix ; Fig. 5E View FIGURE 5 ) shows that polypide recycling can happen in this stable environment, so perhaps peristomial shape might be labile. Indeed, conventional gonozooids begin as normal autozooids, modifying during further growth.
Third, the lack of a capacious incubation chamber in any of the numerous specimens of Rallocytus ridiculu s n. gen., n. sp. (and Anyuta anastema n. gen., n. sp.) suggests that embryonic cloning (polyembryony) might be secondarily suppressed (wholly or partly) in these taxa—the dimorphic zooid, being the same size as an ordinary autozooid, would have little volume for cloning (unlike in Cinctiporidae ). If the ancestrular zooid can produce eggs precociously in R. ridiculu s, then the apparent strategy could be that of reproducing one or a few larvae early and several times therafter, instead of producing a capacious gonozooid for the delayed mass production of embryos, either during a very narrow time period or, as in Filicrisia geniculata ( Jenkins et al. 2017) , over an extended period of time.
Distribution. Recorded from 21 stations within coordinates 12.65742– 14.41137° N, 129.08067– 134.56370° W, at depth range 4640–5213 m.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |