Tethysimyia deemingi ( Ebejer, 1996 ), 2009
|
publication ID |
https://doi.org/10.5733/afin.050.0208 |
|
persistent identifier |
https://treatment.plazi.org/id/567B87D1-1B61-FFBA-080B-FE0DFC31FB60 |
|
treatment provided by |
Felipe |
|
scientific name |
Tethysimyia deemingi ( Ebejer, 1996 ) |
| status |
comb. nov. |
Tethysimyia deemingi ( Ebejer, 1996) , comb. n.
Figs 60 View Fig , 61 View Fig 61
Aphaniosoma deemingi: Ebejer 1996: 289 View in CoL .
This small species was described from a female taken in Oman. With additional specimens of both sexes now available, its distinctive characters may be better appreciated. The male is described and the postabdomen of both sexes is illustrated.
Description:
Male.
Head: Yellow, large and higher than long, fr broad with barely converging eye margins, antenna small and recessed, gena with numerous setulae and about as broad as height of eye, which is round, numerous fr setulae of equal length, 1 orb set far back and hardly longer than fr setulae, pvt minute.
Thorax: Yellow with faint deeper yellow longitudinal stripes on scutum. Chaetotaxy: numerous fine setulae over whole dorsal surface, 1 pprn, 1 short posthu which is a little less than twice length of adjacent setulae, 1 long dc and a shorter one in front, 1 short prscut pair of acrs, others not differentiated from general scutal setulae, scut with usual 4 marginals, prpl setula absent (specimen pinned through sides, but in other specimens anepisternal seta present and katepisternal absent).
Wing: Translucent and slightly iridescent with broad anal margin; veins very pale yellow to white; R 2+3 and R 4+5 run more or less parallel to each other and distance between them on costa about 0.9 that between R 4+5 and M 1+2, which converge towards apex; costa with stouter spine-like setulae and very few thin setulae; costa thinned out but not broken just beyond hu crossvein, subcosta vestigial, visible only as a fold. Haltere pale yellow. Legs: Not dilated or modified, entirely pale yellow and short yellow setulose; tibia 2 with dark apicoventral seta.
Abdomen: Yellow with light brown bands on middle part of tg 2–5; very short setae on disc of tg, longer and stronger on tg 6; st largely membranous.
Postabdomen: Very small and inconspicuous in dry specimens; tg 6 longer than tg 5 when viewed from above, ep narrow dorsally, but broad laterally and ventrally such that most hypopygial structures lie within its boundaries; cerc relatively large and sclerotized with numerous and very fine setulae, hyp forming bridge between lower incurved margins of ep, surs in form of short lobe arising from posteromedial border of ep, prg very small, arising from posterior edge of hyp, psg not identified, ph simple with short narrow ph apd.
Female.
Similar to male in external characters. Postabdomen: tg 7 complete, tg 8 small and with minute setulae along anterior border; st 8 larger and with 2 setae at posterior border, ventrally; hypr distinct with fine setulae, cerc small and round; s poorly sclerotized, conical and with first part of duct thinly sclerotized.
Holotype (examined): OMAN: ^Dhofar, Khor Rouri [Khawr Rawri, 17°03'S: 54°26'E], 12.xi.1992, J.C. Deeming ( NMWC). GoogleMaps
Other material examined: EGYPT: 1ơ Sinai, Taba , 1.v.1996, A. Freidberg ( TAUI) ; 3^Sinai, Taba , 10 km S, 1.v.1996, I. Yarom ( TAUI) ; 1^Sinai, Ras Umm Burqa’ , 1.v.1996, I.Yarom ( TAUI) . OMAN: 1^N Masirah Island, BERS Camp , 5–7.iii.1995, S.P. Dance ( NMWC) .
Distribution: Egypt, Oman.
PHYLOGENY
The systematic position of the genera within the Chyromyidae is explored further with a cladistic analysis taking the Heleomyzinae as the outgroup taxon. D.K. McAlpine’s proposal (McAlpine 2007) to categorize the Sphaeroceridae and Heleomyzidae into one family, the Hetermyzidae, and consequently alter their rank to subfamily, is not relevant to this analysis, for which I accepted the categorization of the Heleomyzinae as a subfamily. The Heteromyzidae (= Heleomyzidae and Sphaeroceridae ) and the Chyromyidae share synapomorphic characters that justify retaining the latter family in closer phylogenetic proximity to the former rather than to any other acalyptrate family, but there are also characters that the Chyromyidae share with Ephydroidea and Opomyzoidea. However, the last two superfamilies have evolved autapomorphies (for example, several antennal characters, one proclinate fr orb seta, precoxal bridge present, short rigid ph) that distance them from the Chyromyidae . The few similarities have probably co-evolved, for example, spinose costa, absent pterostigma and reduction in spermathecae from three. At present, I find no reason to differ from the opinions of previous authors (D.K. McAlpine 1985; J.F. McAlpine 1989) that the Chyromyidae are closer to the Heteromyzidae .
This study of the African Chyromyidae uncovers more diversity than was hitherto appreciated, although many of the characters are not immediately obvious, particularly if low magnification microscopy is used. Some of these characters are present in the Heteromyzidae , though not uniformly so, and they may not all be homologous. For example, an incurved seta placed anteriorly on the fr vitta and not on the orb plate is found in Elachisoma Rondani, 1880 ( Sphaerocerinae , Limosinini ) and Tapeigaster Macquart, 1847 ( Heteromyzidae , Tapeigastrinae ), but this is not homologous with the incurved seta of Chyromyinae , which is inserted on the orb plate and therefore is a true orb. An anterior incurved seta on the scutum is present in Thoracochaeta Duda, 1918 ( Sphaerocerinae , Limosinini ) and in the Aphaniosominae , though it probably is not a homologous structure. I have called this the ihu seta and not a dc seta because it is not at all in line with the dc row. The anepisternal seta is always present in the Chyromyidae and it is found in Fenwickia Malloch, 1930 and Waterhouseia Malloch, 1936 (Heleomyzinae) . The modified setulae of the anterodorsal aspect of the costa, found in many Heleomyzinae , are commonly also found in some Chyromyinae and rarely in Sphaerocerinae (Elachisoma) . The costal break near the hu crossvein, though very rare in Heleomyzinae , is present in a number of species, e.g., Diplogeomyza spinosa McAlpine, 1967 ( Australia) , Blaesochaetophora picticornis Bigot, 1888 ( Chile) and Gephromyza sp. ( Chile). It is present in many Sphaerocerinae , and within the Chyromyidae , it is universally found in Aphaniosominae . Marshall and Richards (1987) state that the wing in Sphaerocerinae has costagial, hu and subcostal breaks. They illustrate examples. Rohácek (1998) repeats this statement and gives the same illustrations. I examined a number of species in a range of genera from all over the world and I find the hu vein is an unstable character (indeed, also so in the Chyromyidae ), there being genera that include species with no break or with a complete break. Often there is a partial break, i.e., a narrowing and/or desclerotization, but no actual interruption. For example, Lotobia moyoensi s Vanschuytbroek, 1959 ( Nigeria) has a complete break, L. elegans Vanschuytbroek, 1959 ( Nigeria) , a partial break, L. elegans Kim & Han, 1990 ( Nigeria) , no break. A break at the hu crossvein is absent also in Copromyza fumipennis Stenhammar, 1854 ( Pakistan) , C. kibokoensis Vanschuytbroek, 1959 ( Congo) , C. marginatis Adams, 1905 ( Oman) , Crumomyia glabrifrons (Meigen, 1830) ( France) , and C. nitida (Meigen, 1830) (Sicily) . A complete break is present in Pterogramma sp. ( Malaysia) and Sphaerocera sp. ( Yemen) . A partial break is present in Poecilosomella angulata (Thomson, 1869) ( Morocco) .
I propose a closer affinity of the Chyromyidae with the Heteromyzidae than with the Ephydroidea. In this, I agree with J.F. McAlpine (1989). Since I found that many characters of the Chyromyidae are represented in one or other of the subfamilies of the Heteromyzidae , namely the Sphaerocerinae and Heleomyzinae , it was unclear to me which of these might be more closely related to the Chyromyidae . In a preliminary analysis, I used both as outgroup taxa and the Heleomyzinae consistently branched out closer to the Chyromyidae than did the Sphaerocerinae . The former was therefore chosen as the outgroup taxon for the cladistic analysis of the new subfamily and generic classification of the Chyromyidae proposed in this study.
The groundplan conditions of the Chyromyidae that I recognize are listed below:
(1) pale integument
(2) face desclerotized centrally below antennal foveae except for a linear vertical ridge
(3) true vibrissa absent
(4) pvt setae convergent
(5) two or more reclinate orb setae
(6) setulose fr
(7) second antennal segment not slit dorsally
(8) prosternum narrow with median groove
(9) one or more dc seta
(10) posthu seta well-developed
(11) two subequal pairs of scut setae
(12) one anepisternal and one katepisternal seta
(13) anepimeron and meron bare
(14) tibiae without preapical posterodorsal seta and without any developed setae along shaft (15) metatarsus of anterior, middle and posterior legs of more or less equal length
(16) wing with costal break at R 1
(17) R
1
not dilated at junction with costa
(18) subcosta merging with R 1 close to junction with costa
(19) veins R 2+3 and R 4+5 convergent
(20) anal and posterior basal cells closed
(21) male abdomen with six and female abdomen with seven pregenital segments
(22) postabdomen symmetrical in both sexes
(23) female postabdominal segments barely extensile, st 8 divided and epiproct desclerotized (24) two spermathecae
(25) male hypopygium with U-shaped hyp
(26) surs, prg and psg not fused with their respective basal hypopygial sclerites
The autapomorphies that characterize the Chyromyidae with respect to the Heteromyzidae are: a pale yellow integument; membranous face; vibrissa absent; wing with veins R 2+3 and R 4+5 convergent; dorsal preapical tibial seta absent; postabdomen symmetrical in both sexes; two spermathecae; articulated surs; prg and psg; and halophilous habits. Synapomorphies shared with the Heteromyzidae include: discoid third antennal segment; second antennal segment without dorsal slit; orb setae reduced to 2 or 3; subcosta reaching costa or merging with R 1 at junction with costa; subcostal break present; A 1 not reaching wing margin; distiph complex and enlarged; female postabdomen not or barely extensile.
The much greater diversity within the Heteromyzidae adds to the problem of interpreting the polarity of characters, although ultimately this would not affect the result of the cladistic analysis. For the purpose of this exercise, I have limited myself to relatively few and easily interpreted characters. I examined a limited number of species from a range of genera of Heleomyzinae and Sphaerocerinae from across the world, but for the Chyromyidae , I examined all the genera and, within each, a great majority of the species (several of them undescribed) from most zoogeographical regions (Table 2). Where I was unable to examine specimens, I did not rely on descriptions, as some of the chaetotaxic characters were either not mentioned or are subject to misinterpretation or at least to an interpretation different from mine.
The only genus, indeed the only species, of the Chyromyidae ever properly considered for phylogenetic study has been Chyromya flava . The other genera have not been considered in any detail and, with regard to the male and female postabdominal characters, have not been considered at all. At the very least, Aphaniosoma must now be taken into account as this significantly widens the concept of the Chyromyidae . Aphaniosoma B (Table 2) consists of those species with a strong presutural dorsocentral seta and other defining features that possibly justify raising this group to generic status. The cladistic analysis reported in this article lends support to this. However, this requires further investigation and is beyond the scope of the present study, as several more species awaiting description are known to me.
For each character assessed within the Chyromyidae , I accepted only 100 % concordance among all the species examined within each genus, with one exception. In Gymnochiromyia , a presutural dc seta is found in a minority of species. This being the only character state that I found to be at odds with the remaining characters of the genus, I considered it a reversal when present. The interpretations of the character states are listed below. The numbering starts at zero to match the first character as it appears on the cladogram generated by the program TNT. Cladistic analysis was performed using TNT version 1.1 ( Goloboff et al. 2008). This program is made available for use by the Willi Hennig Society (http://www.zmuc.dk/public/phylogeny/TNT; downloaded 15.iii.2008).
Character 0. Concave ocp. Across the order, and particularly in the Heleomyzinae , a convex ocp is the usual form. Therefore, I consider a concave ocp an apomorphy within the Chyromyidae .
Character 1. Postgenal seta. This small seta or setula lies posteroventrally on the inferior aspect of the ocp region of the head. It is a subtle character in the Chyromyidae because of their small size. It is rarely mentioned in descriptions of other acalyptrates. Nevertheless, it is widespread and common in many families. I consider its loss in some Chyromyidae an apomorphy.
Character 2. Incurved lower fr-orb. A very few Sphaerocerinae exhibit this character state (seta on the fr vitta), but I consider it a homoplasy rather than a homology with the character state (seta on the orb plate) in the Chyromyinae (in which taxon it is universal). I consider it an apomorphy in the Chyromyidae , it being even more unusual in the other acalyptrates.
Character 3. orb reduced to setulae or absent. Having well-developed orb is the usual character state in the great majority of acalyptrates. Wherever a loss or reduction occurs, often with loss or reduction of other somatic setae, it is considered to be an apomorphic state.
Character 4. Pair of long fr setae. This pair of usually divergent setae is placed in the middle of the fr in front of the oc triangle. It appears sporadically in a number of genera in several families of the acalyptrates. I consider it an apomorphy within the Chyromyidae as it appears in only one species group.
Character 5. Disc of ocp bare. Here, I refer to the space on the ocp between the postocular row(s) of setae or setulae and those situated strictly along the margin of the ocp foramen. This character is common in the Heleomyzinae and Sphaerocerinae , as well as in many other acalyptrates. I therefore consider this absence of setae within the Chyromyidae an apomorphy.
Character 6. pvt setae reduced to setulae or absent.Across the whole suborder, the usual character state is the presence of pvt setae. Their absence, being also uncommon within the Chyromyidae , confirms their apomorphic state.
Character 7. Well-developed facial carina. Although this character state appears in a number of genera within a range of families of the acalyptrates, it is not the norm. A tendency to form a shallow carina is common within the Chyromyidae , but its marked development in some cases suggests this is an apomorphy.
Character 8. Absent vibrissa. The loss of the vibrissa is uncommon in the Schizophora and in the Chyromyidae this is an apomorphy relative to the outgroup.
Character 9. Costa with a complete hu break. This character is often found in the Sphaerocerinae , but rarely in the Heleomyzinae . It is an apomorphic state in the Schizophora. Within the Chyromyidae , it occurs in the four genera of Aphaniosominae , but is rare and incomplete in the Chyromyinae . I consider it an apomorphy within the family.
Character 10. Spine-like setulae on costa. These are common in the Heleomyzinae and rare in the Sphaerocerinae .They are a feature of the Chyromyinae , but not the Aphaniosominae . I consider them to be synapomorphic for the Chyromyinae and Heleomyzinae .
Character 11. R 2+3 and R 4+5 convergent. The convergence of these two longitudinal veins is an apomorphy.
Character 12. R 4+5 and M 1+2 divergent. Although this is the norm in acalyptrates, I consider it to be possibly an apomorphy within the Chyromyidae (a reversal?).
Character 13. Absent presutural dc seta. This is an apomorphy among the higher Diptera . In Gymnochiromyia it is most often absent and I consider its presence in the minority of species a reversal.
Character 14. prsut ia seta. This is not the same seta referred to by Rohácek (1998) as synonymous with the posthu seta in the Sphaerocerinae . In the Chyromyidae , all species have a true posthu. The true prsut ia, when it occurs, is inserted medially and slightly posterior to the posthu. This is a reversal among the higher Diptera especially among the acalyptrates. Therefore, in the Chyromyidae it is likely an apomorphy.
Character 15. Lost or reduced post ia seta. A post ia seta is present in most Sphaerocerinae and Heleomyzinae . Its loss in the Chyromyidae is an apomorphy.
Character 16. Absent postsutural pra seta. This seta is very common in many higher Diptera . I consider its loss in most Chyromyidae an apomorphy, but equally it could be considered a reversal, given its presence in only two genera.
Character 17. Absent sa seta. A seta in this position is a very widespread character in the Diptera . I consider its loss within the Chyromyidae an apomorphy.
Character 18. scut setae reduced to 2 pairs of marginals is the plesiomorphic state in the acalyptrates. The increase in number in a few genera of the Chyromyinae , and also in a few genera of Heleomyzinae and Sphaerocerinae , suggests that the presence of more than two pairs is apomorphic.
Character 19. Absent setulae on disc of scut. This is probably an apomorphy among the higher Diptera and within the Chyromyidae . These setulae are not of the same structure as very short setae.
Character 20. acrs setulae in 2 rows. While there is a very wide range of states among the acalyptrates, from a completely bare to a densely setulose scutum, at least in comparison to the outgroup taxa, reduction to two rows of setulae appears to be an apomorphy.
Character 21. Absent prpl seta/setula. An apomorphic state among the acalyptrates.
Character 22. Absent anepisternal seta. An apomorphic state among the acalyptrates; absent in most genera of the Heleomyzinae and in almost all Sphaerocerinae .
Character 23. Absent katepisternal seta. This seta is most often present in higher Diptera . I consider its loss among the acalyptrates and within the Chyromyidae an apomorphic state.
Character 24. Dilated femora.This feature occurs sporadically across a wide range of acalyptrates. I consider it an apomorphic state among the Chyromyidae .
Character 25. Mid tibia with reduced or absent apicoventral seta. A majority of Diptera in all families have one or more such setae. Their absence is an apomorphy.
Character 26. Tibia without dorsal preapical seta. The presence of this seta is generally considered to be an apomorphy among the Heteromyzidae . Its loss in the Chyromyidae is probably secondary (a reversal) and therefore an apomorphy relative to the chosen outgroups.
Character 27. Internalised ep. This highly complex development in Aphaniosoma is undoubtedly an apomorphic state.
Character 28. Modified cerc in males. This is an apomorphic state in acalyptrates and the Chyromyidae .
Character 29. Loss of ej apd. I consider this an apomorphic state within the Chyromyidae , given its presence in one subfamily and in the chosen outgroup taxa.
Character 30. ph apd extensively fused with hyp. The plesiomorphic state expecially in the outgoup taxon is a long and narrow ph apd that remains free from the hyp almost to its apex, where it articulates with the basiph.
Character 31. Two spermathecae. This is an apomorphic state in the Chyromyidae .
Character 32. Female postabdomen with well-developed fleshy lobes medial to, and attached to, sclerites of st 8. This subtle character is found in only one genus of Chyromyinae (Somatiosoma) . I consider it an apomorphy.
The characters listed above are given in the matrix (Table 2) used for the analysis. Parsimony analysis using “Implicit enumeration” methodology in TNT generated three equally parsimonious trees (score 45) of identical topology with a different interpretation of only a small number of characters. The only “phylogenetic” difference between them was the placement of the genera Oroschyromya , Notiochyromya and Somatiosoma relative to each other. The strict consensus tree is depicted in Fig. 62 View Figs 62, 63 . Tree 0 was one step shorter than the others and it was supported by the cladogram generated by the Majority Rule ( Fig. 63 View Figs 62, 63 ). It was therefore selected and the synapomorphies were superimposed on the clades ( Fig. 64 View Figs 64, 65 ). Bootstrap support was calculated using 1,000 replicate routines and the results superimposed on the strict consensus tree ( Fig. 65 View Figs 64, 65 ). Manual calculations on tree 0 gave a length = 28; a consistency index, CI=0.893; a retention index, R=0.942; and a rescaled consistency index, RC=0.841.
This analysis provides support for the proposed generic grouping into two subfamilies and gives an indication of the phylogenetic relationships of the genera of the Chyromyidae . The tree supports well the phylogenetic position of the genera within the Aphaniosominae , with bootstrap scores of 87 % or above, with the exception of Paraphaniosoma . I consider their relationship to each other, as well as to the subfamilies, to be well resolved. Paraphaniosoma presents at least two very strong characters that convince me it belongs to the Aphaniosominae close to Aphaniosoma A and Aphaniosoma B, rather than Tethysimyia or Krifomyia . These characters are the arrangement of structures of the hypopygium and those of the head. The presence of the pra seta is most probably a reversal or homoplasy. Support is weak for the genera Gymnochiromyia , Oroschyromya , Notiochyromya and Somatiosoma . On general appearance and detailed examination of the postabdomen of both sexes, it appears to me that Notiochyromya is closest to Chyromya , and Oroschyromya closest to Gymnochiromyia . The position of Somatiosoma is as yet unclear, although I suspect it will eventually turn out to be an offshoot of Notiochyromya . At this stage, I cannot consider that the relationship of these four genera to each other or within the Chyromyinae is resolved. This may be partly due to not using more characters of the male and female postabdomen and to an avoidance of other somatic characters where definition is difficult, for example, the relative head shape: prognathous versus opisthognathous, the degree of convergence of eye margins, and the subtle wing characters. These three genera are actually quite easy to separate (with a little practice and experience) in both sexes without dissection, relying only on somatic as opposed to hypopygial structures. To resolve the phylogenetic relationships within the Chyromyinae , a study of more species from other regions of the world may be helpful, but most of the species are undescribed. The choice of an outgroup taxon to perform such an analysis is not easy, as some somatic and hypopygial structures, though clearly very differently developed in the Aphaniosominae , are not easily defined in a categorical way across the family (i.e., present or absent) as is required to run a cladistic analysis. For example, the breadth and depth of the basal half of the phallapodeme is developed differently in the Chyromyinae . It may also be described as broad and deep in Aphaniosoma , but here it clearly presents a very different modification. The same applies to the modification of the ejaculatory apodeme in the genera of the Chyromyinae .
Support for the monophyly of the Chyromyidae is very good, with a supporting bootstrap value of 100 %. Similarly, the division into two subfamilies appears to be well supported, with a bootstrap value of 93 %. Based on the chosen characters and the interpretation of their polarity, the Chyromyidae appear to be closer to the Heleomyzinae rather than the Sphaerocerinae . However, a further cladistic analysis using a larger character set, may be needed to establish the relationships between these and other closely related taxa, such as the Ephydroidea.
ZOOGEOGRAPHY AND ECOLOGY
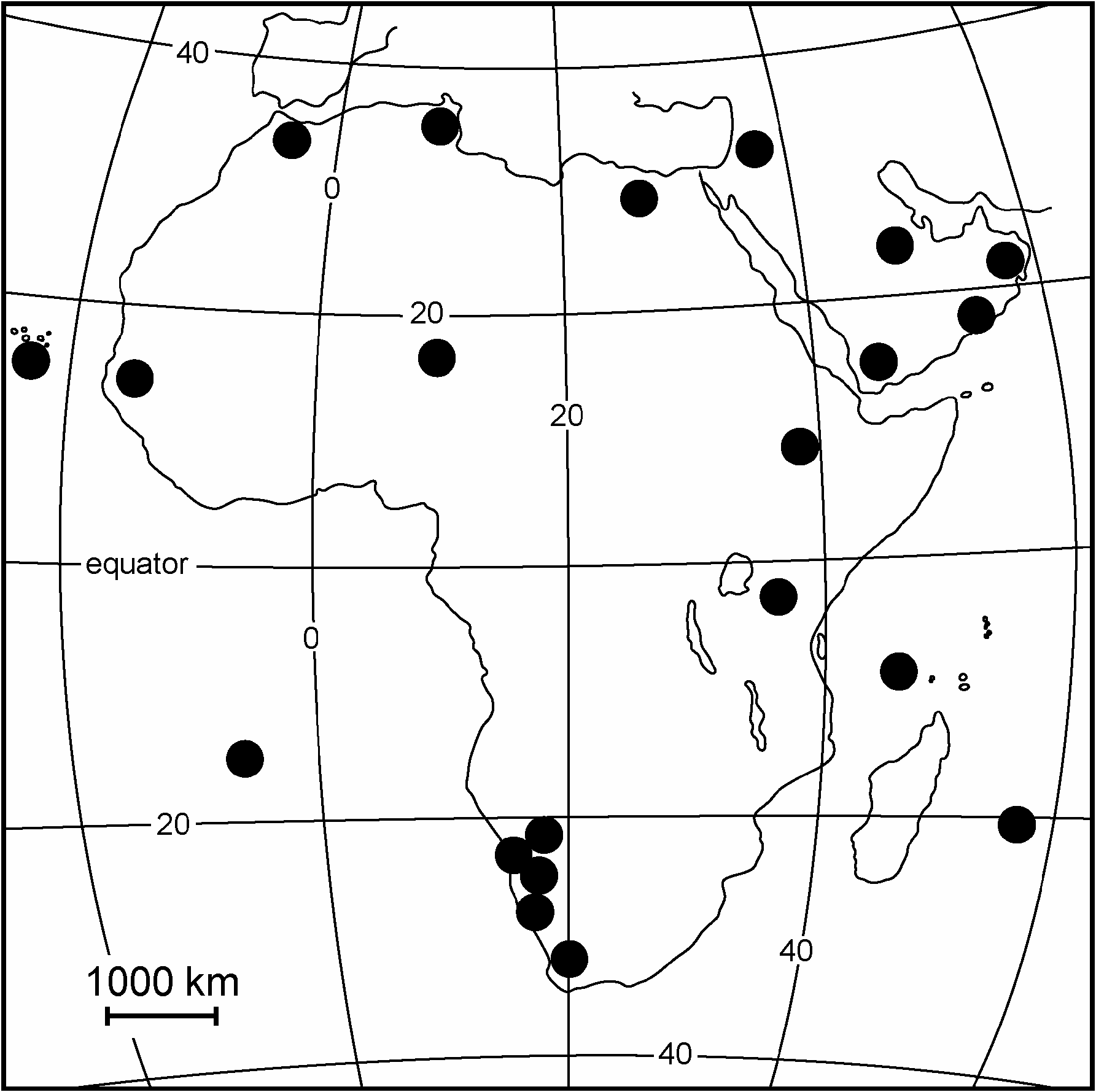
The family is known from all continents and zoogeographical regions except the Arctic and Antarctica (Cogan 1980; McAlpine 1965; Pitkin 1989; Soós 1984; Steyskal 1977). I have seen scanty material from mainland South America, but I know of much more collected material that awaits study. I am unaware of any published information other than a study on the Galapagos species ( Wheeler & Sinclair 1994). Specimens have been collected on a number of remote Atlantic and Pacific islands ( Frey 1945; Hardy & Delfinado 1980). Many are still undescribed and therefore zoogeographical affinities are speculative at best.The distribution of the genera of Chyromyidae in Africa is summarised in the maps given in Figs 66–70 View Fig View Fig View Fig View Fig View Fig .
The genus Aphaniosoma is widespread in the Holarctic and Afrotropical regions, with most species diversity being in the Mediterranean ( Carles-Tolrá 2001; Ebejer 1998; 2005; Ebejer & Baez 2001), Central Asia (Ebejer 2006) and southern Africa (this paper), in that order. There are some affinities between groups of species in the eastern Mediterranean and Africa and between other groups of species in Central Asia and the Mediterrane- an, but not between Africa and Asia. On current knowledge, therefore, it appears that perhaps most species radiation has occurred from the northern part of Africa (e.g., A. approximatum and A. gallagheri ). The central Sahara and the tropical rainforests of central Africa may have been early separating factors for some species groups, whereas the Rift Valley and the savannah zone from southwest Africa to northeast Africa may still act as natural corridors facilitating the spread of species (e.g. Aphaniosoma fissum , Somatiosoma eremicolum , S. nitescens and Oroschyromya affinis ). The countries between the Mediterranean part of the Middle East and the central Asian states of Mongolia and the former Soviet Union have not been investigated. For example, Iraq, Iran, Afghanistan and Pakistan may be rich in species of this genus.
The genera Somatiosoma and Oroschyromya appear to be typically Afrotropical, but the Indian subcontinent has not been investigated for Chyromyidae . If they were truly restricted to the Old World tropical and subtropical areas, both genera might still be expected to occur in the Indian subcontinent. Somatiosoma favours hot, arid habitats and such habitats occur in northwest and central India. The genus has been found in all parts of the Arabian Peninsula. Oroschyromya , with its apparent affinity for wetter regions, might occur in the Western Ghats. Notiochyromya , on the other hand, is represented in all geographical areas of the southern hemisphere and as studies continue and more species are discovered, this genus may turn out to be rich, diverse and inhabiting a wide range of habitats extending into Central America and the southern states of the USA. The Chyromyidae of the Oriental and Australasian regions ( Steyskal 1977; Pitkin 1989) are too poorly known for further comments to be made at present.
As far as is known, most speciation in Gymnochiromyia appears to be in the Mediterranean (Ebejer 1998) and in southern Africa (Ebejer 2008 b). This suggests a disjunction and lends support to the theory that the African fauna includes the Mediterranean, with the Sahara being only a recent barrier ( Kirk-Spriggs & McGregor 2009). The genus, as originally interpreted, has been recorded from North America. I have seen limited material. Very little work has been done on Nearctic Chyromyidae ( McAlpine 1965, 1987) and it is difficult to speculate as to the richness or otherwise of the fauna there, or even as to what affinities this might have with the fauna of Europe and Africa.
| NMWC |
National Museum of Wales |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Tethysimyia deemingi ( Ebejer, 1996 )
| Ebejer, Martin J. 2009 |
Aphaniosoma deemingi: Ebejer 1996: 289
| EBEJER, M. J. 1996: 289 |