Pristimantis marmoratus ( Boulenger, 1900 )
|
publication ID |
https://doi.org/ 10.5852/ejt.2018.397 |
|
publication LSID |
lsid:zoobank.org:pub:783D763E-C553-42B7-954B-BB5CB6DB913A |
|
DOI |
https://doi.org/10.5281/zenodo.5976923 |
|
persistent identifier |
https://treatment.plazi.org/id/5A0CB016-FF88-FFC9-B903-FC78FAFEFB4B |
|
treatment provided by |
Plazi |
|
scientific name |
Pristimantis marmoratus ( Boulenger, 1900 ) |
| status |
|
Pristimantis marmoratus ( Boulenger, 1900) View in CoL
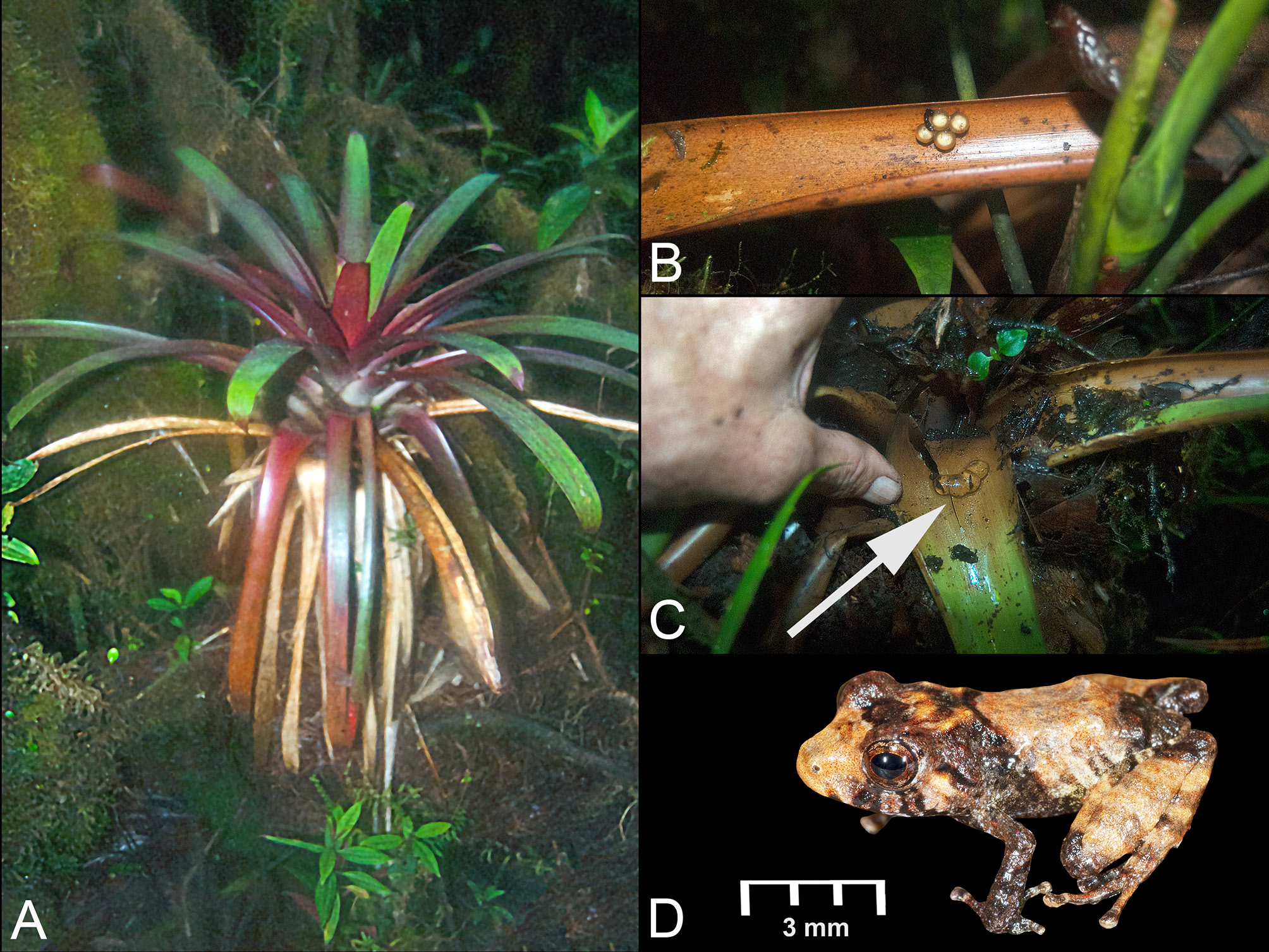
Figs 2–5 View Fig.2 View Fig. 3 View Fig.4 View Fig. 5 , 6D View Fig. 6
Diagnosis
Pristimantis marmoratus ( Boulenger, 1900) has historically been assigned to the polyphyletic “ unistrigatus species group” (sensu Hedges et al. 2008), which is mainly characterized by having Finger I shorter than II, Toe V longer than III, extending to the distal edge of the distal subarticular tubercle of Toe IV when toes are adpressed, and by the absence of cranial crests and the presence of vomerine teeth. Pristimantis marmoratus is characterized by the following unique combination of characters:
(1) body small, adult males 17.6–20.4 mm SVL (n = 5), adult female 27.9 mm SVL (n = 1);
(2) dorsal skin granular/ pustulate, usually with distinctly enlarged tubercles (less pustulate in female), belly skin granular (granules not as closely set as in areolate skin sensu Duellman & Lehr 2009), sometimes becoming smooth in preservative;
(3) presence of well-developed oblique scapular ridges in males (less prominent in female);
(4) tympanum present, tympanic membrane not or only poorly differentiated, and tympanic annulus only partially visible externally, obscured by supratympanic fold, TYM 22–34% of EL;
(5) small pharyngeal ostia present;
(6) TIL 1.9–2.0 times HAND;
(7) snout rounded to subovoid in dorsal view, slightly sloping in profile, canthus rostralis nearly straight in dorsal view, rounded in cross section, loreal region concave, flaring slightly at upper lip;
(8) upper eyelid granular, with 1–3 distinctly enlarged tubercles on each eyelid;
(9) choanae small, round, dentigerous processes of vomers small, sometimes barely visible or even not detectable, slightly oblique, ovoid, posterior and medial to choanae, each bearing 3–5 teeth when present;
(10) presence of vocal slits in male, vocal sac single, subgular;
(11) tongue cordiform;
(12) one unpigmented whitish nuptial pad located on the preaxial side of the thenar tubercle on each thumb in male, a second pad is sometimes present (on one thumb or on both) on the preaxial side of the first subarticular tubercle;
(13) Finger I shorter than II, FI 75–83% of FII in males, 86% of FII in female;
(14) fingers basally webbed, with moderately developed lateral fringes, usually more conspicuously developed preaxially on Finger II;
(15) palmar tubercle V-shaped, often broken in three distinct tubercles;
(16) axillary tubercles (sensu Myers & Donnelly 2001) absent;
(17) small ulnar tubercles present, in line;
(18) small tarsal tubercles present, 1–3 slightly enlarged calcar tubercles present;
(19) inner metatarsal tubercle oval, about 3–4 times the size of the round, projecting outer metatarsal tubercle;
(20) Toe V longer than III, extending to the distal edge of the distal tubercle on Toe IV when toes are adpressed;
(21) toes with lateral fringes, best developed preaxially on Toes II–IV, webbing basal between Toes II–V;
(22) discs broadly expanded, elliptical;
(23) in life, main dorsal colour pattern ranges from large, weakly visible, dark brown or grey-brown blotches to nearly uniformly brownish grey, ventral colouration ranges from whitish to grey or dark brown with series of tiny, dark brown punctuations and light grey flecking;
(24) in preservative, main dorsal colour pattern similar to when alive, but with the melanin more prominent, revealing large, indistinct blotches or more or less uniformly distributed medium brown colour, ventral colouration consists in a uniformly distributed series of tiny, warm brown punctuations on the chin, belly and undersurfaces of the legs, feet and toes;
(25) in life, anterior and lower posterior surfaces of thighs brown to dark brown with light grey flecking (brown with white flecking in preservative);
(26) in life, diffuse yellow or pale green wash (white in preservative) on groin and absence of flashy colour on axillary /pre-axillary region;
(27) in life, iris pale gold to bronze with fine black reticulations, a median horizontal brown to reddish brown bar, and a vertical streak running across the iris;
(28) advertisement call consists in a single note repeated at a rate of ca 11 calls/ min with a dominant frequency ranging from 2756 to 3101 Hz;
(29) males call exclusively at dusk, usually upside down on mossy tree trunks of low diameter;
(30) known elevational distribution ca 600 to 1800 m.
Material examined
Holotype
VENEZUELA: ♂, Bolívar State, foot of Mount Roraima , F.V. McConnell and J.J. Quelch leg., Aug.– Oct. 1898 ( BMNH 1947.2.16.92 , formerly 99.3.25.19).
Other material
GUYANA: 2 ♂♂, 1 juvenile, Cuyuni-Mazaruni District, slopes of Maringma-tepui , Philippe J.R. Kok leg., 20–22 Nov. 2007 ( IRSNB 12862 View Materials , IRSNB 17916 View Materials , IRSNB 17939 View Materials ); 3 ♂♂, 1 ♀, Potaro-Siparuni District, Kaieteur National Park , Philippe J.R. Kok leg., 26–28 Jun. 2006 ( IRSNB 14471-74 View Materials ) .
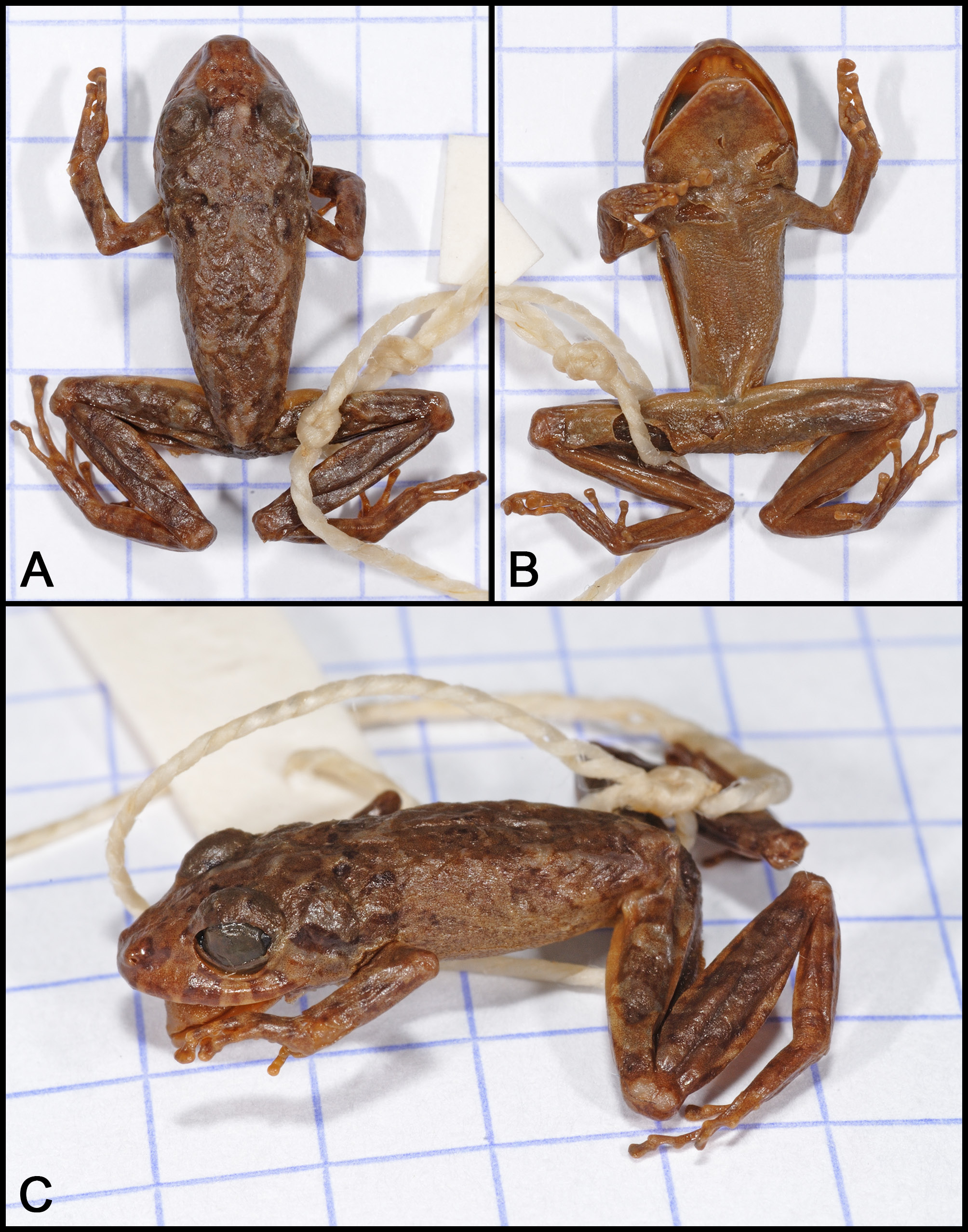
The holotype ( BMNH 1947.2.16.92, formerly 99.3.25.19; Fig. 2 View Fig.2 ) is an adult male with large vocal slits (gender of the holotype was not provided in the original description).
Description of adult
Measurements are provided in Table 2. Adult males 17.6–20.4 mm SVL (n = 5), distinctly smaller than adult female 27.9 mm SVL (n = 1). Head slightly longer than wide, wider than body; HW 36–41% of SVL; HL 37–45% of SVL; cranial crests absent. Snout longer than eye length (SL 102–133% of EL), rounded to subovoid in dorsal view, slightly sloping in profile; canthus rostralis nearly straight in dorsal view, rounded in cross section, loreal region concave, flaring slightly at upper lip; EN 80–110% of EL. Nares protuberant, directed posterolaterally, visible in front, dorsal and usually ventral views. Widest upper eyelid width equal or subequal to interorbital distance. Upper eyelid granular, with 1–3 distinctly enlarged tubercles on each eyelid. Tympanum present, tympanic membrane not or only poorly differentiated, tympanic annulus only partially visible externally, obscured by supratympanic fold, TYM 22–34% of EL; small pharyngeal ostia present. Choanae small, round, not concealed by palatal shelf of maxillary arch; dentigerous processes of vomers small, sometimes barely visible or even not detectable (e.g., in IRSNB 14473 on both sides, in IRSNB 17939 on left side, in IRSNB 14471 on right side), slightly oblique, ovoid, posterior and medial to choanae, each bearing 3–5 teeth when present. Tongue cordiform, slightly longer than wide, posterior half free. Vocal slits present in male, vocal sac single, subgular.
Dorsal skin texture fairly variable (probably depending on activity, as observed in several species of the genus; see for example Guayasamin et al. 2015), from granular to pustulate, including on head, usually with distinctly enlarged tubercles (dorsal skin less pustulate in female); well-developed oblique scapular ridges in males (less prominent in female); middorsal raphe not, or barely, detectable; supratympanic fold conspicuous in life, slightly arched, originating at posterior corner of eye, failing to reach shoulder; large post-rictal tubercles present; a weak dermal fold often present along upper flank; flanks granular. Throat surface smooth; upper chest smooth; weak thoracic fold; belly skin granular, those granules not as closely set as in so-called areolate skin (sensu Duellman & Lehr 2009), sometimes becoming smooth in preservative; weak discoidal fold anterior to groin; posteroventral thigh and cloacal region areolate; cloacal sheath absent.
Hand length 26–30% of SVL. Finger I 75 –83% of FII in males, 86% of FII in female. Relative length of fingers III> IV> II> I; adpressed Finger I fails to reach proximal edge of digital pad of Finger II; adpressed Finger IV reaches beyond distal subarticular tubercle. One unpigmented whitish (translucent when wet) nuptial pad located on preaxial side of thenar tubercle on each thumb in male, a second pad is sometimes present (on one thumb or on both) on preaxial side of first subarticular tubercle. Fingers basally webbed, with moderately developed lateral fringes, usually more conspicuously developed preaxially on Finger II. Finger discs broadly elliptical, broader than long, circumferential groove conspicuous; disc on Finger III ca three times as wide as distal end of adjacent phalanx. Palmar tubercle V-shaped, usually pigmented, often broken in three distinct small tubercles; thenar tubercle large, ovoid, protuberant; supernumerary tubercles few, large, subequal to subarticular tubercles, round and protuberant: subarticular tubercles large, round and protuberant, one each on FI and FII, two each on FII and FIV ( Fig. 3 View Fig. 3 ). Ulnar tubercles small, in line. Axillary tubercles absent.
Hind limbs moderate in length, heels slightly overlap when held at right angles to sagittal plane; TIL 50–59% of SVL; FL 32% of SVL in female, 40–50% of SVL in males. Relative length of toes IV> V> III> II> I; tip of Toe V extends to distal edge of distal tubercle on Toe IV when toes are adpressed. Lateral fringes on all toes, best developed preaxially on Toes II–IV; webbing basal between Toes II–V. Toe discs subequal to finger discs, WTD /WFD 0.9; toe discs broadly expanded, elliptical, broader than long, circumferential groove conspicuous. Inner metatarsal tubercle elongate, oval, about 3–4 times size of round, projecting outer metatarsal tubercle; subarticular tubercles round, large and protuberant; supernumerary plantar tubercles small, low and round, increasing in size distally ( Fig. 3 View Fig. 3 ). One to three slightly enlarged calcar tubercles present; outer tarsal tubercles few, low, barely detectable in preservative; inner tarsal fold short, straight to slightly curved.
Colour in life
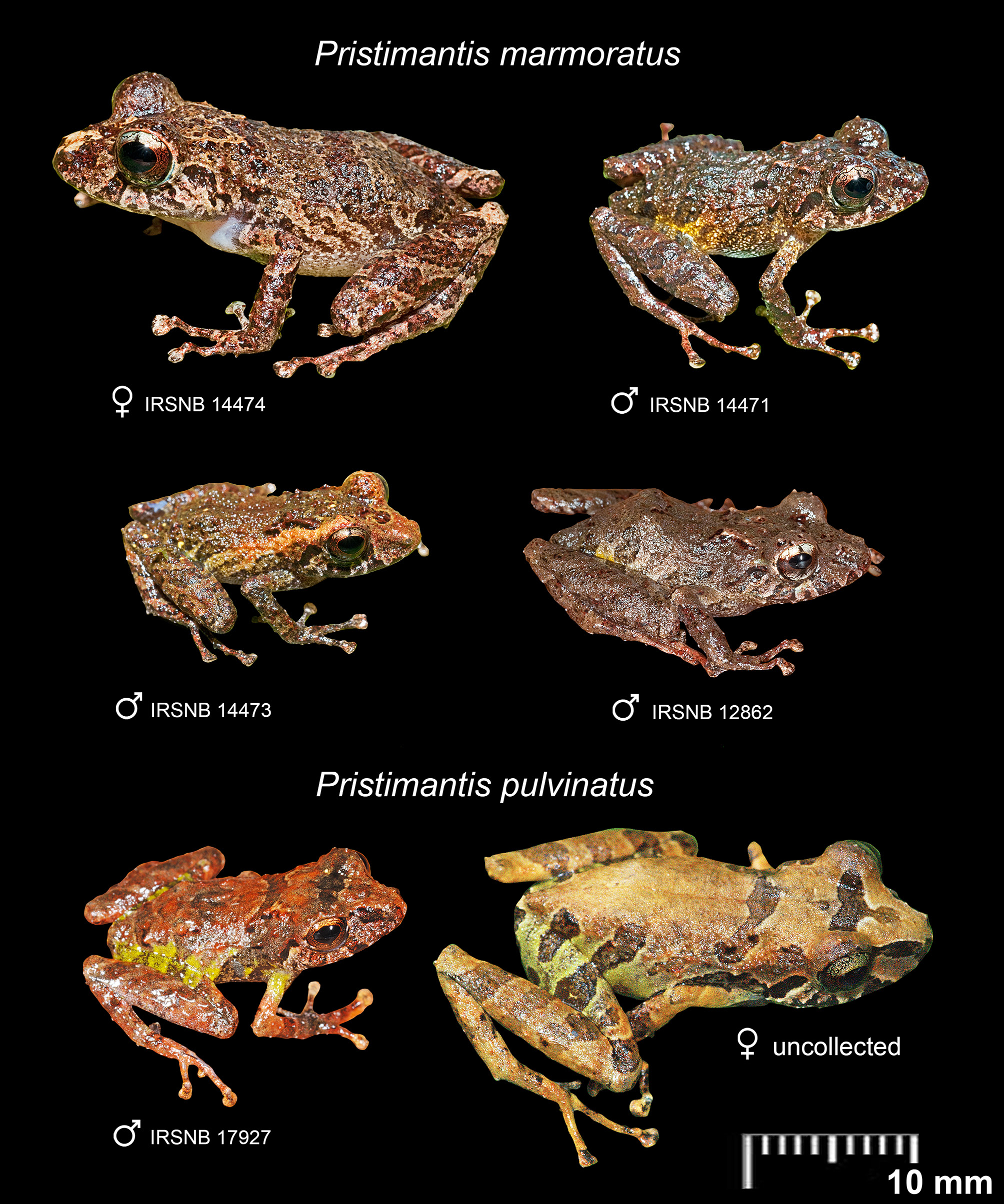
The dorsal pattern of living specimens ranges from large, weakly visible, dark brown or grey-brown blotches to nearly uniformly brownish grey. Dorsolaterally, the pair of oblique scapular ridges is light grey on top and edged with black pigment, running from the level of the posterior of the eye to the level of the armpit. One specimen (IRSNB 14473) has distinct beige oblique lateral stripes running from tip of snout (where they fuse) to groin. Top of the forearm with a single, medial, dark brown band about onethird to one-half the width of the forearm; top of the thigh with three wide dark brown bands alternating with three light grey or reddish brown bands, all continuing onto the top of the tarsus. Diffuse yellow or paler green wash on groin. A dark blotch runs from over the ear to the angle of the mouth and two wide, dark vertical patches span from the eye to the upper lip ( Fig. 4 View Fig.4 ). Canthus rostralis not set off by colour from the snout or upper lip as in some other Pristimantis (e.g., in P. pulvinatus , see Fig. 4 View Fig.4 ). Iris pale gold to bronze with fine black reticulations, a median horizontal brown to reddish brown bar, and a vertical streak running across the iris ( Fig. 4 View Fig.4 ). The ventral pattern is fairly variable and may change depending on light intensity, varying from whitish to grey or dark brown with series of tiny, dark brown punctuations and light grey flecking, the latter mostly on the belly. Skin on belly is translucent with some organs visible through it. Anterior and lower posterior surfaces of thighs are brown to dark brown with light grey flecking. Palms and soles are grey to dark brown.
Colour in preservative
Dorsal colour in specimens after ca 10 years in preservative is similar to when alive, but with the melanin more prominent, revealing the large, indistinct blotches on some specimens and a more or less uniformly distributed medium brown colour on others. Relatively distinct light areas in the shape of a V extend backward from the posterior of the orbit with the apex of the V terminating at the scapular ridges at the level of the insertion of the forelimbs; margins of the light V patch are usually edged with black pigment. The dark transverse bands on the forearm and legs described for living frogs are much more pronounced in preserved specimens. Anterior and lower posterior surfaces of thighs are light brown with white flecking. The diffuse yellow/ pale green wash on the groin present in life becomes immaculate white in preservative. Ventral colouration is a uniformly distributed series of tiny, warm brown punctuations on the chin, belly, and undersurfaces of the legs, feet, and toes ( Fig. 5 View Fig. 5 ). The preserved holotype (> 115 years in preservative, Fig. 2 View Fig.2 ) has a similar colour pattern as the other specimens examined, except that its skin has turned completely brown dorsally and ventrally, and some markings have faded.
Colour in juvenile
The colour pattern of one live juvenile ( Fig. 6D View Fig. 6 ) was similar to that of the adults, except that a wide band of black pigment wrapped around the anterior one-third of the body from the middle of the belly to between the eyes and the top the orbits, including the forearms and feet. Another blotch of black pigment covered one-fifth of the posterior of the back and cloaca as well as on the opposing parts of the heels and tips of the knees. Light brown colour with a faint stippling of tiny dark brown dots formed a dorsal saddle between the darkly pigmented areas and coloured the entire snout, top of the hind limbs, a roundish blotch over the ear, and on top of the two small warty scapular ridges. As in the adults, three medium brown, transverse crossbands are evident on top of all three segments of the hind limbs, continuing from the sides of the body. Two prominent, black-pigment-fringed, medium brown dorsoventral streaks run from the bottom of each orbit to the upper lip. As in adults, the iris is bronze with fine black reticulations, a median horizontal reddish brown bar and a vertical streak running across the iris. The ventral parts of the juvenile were completely black covered by light grey and light greenish brown flecks.
Sexual dimorphism
Sexual dimorphism is evident in size ( Figs 4–5 View Fig.4 View Fig. 5 ), with the only known adult female being much larger than adult males (max. 20.4 mm SVL in males vs 27.9 mm SVL in female), and by the presence of 1–2 whitish nuptial pads in males. Since only one female is known, possible sexually dimorphic characters are hard to evaluate, but we nevertheless note differences in the dorsal skin texture (less tuberculate in the female, with less prominent oblique scapular ridges; Figs 4–5 View Fig.4 View Fig. 5 ). No evident sexual dichromatism is detected, although the only known female has a large whitish unpigmented blotch on the lower throat and upper chest, which is absent in males ( Fig. 5 View Fig. 5 ). Compared to SVL, hands and tibia are slightly longer in males than in the only known female (HAND 27–30% vs 26% of SVL in female; TIL 54–59% vs 50% of SVL in female), and feet are longer in males than in the only known female (FL 40–50% vs 32% of SVL in female). No additional significant difference was detected in other size ratios.
Comparison with other species from the Guiana Shield
The combination of FI <II, SVL ≤ 20.4 in males, presence of vocal slits in males, granular / pustulate dorsal skin with well-developed scapular ridges, basal webbing between fingers, fringes on fingers and toes, crossed iris, diffuse yellow or pale green wash on groin, absence of flashy colour on axillary/ preaxillary region, and absence of conspicuous canthal stripe immediately distinguish Pristimantis marmoratus from all described congeners reported from the Guiana Shield, lowlands included.
More specifically compared to the 23 described species of Pristimantis currently reported from the Guiana Shield uplands and highlands (i.e., between ca 400 and 2900 m elevation), Pristimantis marmoratus is immediately distinguished from P. abakapa Rojas-Runjaic, Salerno, Señaris & Pauly, 2013 , P. aureoventris Kok, Means & Bossuyt, 2011 , P. auricarens (Myers & Donnelly, 2008) , P. cantitans (Myers & Donnelly, 1996) , P. dendrobatoides Means & Savage, 2007 , P. imthurni Kok, 2013 , P. jamescameroni Kok, 2013 , P. jester Means & Savage, 2007 , P. marahuaka (Fuentes-Ramos & Barrio-Amorós, 2004) , P. muchimuk Barrio-Amorós, Mesa, Brewer-Carías & McDiarmid, 2010 , P. saltissimus Means & Savage, 2007 , P. yaviensis (Myers & Donnelly, 1996) , and P. yuruaniensis Rödder & Jungfer, 2008 , by the presence of large vocal slits in males (absent in the aforementioned species); from P. guaiquinimensis (Schlüter & Rödder, 2007) (male unknown, see Kok & Barrio-Amorós 2013) mainly in having granular/ tuberculate skin (smooth to finely granular in P. guaiquinimensis ), fringes on fingers and toes (absent in P. guaiquinimensis ), basal webbing between most fingers and toes (absent), and smaller SVL in female (27.9 mm in P. marmoratus vs 32.4–34.7 in P. guaiquinimensis ); and from P. vilarsi (Melin, 1941) and P. zeuctotylus (Lynch & Hoogmoed, 1977) mainly in having FI <II (FI> II in P. vilarsi and P. zeuctotylus ). The remainder seven described Pristimantis species found in the Guiana Shield uplands and highlands also have vocal slits in males. Compared to those, P. marmoratus is mainly distinguished from P. avius (Myers & Donnelly, 1997) (endemic to the Sierra Tapirapecó, Amazonas State, Venezuela) by its smaller size (17.6–20.4 mm SVL in males, 27.9 mm SVL in female in P. marmoratus vs 20.0–24.0 mm SVL in males, 31.0–33.0 mm SVL in females in P. avius ), absence of yellow or orange colouring on ventral surface (present in P. avius ), presence of a vertical streak running across the iris (absent in P. avius ), and presence of fringes on fingers and toes (absent in P. avius ); from P. cantitans (Myers & Donnelly, 1997) (endemic to Cerro Yaví, Amazonas State, Venezuela) mainly by its much smaller size (17.6–20.4 mm SVL in males, 27.9 mm SVL in female in P. marmoratus vs 24.9–34.5 mm SVL in males, 31.9–44.7 mm SVL in females in P. cantitans ), presence of a vertical streak running across the iris (absent in P. cantitans ), diffuse yellowish /pale green colouration on groin and absence of colourful marking on posterior thigh surface ( P. cantitans has the posterior thigh surface blackish with yellow flecking, and with a rose wash in the groin, anterior thigh, and concealed portion of the shank); from P. espedeus Fouquet, Martinez, Courtois, Dewynter, Pineau, Gaucher, Blanc, Marty & Kok, 2013 (reported only between 200 and 700 m elevation in French Guiana) mainly by its smaller size (17.6–20.4 mm SVL in males, 27.9 mm SVL in female in P. marmoratus vs 20.7–24.8 mm SVL in males, 29.4 mm SVL in female in P. espedeus ), diffuse yellowish / pale green colouration on groin ( P. espedeus has the anterior surface of thighs and groin reddish), no sharp demarcation between dorsal and flank colours (present in P. espedeus ) and presence of a vertical streak running across the iris (absent in P. espedeus ); from P. memorans (Myers & Donnelly, 1997) (reported only from the Sierra Tapirapecó in Amazonas State, Venezuela, and adjacent Amazonas State in Brazil) by the presence of fringes on fingers and toes (absent in P. memorans ), presence of a vertical streak running across the iris (absent in P. memorans ) and in having a diffuse yellowish / pale green colouration on groin (absent in P. memorans ); from P. pruinatus (Myers & Donnelly, 1996) (known only from Cerro Yaví, Amazonas State, Venezuela) by the presence of fringes on fingers and toes (absent in P. pruinatus ), in having the tip of Toe V extending to the distal edge of the distal tubercle on Toe IV when toes are adpressed (extends only midway to proximal edge of ultimate subarticular tubercle on Toe IV in P. pruinatus ), presence of a vertical streak running across the iris (absent in P. pruinatus ), absence of dark orange brown on ventral surface and anterior and posterior thigh surfaces (present in P. pruinatus ), and discernible dorsal colour pattern in preservative (dorsum patternless, blackish grey in P. pruinatus ); from P. pulvinatus ( Rivero, 1968) , reported from the uplands of the Gran Sabana region of south-eastern Venezuela to adjacent western Guyana, therefore sympatric with P. marmoratus , mainly in having a vertical streak running across the iris (upper part of streak absent in P. pulvinatus , see Fig. 4 View Fig.4 ), prominent scapular ridges in males (absent or usually fainter in P. pulvinatus , see Fig. 4 View Fig.4 ), canthus rostralis not conspicuously marked (dark canthal stripe present in P. pulvinatus , see Fig. 4 View Fig.4 ), absence of broad, conspicuous, dorsal dark bands between eyes and between axilla (usually present in P. pulvinatus , see Fig. 4 View Fig.4 and Rivero 1968: fig. 1), and in lacking any flashy colour on axillary/ pre-axillary region (present, green in P. pulvinatus , see Fig. 4 View Fig.4 ); and from P. sarisarinama Barrio-Amorós & Brewer-Carías, 2008 (endemic to Sarisariñamatepui, Bolívar State, Venezuela) mainly by the presence of fringes on fingers and toes (absent in P. sarisarinama ), in having a vertical streak running across the iris (absent in P. sarisarinama ) and in having a diffuse yellowish/ pale green colouration on groin (absent in P. sarisarinama ).
Advertisement call
Temporal structure
The advertisement call of Pristimantis marmoratus consists of a single, unpulsed (tonal) note repeated at a rate of 11.25–11.87 calls /min. Mean call duration is 0.0184 ± 0.002 s (0.016– 0.023 s). The intercall silent interval is relatively uniform and has a mean of 6.011 ± 0.9 s and a range of 5.023– 7.958 s ( Figs 7–8 View Fig. 7 View Fig. 8 ).
Spectral structure
Four to five harmonics are developed, with the dominant frequency located in the first (fundamental) harmonic (mean: 3047 ± 121 Hz; range: 2756–3101 Hz) ( Figs 7–8 View Fig. 7 View Fig. 8 ). Calls show a downward followed by a slightly upward frequency modulation ( Fig. 8 View Fig. 8 ).
Comparison with other Pristimantis calls in the Guiana Shield uplands and highlands
To the best of our knowledge, only three uplands /highlands Pristimantis species for which documented calls are known produce a single note per call: P.aureoventris from above 2200 m elevation onWei-Assiputepui and on the highest slopes of Mt. Roraima ( Kok et al. 2011), P. muchimuk Barrio-Amorós, Mesa, Brewer-Carías & McDiarmid, 2010 from above 2300 m elevation on Churí-tepui, and P. yuruaniensis Rödder & Jungfer, 2008 from above 2300 m on Yuruaní-tepui. The call of P. aureoventris mainly differs in having a lower dominant frequency (2180–2430 Hz vs 2756–3101 Hz in P. marmoratus ), and a higher call rate (18 calls /min vs ca 11 calls / min in P. marmoratus ). The call of P. muchimuk is longer (0.027– 0.062 s vs 0.016– 0.023 s in P. marmoratus ) and emitted at a much faster pace (> 100 calls/ min vs ca 11 calls/ min in P. marmoratus ). The call of P. yuruaniensis is longer (0.093– 0.139 s vs 0.016– 0.023 s in P. marmoratus ) and the dominant frequency is at 1980 Hz (2756–3101 Hz in P. marmoratus ).
Interestingly, the call of Pristimantis marmoratus is extremely similar to the call of P. inguinalis (Parker, 1940) , a species known from the lowlands of the eastern Guiana Shield, east of the Essequibo River. Both species have an unpulsed single note call with a dominant frequency within the same range. Based on our analysis of a call of P. inguinalis provided in Marty & Gaucher (1999) and partial data from Fouquet et al. (2013), the only differences found are (1) a slightly longer call length in P. inguinalis (0.023– 0.032 s vs 0.016– 0.023 s in P. marmoratus ); (2) a slightly higher call rate in P. inguinalis (17.91 calls/ min vs 11.25–11.87 calls/ min in P. marmoratus ); and (3) a slightly shorter inter-call silent interval in P. inguinalis (3.16– 3.85 s vs 5.02– 7.96 s in P. marmoratus ). See Discussion for further comments about distribution and phylogenetic relationships; see also fig. 6 in Fouquet et al. (2013) for comparison.
Distribution
Pristimantis marmoratus is currently known only from – west to east – the La Escalera region in Venezuela, the southern base of Mount Roraima in Venezuela (type locality) and the north-eastern base of Mount Roraima in Guyana (Double Drop Falls), the slopes of Maringma-tepui along the border between Guyana and Brazil, Mount Ayanganna in Guyana ( MacCulloch & Lathrop 2009), the Wokomung Massif in Guyana and Kaieteur National Park in Guyana ( Fig. 1 View Fig. 1 ). The species occurs between ca 600 and 1800 m above sea level, and is probably restricted to the pristine submontane and montane rainforests of the Pantepui uplands and highlands, east of the Gran Sabana, i.e., the Eastern Pantepui District of McDiarmid & Donnelly (2005). Its occurrence in northern Brazil is very likely, since parts of the slopes of Maringma-tepui are in Brazil ( Fig. 1 View Fig. 1 ).
Natural history
All specimens were found in undisturbed submontane or montane rainforest ( Fig. 9 View Fig. 9 ), active on small trees 50–300 cm above the ground, exclusively at dusk. Pristimantis marmoratus is not a common species; only a few specimens have been found at each locality of occurrence. Males were found calling (in June and November) upside down from mossy tree trunks of low diameter (<10 cm) between 120 and 300 cm above the ground, except one male (IRSNB 14473), which was calling from the top of a green leaf 50 cm above the ground. The “upside down” call posture is also found in the closely related P. sp. 1 of Fouquet et al. (2013) (as recovered in our preliminary molecular phylogenetic analysis, see below), and in P. espedeus and P. inguinalis .
In June 2012, which corresponds to the rainy season in the area, a cluster of four Pristimantis marmoratus eggs ( Fig. 6B View Fig. 6 ) was found by one of us (DBM) attached to the inside part of a leaf of a bromeliad, Guzmania cf. sphaeroidea (André) André ex Mez ( Fig. 6A View Fig. 6 ), 150 cm above the ground, on Mount Kopinang, Wokomung Massif, near the top of Kamana Falls at about 1600 m elevation (04°59′58″ N, 59°52′49″ W). Molecular analyses confirmed conspecificity of these eggs with P. marmoratus (Appendix 3). The large, white eggs did not have visibly developed embryos. After photographing and preserving the eggs, the small plant was investigated for inhabitants of the aquatic portion of the phytotelmata. Immediately a small frog jumped out and disappeared into the deep ground litter, and eggs and tadpoles of Anomaloglossus beebei (Noble, 1923) were found in the water of the phytotelmata of the same small bromeliad and in the water of five other bromeliads nearby (egg /frog identifications confirmed by molecular analyses). Pristimantis marmoratus and Anomaloglossus beebei thus share the same bromeliad as an oviposition site on the Wokomung Massif ( Fig. 6C View Fig. 6 ). Other Pristimantis species found in syntopy with P. marmoratus were P. dendrobatoides (above 1600 m elevation), P. jester (above 1300 m elevation), P. saltissimus (above 1000 m elevation), and P. pulvinatus (above 1000 m elevation).
Phylogenetic relationships
Other Pristimantis species have often been confused with Pristimantis marmoratus (see below and Table 1). Available phylogenetic analyses using samples from misidentified specimens are thus unreliable for that taxon (e.g., Hedges et al. 2008; Pyron & Wiens 2011; Canedo & Haddad 2012; Kok et al. 2012; Padial et al. 2014; Mendoza et al. 2015; see below).
Our molecular phylogenetic analysis based on a single mitochondrial gene (16S) of correctly identified – or reidentified – specimens of the Pristimantis “ unistrigatus group” in the Guiana Shield recovered Pristimantis marmoratus as unambiguously distinct from all the other included members of the group ( Fig. 10 View Fig. 10 ). The gene fragment we used is short and many members of the “ unistrigatus group” occurring outside the Guiana Shield have not been included. Therefore, our analysis remains limited in deciphering the phylogenetic position and affinities of P. marmoratus with the other species of the genus. Nevertheless, our analysis recovered P. marmoratus as the sister species (with strong support, pp = 0.98) of an unnamed Pristimantis species from the lowlands of French Guiana and Amapá State ( Brazil), called P. sp. 1 by Fouquet et al. (2013). However, the lowest genetic distances (0.077) are found between P. marmoratus and other Pantepui species ( P. abakapa and P. sp. Ayanganna, see Table 3).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Brachycephaloidea |
|
Family |
|
|
Genus |