Ramazzottius subanomalus ( Biserov, 1985 )
|
publication ID |
https://doi.org/10.11646/zootaxa.4300.3.4 |
|
publication LSID |
lsid:zoobank.org:pub:59A51737-B46B-4094-84CC-565C01D01FAD |
|
DOI |
https://doi.org/10.5281/zenodo.6018180 |
|
persistent identifier |
https://treatment.plazi.org/id/566E2528-3D52-FFC7-FF1F-FD2BFD4E2110 |
|
treatment provided by |
Plazi |
|
scientific name |
Ramazzottius subanomalus ( Biserov, 1985 ) |
| status |
|
Ramazzottius subanomalus ( Biserov, 1985) View in CoL
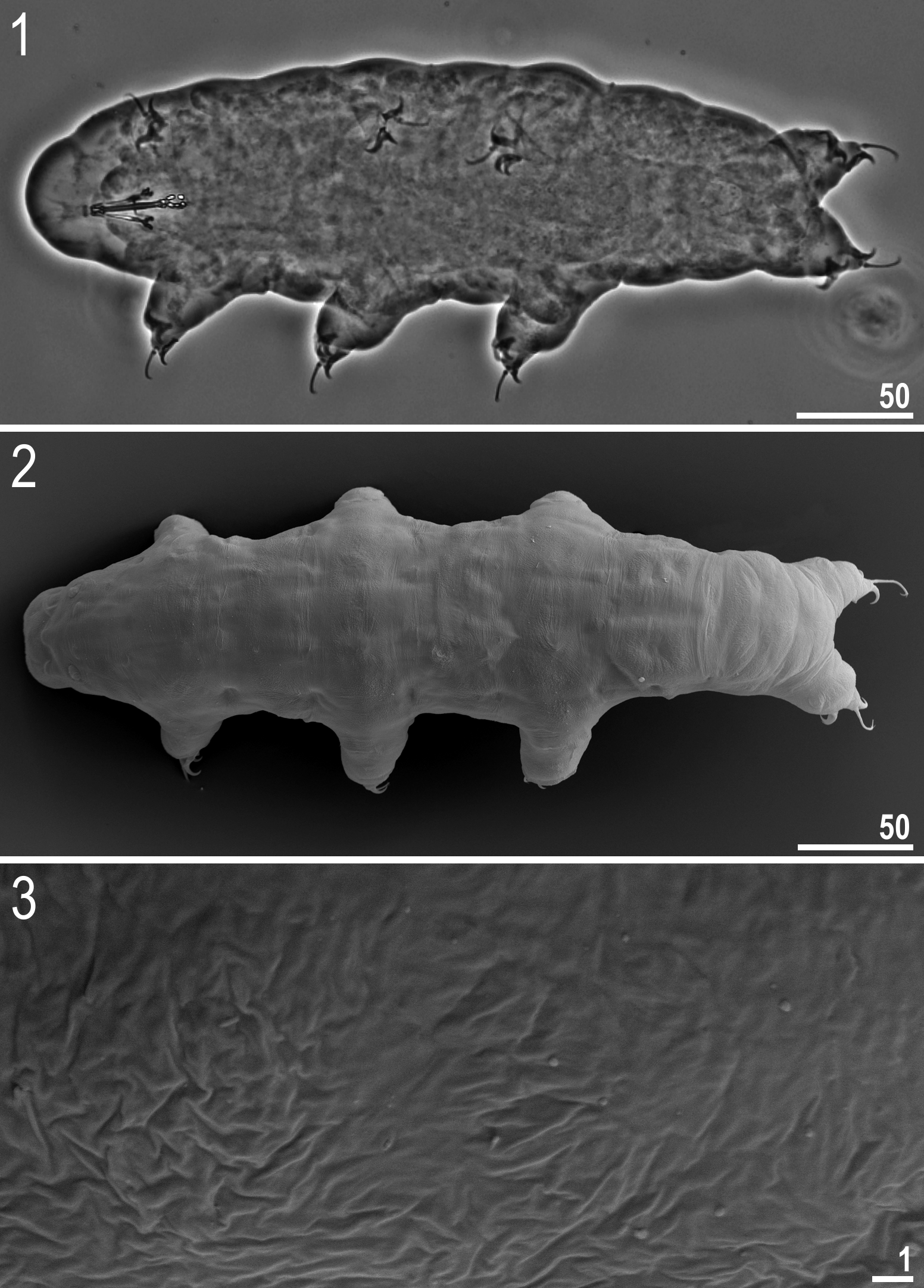
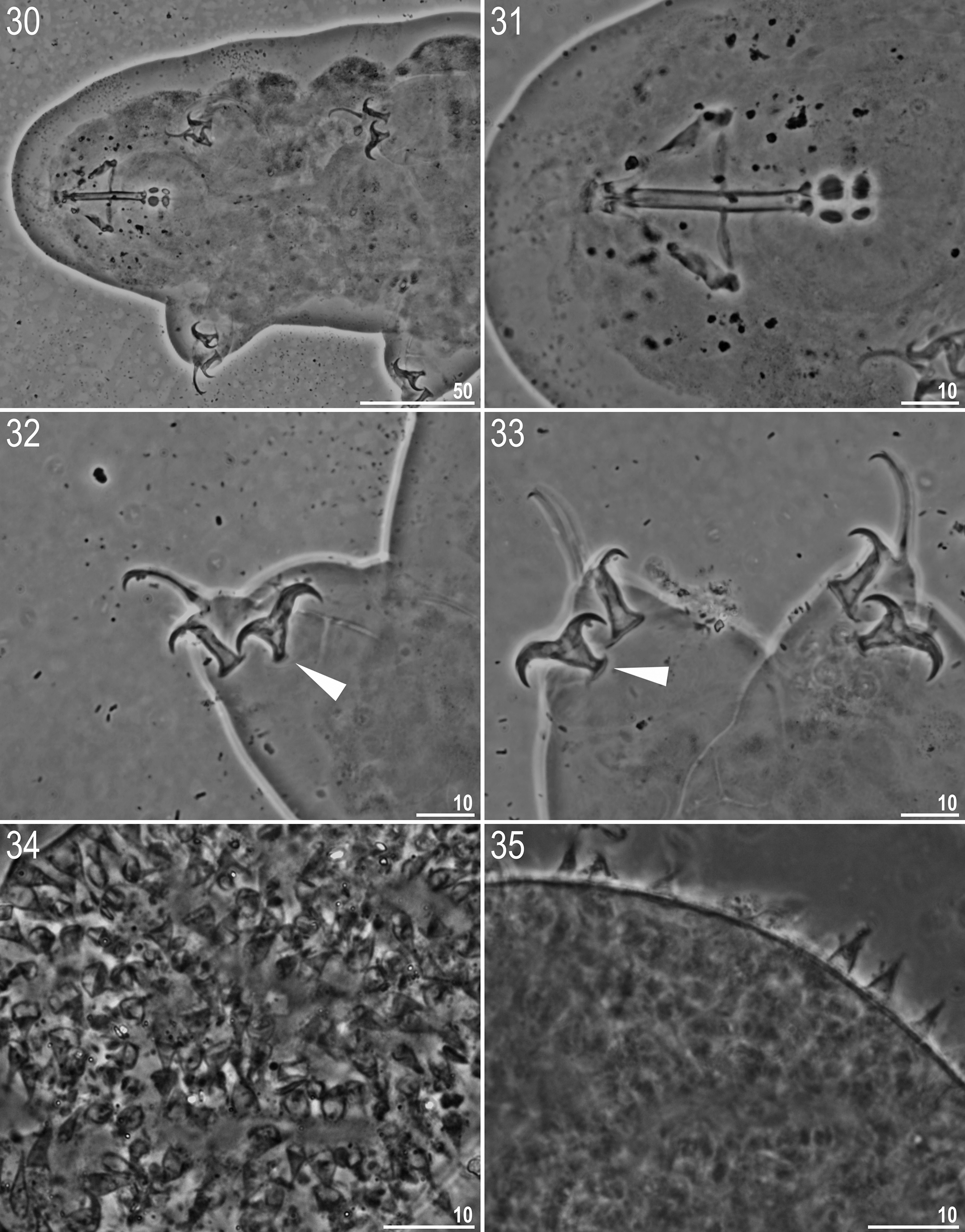
Locus typicus: Yango-Asker, Astrakhanskaya Oblast , Russia ( Tables 4–5, Figures 1–33 View FIGURES 1 – 3 View FIGURES 4 – 7 View FIGURES 8 – 10 View FIGURES 11 – 12 View FIGURES 13 – 15 View FIGURES 16 – 29 View FIGURES 30 – 35 )
Description. Animals (morphometrics in Table 4): Pigmentation of the cuticle from light-red to red-brown chequered pattern on the dorsum. Cuticle smooth (without sculpture, granulation or gibbosities) ( Figs 1–3 View FIGURES 1 – 3 ). Eyes absent in live animals. Two elliptical organs on head only clearly visible under SEM ( Fig. 2 View FIGURES 1 – 3 ).
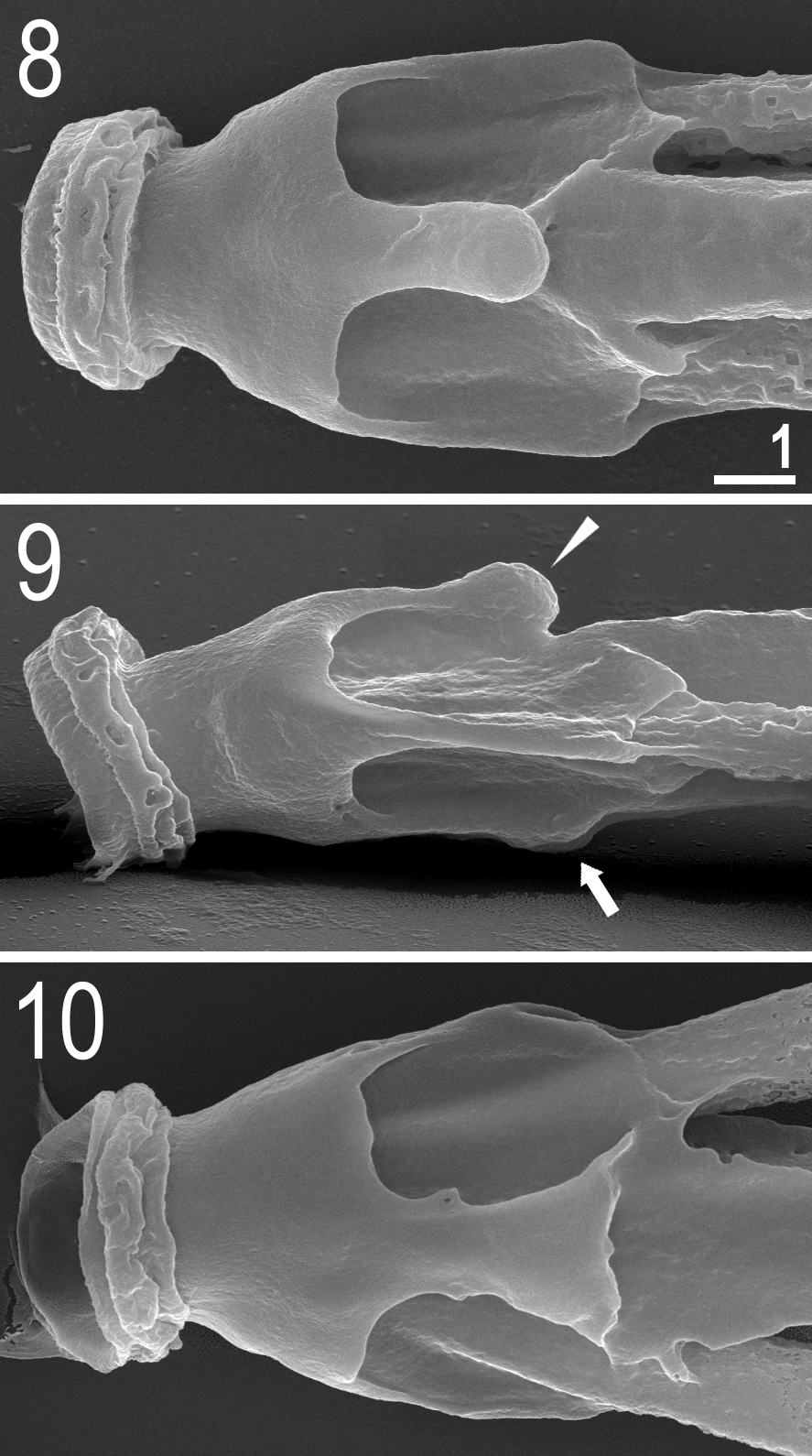
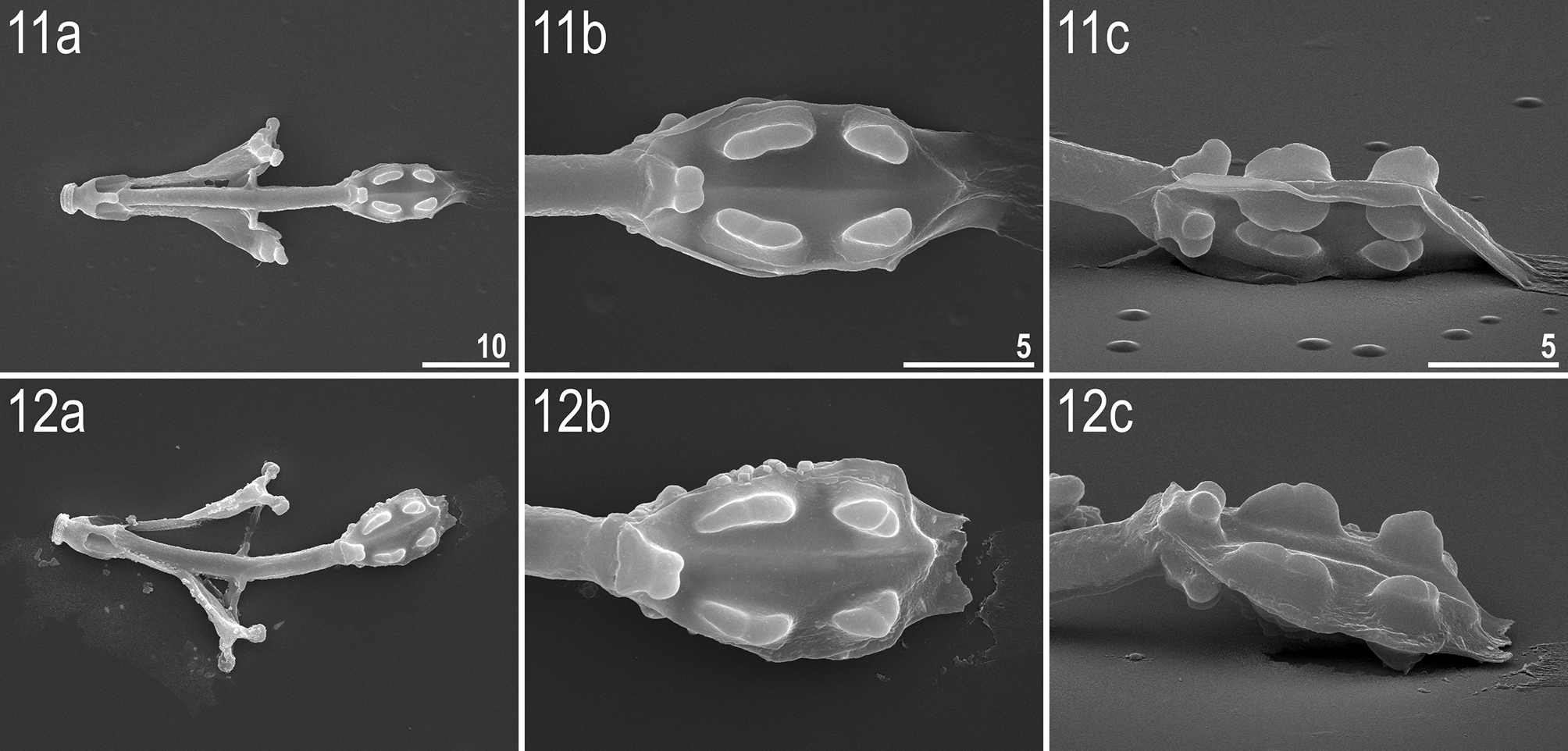
Bucco-pharyngeal apparatus of the Ramazzottius -type ( Figs 4–12 View FIGURES 4 – 7 View FIGURES 8 – 10 View FIGURES 11 – 12 ). Mouth opening antero-ventral. Oral cavity armature only partially visible under PCM, clearly visible under SEM. The armature composed of two bands of teeth, both located in the posterior oral cavity ( Figs 4–5 View FIGURES 4 – 7 ). The first band of teeth absent. The second band composed of very small cone-shaped teeth arranged in 3–5 irregular rows, located on the ring fold ( Fig. 5 View FIGURES 4 – 7 , arrowhead). The third band composed of a single row of ten large and regularly spaced cone-shaped teeth located just behind the second band of teeth ( Fig. 5 View FIGURES 4 – 7 , arrow). The second band of teeth not visible under PCM, the third band barely detectable under PCM in only some specimens. Apophyses for the insertion of stylet muscles (AISM) in the shape of blunt hooks and asymmetrical in size and shape with respect to the frontal plane ( Figs 8–10 View FIGURES 8 – 10 ). Stylet furcae with rounded ends ( Fig. 7 View FIGURES 4 – 7 ). Buccal tube with a posterior bend and thickened walls posteriorly from the stylet support insertion point. Pharyngeal bulb (bulbus) almost oval, with apophyses and two clearly separated macroplacoids. Pharyngeal apophyses triangular, smaller than macroplacoids. First macroplacoid slightly elongated, second roundish, but the size and shape of placoids exhibit considerable variation; particularly obvious under SEM ( Figs 11 View FIGURES 11 – 12 a,b,c–12a,b,c). Macroplacoids appear without constrictions under PCM, but slight constrictions in both macroplacoids are visible in the majority of buccal apparatuses observed under SEM ( Figs 6 View FIGURES 4 – 7 , 11 View FIGURES 11 – 12 a,b,c–12a,b,c). Macroplacoid length configuration 2<1. Microplacoid and septulum absent ( Figs 6 View FIGURES 4 – 7 , 11–12 View FIGURES 11 – 12 ).
Claws of the Ramazzottius - type. Primary branches of external and posterior claws long and thin. Internal and anterior claws much smaller and of a different shape than external claws ( Fig. 13–15 View FIGURES 13 – 15 ). Base of external and posterior claws distinctly enlarged, internal and anterior claws with small and smooth pseudolunules. Accessory points on primary branches of all claws, present. Bars and other cuticular thickenings on legs, absent.
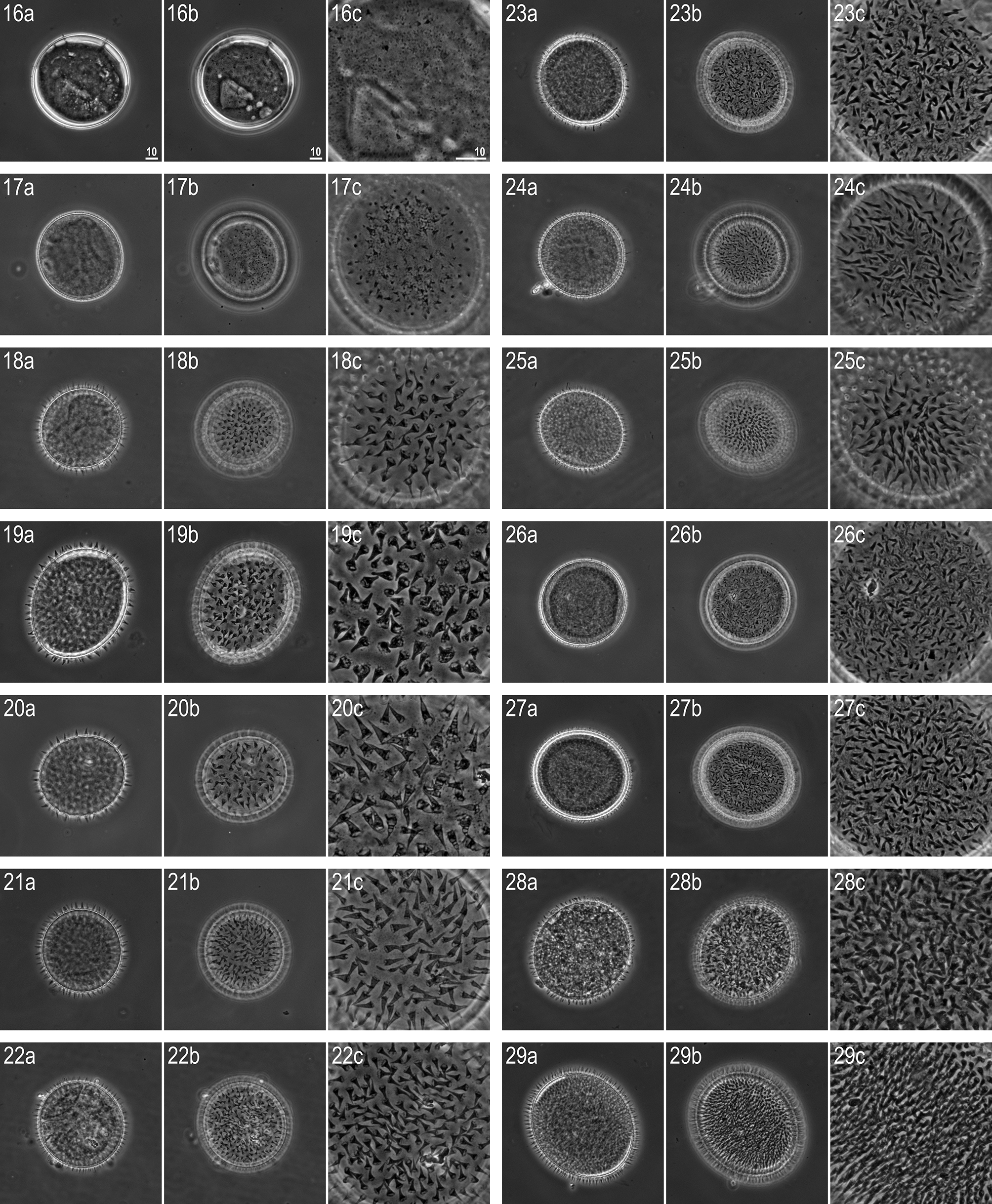
Eggs (morphometrics in Table 5): Laid freely, white and spherical, covered with numerous small, thorn-, spine- or filament-shaped processes ( Figs 16–29 View FIGURES 16 – 29 ). Always triangular in shape, processes exhibit extreme diversity in size (both height and base width), morphology (e.g. single examples exhibiting transverse septa), as well as in their distribution on the egg surface ( Figs 16–29 View FIGURES 16 – 29 ). The greatest variability was observed between eggs, however exceptional variability can also sometimes be observed within an individual egg. There is also a considerable variability in process numbers on the egg surface—from fully covered to almost bald eggs with sparse and poorly developed processes. Egg processes and surface between processes smooth ( Figs 16–29 View FIGURES 16 – 29 ).
DNA: Obtained sequences were identical across the three analysed individuals (i.e. only a single haplotype was found).
The obtained COI sequence (GenBank: MF001999 View Materials ) is 822 bp long and it encompasses a longer fragment than the 358 bp long COI sequence provided in Stec et al. 2016a (i.e. KU900021 View Materials ):
TATATGGAACATTATATTTTATTTTCGGCATTTGAGCCGCCACTGTGGGCACCTCCTTAAGAATAATTATTCGATC AGAATTAAGAGAACCCGGATCATTGTTCGCGGAAGAACAACTATACAATGTAACAGTAACTAGACACGCTTTCATT ATGATTTTTTTCTTTGTTATACCCATCCTGATTGGAGGTTTTGGAAATTGATTAGTGCCTCTTATGGTTGGCGCGC CGGATATAGCTTTCCCCCGAATAAATAACTTAAGATTTTGACTACTACCCCCGTCATTTTTATTAATTTCTACAAG CACTATGAGAGAGCAGGGCGCAGGCACCGGATGAACAGTATACCCACCCCTCTCAAACTATTTCGCACATAGGGGA CCTGCCGTAGATTTAACTATTTTTTCTTTACATATTGCAGGTGTTTCCTCTATTTTAGGGGCCATTAATTTTATTT CCACAATTATAAATATACGAACCCCGTCAGTCAGATTAGAGAACATATCTCTCTTCGTTTGATCAGTCCTAATTAC TGCAGTATTATTACTTTTAGCGCTCCCAGTGCTCGCAGGAGCGATTACAATACTACTTCTAGATCGAAATTTTAAT ACCTCATTTTTTGATCCGGCAGGAGGTGGAGATCCGATTCTTTACCAACACCTGTTTTGATTTTTCGGACATCCAG AAGTTTACATTCTTATTTTGCCTGGTTTCGGTTTAGTGTCACAAATTATTGTCCACTACAGAGGGAAACACCTTAC GTTTGGACACTTAGGAATAATTTATGCTATAAGAACTATCGGTTTATTGGGGTTCATCGTTT
The obtained 28S rRNA sequence (GenBank: MF001998 View Materials ) is 787 bp long:
TACTAAGCGGAGGAAAAGAAACCAACGGGGATTCCCATAGTAACTGCGAGTGAAAGGGGAAGAGCCCAGCGCCGAA TCCTACTGCTGGCAACGGTGGTAGGAACTGTGGCGTGAAGACAGTCTATTCCAGTGCGGCTAGCTTGCGTAAGTTC TCCTGAGTGAGGCTCCATCCCATGGAGGGTGCAAGGCCCGTATCGTAAGCAGCTGGTGCTGGTATCAGCTGTCGGA GAGTCGCCTTGTTTGCGAGTACAAGGTGAAGTCGGTGGTAAACTCCATCGAAGGCTAAATATGACCACGAGTCCGA TAGCGAACAAGTACCGTGAGGGAAAATTGAAAAGCACTTTGAAGAGAGAGCGAAACAGTGCGTGAAACCGCTTAGA GGCAAGCAGATGGGGCCTCGAAGGCAAAGCAGTGAATTCAGCTGGTAGTCCGTAGTGCCGGCCTGCATTACAGATC GCAAGACTGTGGCAGGTTGTGAGGCTGCGGCTGCTAGTGCACTTTCACTGTTTGTACGCCACCGCCGTTGAGCGAG CGTCCGTCTAGCTGGCGTGTGAAGCCTTGTTCCCTTTACGGGGTTACAGGTGTCTTACTGCCGGTCACGACGCGTT CGCACCTCAACCGGTCATGTCAGCGTGTGCCAGCGTATTGCGTTGGGCTCGTTCACCCTGGTGTGCGTCGGAGATG ACAAGCTCGCTTGGCTCACTGGCGTATTGCCTGGGAATGGGCGGGTTTGCAACGTAGGCACATTGTCGATTCGGTG GCGAGTAGACGGCTGCCCATCTAACCC
The obtained 18S rRNA sequence (GenBank: MF001997 View Materials ) is 1034 bp long:
AGATCGTACAGTTTACATGGATAACTGTGGTAATTCTAGAGCTAATACATGCATTCAGCTCGCTCTCTCGGGAGCG AGCGCAGTTATTAGAATAAAACCAATCCGGCCTTCGGGTCGGTAAAATTGGTGACTCTGAATAACCGAAGCGGAGC GCATGGTCTCGTACCGGCGCCAGATCTTTCAAGTGTCTGACTTATCAGCTCGTAGGTAGGTTATGTGCCTACCTAG GCTCTTACGGGTAACGGGGTGTCAGGGCCCGACACCGGAGAGGGAGCCTGAGAAACGGCTACCACATCCAAGGAAG GCAGCAGGCGCGCAAATTACCCACTCCCGGCACGGGGAGGTAGTGACGAAAAATAACGATGCGAGAGCTTTTAGCT TTTCGTAATCGGAATGGGTACACTTTAAATCCTTTAACGAGGATCTATTGGAGGGCAAGTCTGGTGCCAGCAGCCG CGGTAATTCCAGCTCCAATAGCGTATATTAAAGTTGCTGCGGTTAAAAAGCTCGTAGTTGGATCTGGGTTGTTCGA GCGAGCGGTGCGTCTTCACGGCGTAACTGTTTGTTCGGCACCACAGCCCGGTTATGTCTTGCATGCCCTTCACTGG GTGTGCTTGGCGACCGGAACGTTTACTTTGAAAAAATTAGAGTGCTCAAAGCAGGCGTATGGCCTTGCATAATGGT GCATGGAATAATAGAATAGGACCTCGGTTCTATTTTGTTGGTTTTCGGAGCTCGAGGTAATGATTAATAGGAACAG ACGGGGGCATTCGTATTGCGGCGTTAGAGGTGAAATTCTTGGATCGTCGCAAGACGCACTACTGCGAAAGCATTTG CCAAGAATGTTTTCATTAATCAAGAACGAAAGTTAGAGGTTCGAAGGCGATCAGATACCGCCCTAGTTCTAACCAT AAACGATGCCAACCAGCGATCCGTCGGTGTTTGTTTTATGACTCGACGGGCAGCTTCCGGGAAACCAAAGTGTTTA GGTTCCGGGGGAAGTATGGTTGCAAAGCTGAAACTTAAAGAATGAC
See also Stec et al. (2016a) for the sequences of the two known ITS-2 haplotypes ( KU900019 View Materials –20).
CHARACTER R. anomalus View in CoL R. subanomalus R. subanomalus View in CoL ( holotype) ( holotype) (a Polish specimen) Intraspecific variability in morphology and morphometry. In the same population of R. subanomalus View in CoL as used for this study, Stec et al. (2016a) analysed intra-specific variability in the standard morphometric traits of both animals and eggs in relation to the genetic variation uncovered. However, Stec et al. (2016a) concentrated on analysing the determinants of eggshell variability, and thus their study lacked a comparison of the recorded variability with that described by Biserov (1985) in the original description of R. subanomalus View in CoL . Compared to the type series (measurements by Biserov 1985 provided in Table 6 and new photomicrographs in Figs 30–35 View FIGURES 30 – 35 ), our Polish population of R. subanomalus View in CoL exhibited a wider range for several morphometric traits, e.g. macroplacoid I length (2.3–4.5 µm in the type population vs. 3.8–6.0 µm in the Polish population), egg process height (3.0–6.6 µm in the type population vs. 1.5–12.5 µm in the Polish population), egg process base diameter (2.0–4.5 µm in the type population vs. 0.5–9.9 µm in the Polish population). Thus, the most striking variability in R. subanomalus View in CoL was observed not in the animals, but in the number, size and shape of the egg processes ( Figs 16–29 View FIGURES 16 – 29 ; see also Stec et al. 2016a). Biserov (1985) described R. subanomalus View in CoL eggs as covered with cone-shaped processes of similar size, but our observations considerably widen the range of chorion morphology in this species. We suspect that if, for example, eggs shown in Figs 16, 20, 26 and 29 View FIGURES 16 – 29 were found in different samples, it is very likely that they would be classified as four different species. Even though egg morphology is considered to provide key taxonomic traits for the differentiation of many eutardigrade taxa, including the genus Ramazzottius View in CoL , the evidence presented in this paper plus that provided by Biserov (1996) for Ramazzottius montivagus ( Dastych, 1983) View in CoL and in Biserov (1997/8) for Ramazzottius caucasicus Biserov, 1998 View in CoL and Ramazzottius ljudmilae Biserov, 1998 View in CoL , suggest egg morphology has a limited value for the differentiation of at least some Ramazzottius View in CoL species. Since the taxonomy of Ramazzottius View in CoL was based solely on alpha taxonomy (i.e. morphology and morphometrics), there is a problem even if the stability of egg traits is assumed. Our study, therefore, underlines the importance of providing an integrated analysis of species, combining classic taxonomy with the molecular approach, to delineate the species in this genus (see also Stec et al. 2016a).
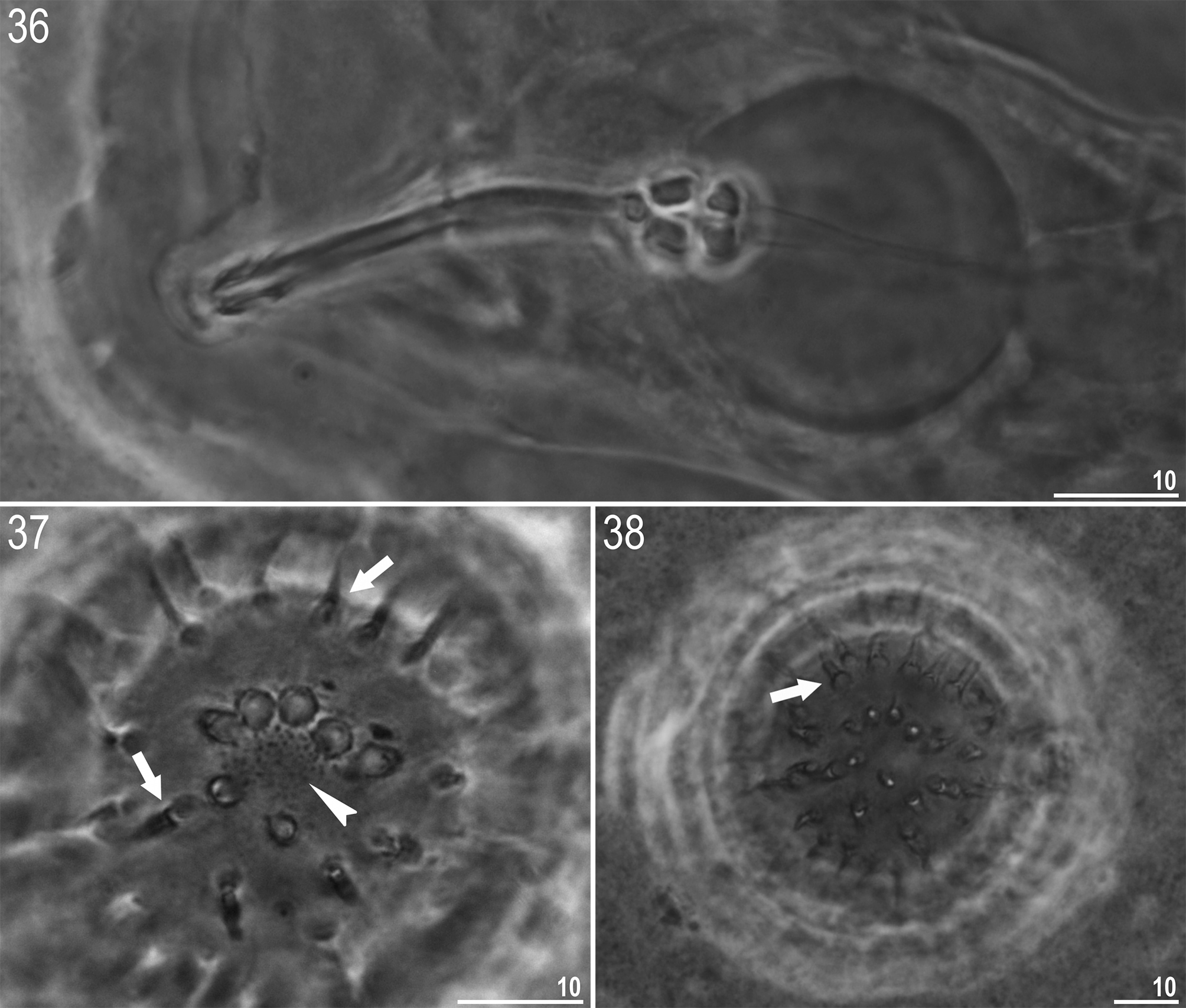
R. subanomalus View in CoL vs. R. anomalus View in CoL . The spine-shaped egg processes and qualitative as well as quantitative characters of the adults, indicate Ramazzottius subanomalus View in CoL is most similar to Ramazzottius anomalus ( Ramazzotti, 1962) View in CoL ( Figs 36–38 View FIGURES 36 – 38 ). The two species are very similar with the only recorded qualitative difference between them being the presence ( R. anomalus View in CoL ; Fig. 37 View FIGURES 36 – 38 , indented arrowhead) or absence ( R. subanomalus View in CoL ; Figs 16–29 View FIGURES 16 – 29 and 34 View FIGURES 30 – 35 ) of a fine granulation on the eggshell surface. It also appears that the egg processes of R. anomalus View in CoL have a more evident septa between their basal and distal parts ( Figs 37–38 View FIGURES 36 – 38 ). In addition, Biserov (1985) also noted that the egg processes are on average 6.7 µm taller in R. anomalus View in CoL than R. subanomalus View in CoL . However, our observations (see Figs 16–29 View FIGURES 16 – 29 and Table 5) now question whether the number, size and shape of egg processes are a reliable taxonomically trait in differentiating the two species: i.e. process height in the Polish population of R. subanomalus View in CoL ranged from 1.5 to 12.5 µm, and thus largely overlap the values reported by Ramazzotti (1962) for R. anomalus View in CoL (i.e. 5.0–12.0 µm).
In the absence of eggs, differentiating between individuals of R. anomalus View in CoL and R. subanomalus View in CoL relies exclusively on three morphometric differences: R. anomalus View in CoL has a shorter buccal tube and placoids (both macroplacoids in absolute values, but only the first macroplacoid in pt values). The remaining morphometric characters are very similar in the two species (see Table 6 for a full comparison). However, as R. anomalus View in CoL was described over half a century ago and tardigrade taxonomy has moved forward, the original description lacks many key measurements ( Table 6), so there could be more morphometric differences between R. anomalus View in CoL and R. subanomalus View in CoL which we are currently unaware of. In other words, a modern integrative re-description of R. anomalus View in CoL is very desirable in order to confidently differentiate the species from other congeners with similar adult and egg morphology. Also, as this is only the second report for R. subanomalus View in CoL , data from other populations of this species are required for the better estimation of the extent of R. subanomalus View in CoL intraspecific variability.
Ramazzottius View in CoL DNA sequences. The COI sequence for the Polish population of R. subanomalus View in CoL proved to be distinct from all the Ramazzottius View in CoL COI sequences that were available from GenBank and suitable for analysis, with p-distances ranging from 19.0 to 20.0% ( EU251379 View Materials and EU251381 View Materials , respectively). This figure is well above the 3% threshold proposed for species delineation ( Cesari et al., 2009, but see also Michalczyk et al. 2012a). Similarly, our 28S rRNA sequence, with the p-distance of 3.1%, was distinct from the single available and suitable Ramazzottius View in CoL sequence ( FJ435768 View Materials ). Regarding the 18S rRNA, the p-distances ranged from 0.4% to 1.1% ( FJ435727 View Materials –8 and HQ604950 View Materials , AY582122 View Materials , respectively). The scarcity of available molecular data for Ramazzottius View in CoL spp. underlines the need for more integrated taxonomy and ecological studies to provide DNA sequences from a variety of populations for any given tardigrade species. Without such large-scale systematic studies, threshold values or barcode gaps for species delineation in tardigrades cannot be confidently estimated or used for species delineation.
Ramazzottius View in CoL in Poland. Hitherto there have been four species of Ramazzottius View in CoL reported from Poland: R. anomalus (in Dastych 1988) View in CoL , R. montivagus (in Dastych 1980) View in CoL , R. oberhaeuseri View in CoL (in Kranz-Leśniewska 1933, Marcus 1936, Węglarska 1959a, b, Hęciak 1976, Węglarska & Korecka 1983, Dastych 1970, 1972, 1979, 1980, 1988, Kaczmarek & Michalczyk 2003ab, Zawierucha 2011, Zawierucha et al. 2012), and R. subanomalus (in Stec et al. 2016a) View in CoL . However, for the following reasons, we think that the Polish record of R. anomalus View in CoL is invalid. First, the R. anomalus View in CoL report by Dastych (1988) was for Jakubowo, which is only ca. 45 km from Poznań (where the population of R. subanomalus View in CoL reported in this paper was found), whereas the type locality for R. anomalus View in CoL was Cerro El Roble in Chile , over 12,500 km away. Second, the measurements provided for the Polish R. anomalus View in CoL (see Dastych 1988) are similar to those of R. subanomalus View in CoL . Third, the eggs of the Polish R. anomalus View in CoL were described as having a smooth chorion between processes (see Dastych 1988), and are granulated in R. anomalus View in CoL (see Fig. 39). Finally, at the time of publishing his monograph, Dastych (1988) was probably unaware of the Biserov (1985) publication in a Russian journal (not cited in Dastych 1988). Thus, it seems reasonable to conclude that the species Dastych (1988) recorded from Jakubowo in 1978 was actually R. subanomalus View in CoL rather than R. anomalus View in CoL . We therefore think that R. anomalus View in CoL should be deleted from the list of Polish tardigrade species, and be substituted with R. subanomalus View in CoL . However, this being an exchange rather than an addition to the record, the overall total number (103) of species recorded for Poland is unaffected.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Ramazzottius subanomalus ( Biserov, 1985 )
| Stec, Daniel, Zawierucha, Krzysztof & Michalczyk, Łukasz 2017 |
R. subanomalus
| in Stec et al. 2016 |
Ramazzottius caucasicus
| Biserov 1998 |
Ramazzottius ljudmilae
| Biserov 1998 |
Ramazzottius
| Binda & Pilato 1986 |
Ramazzottius
| Binda & Pilato 1986 |
Ramazzottius
| Binda & Pilato 1986 |
Ramazzottius
| Binda & Pilato 1986 |
Ramazzottius
| Binda & Pilato 1986 |
Ramazzottius
| Binda & Pilato 1986 |
Ramazzottius
| Binda & Pilato 1986 |
Ramazzottius
| Binda & Pilato 1986 |
Ramazzottius
| Binda & Pilato 1986 |
Ramazzottius subanomalus
| Biserov 1985 |
Ramazzottius subanomalus
| Biserov 1985 |
Ramazzottius subanomalus
| Biserov 1985 |
Ramazzottius subanomalus
| Biserov 1985 |
Ramazzottius subanomalus
| Biserov 1985 |