Simulium ( Inseliellum ) englundi, Craig, Douglas A., 2004
|
publication ID |
https://doi.org/10.5281/zenodo.157955 |
|
DOI |
https://doi.org/10.5281/zenodo.6270790 |
|
persistent identifier |
https://treatment.plazi.org/id/522E5305-9D3B-643B-FEBA-FA3FFD8FFD85 |
|
treatment provided by |
Plazi |
|
scientific name |
Simulium ( Inseliellum ) englundi |
| status |
sp. nov. |
Simulium ( Inseliellum) englundi View in CoL n. sp.
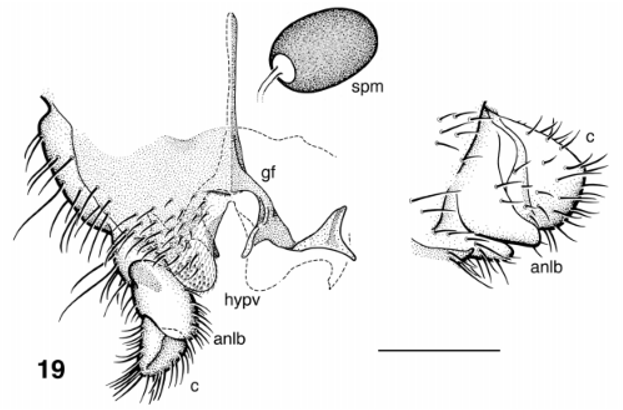
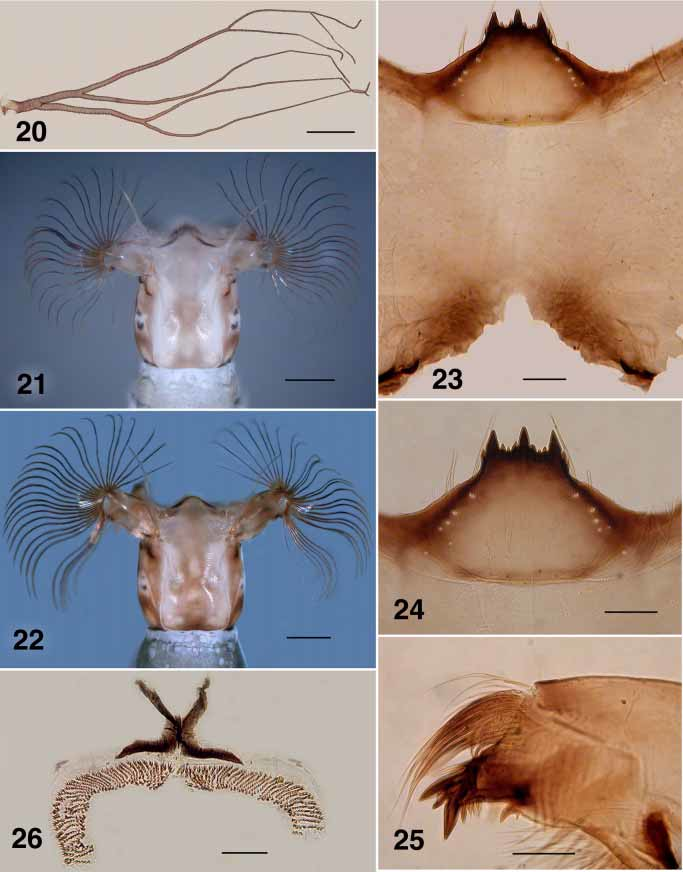
( Figs. 16–20 View FIGURES 16 – 18 View FIGURE 19 View FIGURE 20 , 22–26, 29).
Types
Holotype
Female: pinned. Label data—" Simulium ( Inseliellum) englundi . MARQUESAS , Mohotani (Motane) Island, alt. 378 m. S9° 58.80’ W138° 49.80’. 31viii2001. Coll. R. Englund, S. Jordan. HOLOTYPE. No. 16524" (BPBM). Head, mouthparts and genitalia cleared and in glycerine vial on pin. Thorax slightly damaged. Dried from alcohol via Peldri II®.
Paratypes
Larvae: Last and penultimate instars in alcohol. Label data as for Holotype, but with “ PARATYPE ” (BPBM).
Diagnosis
Adult female: markedly dark brown; vestiture of black hairs; maxillary palpus sensory vesicle poorly developed; posterolateral sclerite of anal fork markedly developed. Pupa: six gill filaments. Larva: head brown; labral fan stem markedly developed.
Description
Adult Female (colours based on specimen in alcohol). Body ( Fig. 16 View FIGURES 16 – 18 ): overall medium to dark brown; 1.92 mm in length. Head ( Fig. 17 View FIGURES 16 – 18 ): eyes, dark red; postocciput, vertex and clypeus evenly dark brownish black; width 0.57 mm, depth 0.44 mm. Eyes: interocular distance 0.05 mm; ommatidia 0.017 mm in diameter, ca. 28 and 30 respectively across and up eye in middle row. Frons: evenly dark brown, no apparent vestiture. Clypeus: slightly longer than wide, vestiture of sparse coarse black hairs. Postocular hairs: sparse, black, barely extended to eye margin. Antenna: total length 0.41 mm; evenly dark brown. Mouthparts: 0.3 length of head depth; mandible poorly sclerotized, as long as labrum, 4 times as long as wide, 19 inner teeth; lacinia with 20–22 teeth; maxillary palpus 0.57 mm long, proportional lengths of 3rd, 4th and 5th articles, 1:1:3.1; sensory vesicle occupying 0.25 width of third article (markedly small), opening 0.3 width of vesicle; third article lacking markedly angular anterodistal margin; cibarium shallowly Ushaped, smooth between substantial, angular, heavilypigmented proximal arms. Thorax: length 0.83 mm, width 1.2 mm; evenly dark brown; scutum lacking vittae, subshiny, vestiture of short black hairs; scutellum concolorous with scutum, apical angle of 90°, posterolateral edges slightly convex, vestiture of long coarse black hairs; postscutellum marginally lighter than scutellum, with darker medial region; pleuron evenly medium brown. Wing: length 1.7 mm, width 0.8 mm; veins generally very pale; stem vein hair tuft sparse. Halter: knob and pedicel pale. Legs ( Fig. 16 View FIGURES 16 – 18 ): coxae light brown; femora with main length yellow, darker distal region; tibiae darker; hind tibiae mainly yellow; tarsi dark; calcipala well developed ( Fig. 18 View FIGURES 16 – 18 ); pretarsal claw with well developed basal tooth. Abdomen: overall dark gray with vestiture of fine black hairs; basal fringe of sparse black hairs extended to second abdominal segment; tergites marginally lighter than tergum; posterior tergites subshiny; sternum evenly gray, except for segment VIII which is concolorous with tergum. Genitalia ( Fig. 19 View FIGURE 19 ): cercus in lateral view flattened dorsally, convex ventrally; anal lobe extended only to base of cercus, with hairs ventromedially; hypogynial valves distinctly rounded apically, extended 0.3 length of anal lobe, with sparse vestiture of short hairs and trifid microtrichia laterally; median space extended anteriorly to branch of genital fork, narrowly rounded anteriorly, slightly wider at middepth, pigmented area at middepth; stem of genital fork narrow, with accessory fluted lobe posteromedially; posterolateral triangular sclerite markedly enlarged, with medial ridge. Spermatheca: ovoid, dark brown, pattern and internal hairs absent, distinct clear area at spermathecal duct junction.
Adult Male (Unknown)
Pupa (based on pharate gill histoblasts)
Gill ( Fig. 20 View FIGURE 20 ): six fine filaments (3+1+2); 1.8 mm maximum length; filaments subequal in length.
Larva (Based on four last instar larvae with dark pharate pupal gills).
Body: evenly medium gray; total length 4.7–4.9 mm. Head (Fig. 22): colour overall light brown; width 0.54 mm, length 0.58 mm; distance between fan stem bases 0.35 mm; lateral margins slightly convex, more so posteriorly; frontoclypeal apotome pale brown anteriorly, darker posteromedially with marked figure 8shaped pattern produced by negative head spots, cuticle lateral of ecdysial line brown, lateral pigmentation medium brown; cervical sclerites small but distinct, not fused to postocciput. Antenna: essentially unpigmented; total length 0.51 mm; distal article 0.17 mm in length, complete article extended well beyond apex of labral fan stem. Labral fans: stem markedly elongated (cf. Figs. 21, 22); 27–31 rays, medial rays 1.06 mm. in length; width 0.01 mm; microtrichia 1.3 times longer than ray width, longer microtrichia with 4–5 smaller ones decreased slightly in size to next long one. Postgenal bridge (Fig. 23): two times longer than cleft depth; colour pale. Postgenal cleft: shallow Vshaped, two times as wide as deep, with small anterior extension. Hypostoma (Fig. 24): 12–13 teeth; median tooth prominent, just extended beyond lateral teeth; 3 sublateral teeth; 1–2 paralateral teeth; 2 very small lateral serrations; 4–5 hypostomal setae per side. Mandible (Fig. 25): apical teeth well developed; seven spinous teeth; distance between spinous teeth and serration marked; serration longer than basal width, curved; sensillum distinct; cuticle posterior to sensillum smooth and convex. Maxilla: tapered; palpus 2.5 times as long as basal width. Mandibular phragma: extended ventrally to 0.3 depth of maxilla base. Abdomen: anteriorly concolourous gray with thorax, expanded posterior segments browner; segments I–IV subequal in size to thorax, expanded gradually at segment V, and further to segment VII, then decreased gradually (elongated amphorashape); posteroventral tubercles small, but distinct; posterodorsal cuticle with no obvious cuticular pattern. Anal sclerite (Fig. 26): posteroventral arms subequal in length to dorsolateral arms, well separated from lateral accessory sclerites. Posterior proleg circlet of hooks: with 71 rows of hooks, 12–14 hooks per row. Rectal papillae: simple.
Etymology
Named after R. Englund, who collected the material.
Comments
Simulium englundi larvae are similar in many characters to those of S. gallinum (Fig. 21) and S. hukaense , but differ in darker head coloration, postgenal cleft shape, more pronounced hypostomal teeth and longer labral fan stem. The female differs markedly from that of S. gallinum (Hiva Oa) in darker colour and vestiture and the lateroposterior triangular sclerite of the genital fork which is markedly enlarged. The pupa has six gill filaments of pattern similar to that in S. hukaense . With poorly sclerotized mandibles and undeveloped maxillary palpus sensory vesicle, it is unlikely that females of S. englundi blood feed.
Mohotani, 15 km directly south of Hiva Oa, is a small ( 12.2 km 2) volcanic island with coastal cliffs and a gentle sloping interior, that reaches an altitude of 520 m. The type locality of S. englundi is a small stream on the northern side of the island, starting as a trickle at 390 m altitude and is only some 400 m in length. The only permanent running water on the island, the stream consists of small cascades 3–4 m in height ( Fig. 29 View FIGURES 27 – 29 ), interspersed with pools. As typical for Marquesan simuliid larvae (Craig 2001), those of S. englundi were taken off leaves only.
Mohotani has feral sheep and the undergrowth is severely overgrazed causing considerable erosion. Craig (2003) discussed conservation of Polynesian simuliids, noting that most running water in Polynesia has been impacted by humans in one manner or another. He concluded, however, that on the larger islands (e.g., Tahiti) that there were still pristine habitats available for simuliids. But, for Mohotani, S. englundi , might be considered an endangered species, since there is only the one small stream.
Discussion
The majority of new species of Tahitian Inseliellum described by Craig (1997) and Craig and Joy (2000) have been from higher altitude localities and so are the two new species described herein. Noticeable also is that the new species have characteristics that tend to bridge the limits of previous established species groups. Perhaps this should be expected since the fauna is young and apparently rapidly evolving ( Craig 2003, Joy et al. 2004) and the basal progenitor species are still extant ( Craig and Currie 1999, Craig et al. 2001). As noted by Craig (1997), it is becoming more apparent that there are seasonal differences in species composition from a given stream, particularly those of higher altitude. The S. sublonckei type locality has been collected extensively; however, typically during the Austral winter. The most recent collection was in the Austral summer and S. sublonckei larvae were markedly more numerous, while those of some species such as S. fararae , collected from that site during winter, were absent.
The occurrence of an endemic species of simuliid ( S. englundi ) on such a small island as Mohotani is unexpected, given that the distance between that island and Hiva Oa is only 15 km. MacArthur and Wilson (1963) hypothesized that adaptive radiation within an island would be a major contributor to species richness, and that this may increase on islands of major distance from the source. Their rationale was that colonization to a far island, although rare, might allow colonists to be less influenced by gene flow from the source populations which would hinder speciation. For example, Moorea, presumably close enough ( 21 km) to Tahiti to be influenced by gene flow from Tahitian species, has not undergone an intraisland species radiation, and while possess 10 species, has only one poorly known endemic species ( Craig and Joy 2000). Raiatea, the second largest of the Society Islands and well removed ( 200 km) from Tahiti, has had a moderate intraisland species radiation (4 endemic species), but was close enough to Tahiti for some colonization (3 species) ( Craig 2003, Spironello and Brooks 2003).
Geologically the average age of Mohotani (2.15 My) is little different from that of Tahuata (1.91 My), merely 23 km to the west, or the larger island of Hiva Oa (1.91 My) just to the north (15 and 5 km respectively) of both of them ( Clouard and Bonneville 2001). Simulium gallinum is widespread in the Marquesas ( Craig et al. 1995) and is assumed to be basal to other Marquesan species ( Craig and Currie 1999, Craig et al. 2001), so without further information, it is not unreasonable to assume that S. englundi diverged from a S. gallinum precursor after the latter had arrived at this group of islands. Speciation appears to have occurred rapidly on Mohotani, since this small island, apart from possessing an endemic simuliid species, has other endemic biota. Ixora marquesas (Rubiaceae) is endemic to Mohotani, with Lebrannecia kakiodes ( Malvaceae ) endemic to Mohotani and Tahuata. Further, Mohotani has two endemic subspecies of birds: Pomarea medozae montanensis (Flycatcher) and Acrocephalus caffer consobrinus (Polynesian Warbler) .
Does this endemism on such a small island, close to a larger one, indicate that interisland gene flow for Marquesas simuliids is lower than that apparent for those of the Society Islands (Craig et al. 2001)? As noted by Craig (2003), there is still much to be learned about the Polynesian Simuliidae . In particular, the Marquesas simuliids are in need of cytological and molecular scrutiny to compliment the preliminary morphologicalbased study by Craig et al. (1995).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
|
SubGenus |
Inseliellum |