Caridina ravisankarani, Vijayamma & Dhamorikar & Manchi, 2021
|
publication ID |
https://doi.org/10.11646/zootaxa.5057.3.5 |
|
publication LSID |
lsid:zoobank.org:pub:30EB798C-44C8-4D11-9834-E051764E93AB |
|
DOI |
https://doi.org/10.5281/zenodo.5593605 |
|
persistent identifier |
https://treatment.plazi.org/id/5A617900-FFDA-FFF1-2BB3-FC18446BE886 |
|
treatment provided by |
Plazi |
|
scientific name |
Caridina ravisankarani |
| status |
sp. nov. |
Species: Caridina ravisankarani View in CoL sp.nov.
Materials examined
Measurements. Holotype male: tl— 28 mm; cl— 9.5mm; rl— 2 mm; paratypes male – tl— 24 mm; cl— 9 mm; rl— 2mm; female: tl— 23mm; cl— 8 mm; rl— 1.8 mm.
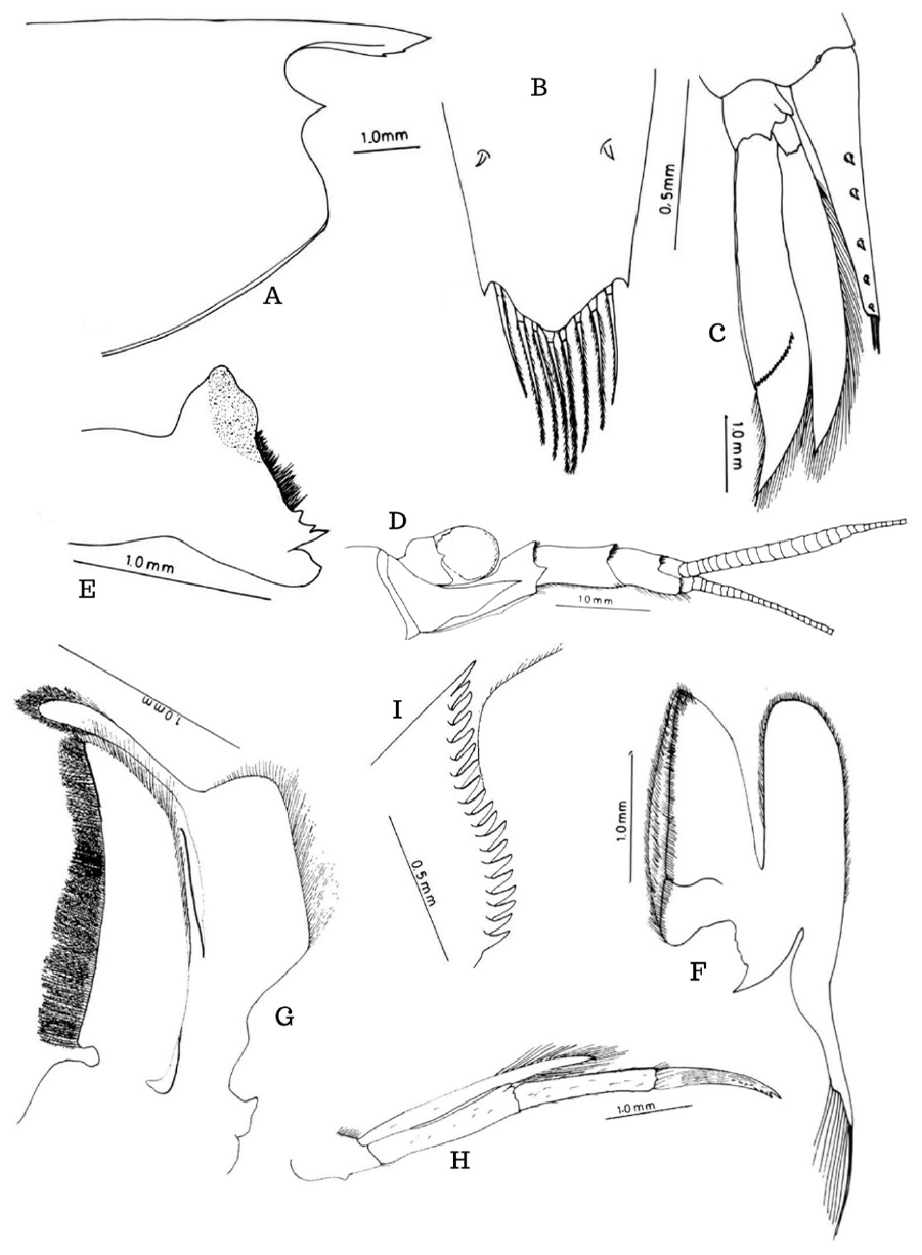
Description. Rostrum short, slender, shallow, reaching as far as the distal end of basal segment of the antennular peduncle, distal end pointed and directed forwards, upper margin straight edentulous, two-third of proximal ventral margin nearly straight and distal third gently curved, bears two very minute teeth, adrostral carina prominent incomplete, situated at the proximal half at the lower region and divides unequally; carapace smooth, orbit broad, antennal spine prominent, pterygostomian angle broadly rounded ( Fig. 1A View FIGURE 1 ).
Eyes with cornea pigmentation variable, from totally absent to a small black spot.
The sixth abdominal segment is a little longer than the fifth. Telson broad basally and narrow distally reaches as far as the outer spine of uropodal exopod, disto-lateral margins pointed; upper surface bears 5 pairs of spines, promixal most pair at about one-third distance from the base, distal pair subdistal in position; disto-median part not spinous, distal region bears 8 long spines and without short spines, outermost pair with setae only on the inner side, remaining bipectinate, the base of each spine with chitinous structures ( Fig. 1B, C View FIGURE 1 ).
Antennular peduncle reaches beyond the level of outer spine of antennal scale, three segments in the ratio 1.00:0.55:0.54; basal segment broad, lateral border ends distally as a characteristic triangular prominence, stylocerite long, sharp and extends as far as 80% of the proximal segment; middle and distal segments almost equalsized; 16 segments at the proximal part of outer flagellum swollen, characteristic, inner flagellum slightly broad ( Fig. 1D View FIGURE 1 ). Antennal scale fully developed, 0.38 times broad as long, the outer lateral spine extends to 0.79 times its length, and flagellar peduncle 0.49 times the length of the scale. Incisor process stronger than molar process with one broad, one sharp, and two smaller teeth, molar process with concavity, a series of short stiff closely packed fine setae in between molar and incisor processes; apophysis long and broad ( Fig. 1E View FIGURE 1 ). Maxillulae with coxa very broad bears a fringe of stiff spinous setae on the inner border, basis also broader with scattered setae, endopod slender. Maxillae with coxa and basis highly flattened with closely set stiff long setae in rows on the inner border, coxa smaller, endopod reduced, and exopod flattened with much elongated setae ( Fig. 1F View FIGURE 1 ).
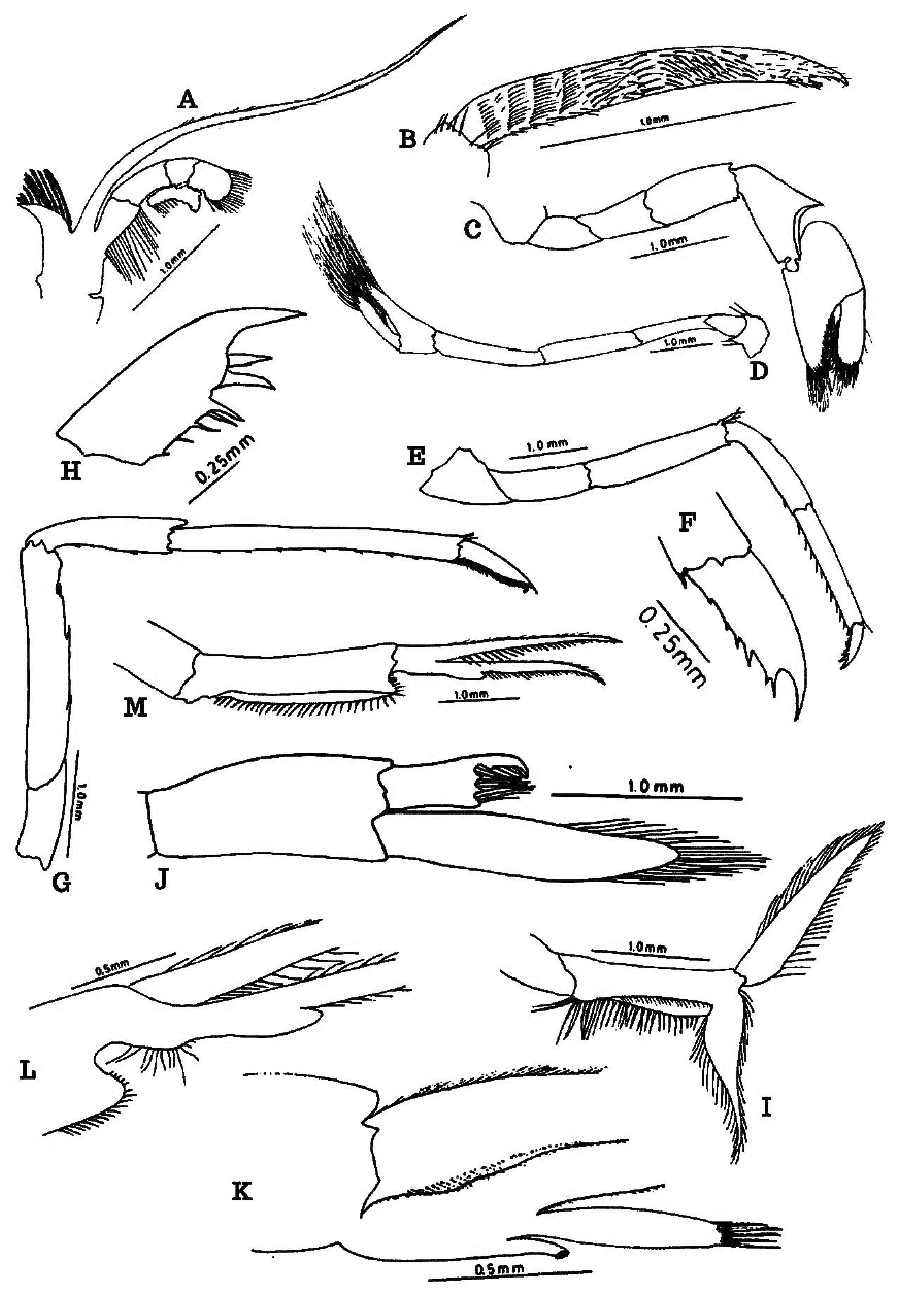
First maxilliped with highly flattened short coxa and long broad basis, inner borders with stiff long closely set stiff setae; endopod short, exopod highly flattened and membranous, basal part with a rectangular caridean lobe bearing long setae and a club-shaped distal portion with very long setae at the tip ( Fig. 1G View FIGURE 1 ). Second maxilliped with endopod reduced in structure, ischium, and merus almost equal-sized, carpus ring-shaped, propodus distally expanded and dactylus highly reduced, inner margin of ischium and the distal surface of propodus bear long stiff spinous setae; exopod slender and highly elongated, more than two times longer than endopod and tip bears very long setae ( Fig. 2A View FIGURE 2 ). Three segments of the third maxilliped in the ratio 35.4:34.3:30.3; distal segment ends in a sharp spine and bears seven spines on distal flexor margin of which distal-most attains the size of the distal spinous part of the segment so that it appears a claw and bunches of plumose stiff long setae along basal 2/3 distance, distal 1/3 with scattered setae, exopod reaches about half of the second segment and with setae on either side distally ( Figs. 1H View FIGURE 1 , 2B View FIGURE 2 ).
First pereopod stronger than second; Ischium, merus, carpus, propodus, palm, and fingers in the ratio 17.1:25.7: 31.4:47.0:25.7:21.4; distal carpus as broad as palm, distal region highly excavated and longer than merus, propodus longer than carpus; palm swollen, fingers slightly shorter than palm, spoon-shaped with characteristic long setae, bushy along the rim of both fingers ( Fig. 2C View FIGURE 2 ). Second pereopods slender, ischium, merus, carpus, propodus, and fingers in the ratio 19.0: 27.4: 32.1:21.4:16.2; merus and carpus longer than propodus; fingers longer than palm and its tips with a very long tuft of setae, characteristic to species ( Fig. 2D View FIGURE 2 ).
Third pereopods with ischium, merus, carpus, propodus, dactylus in the ratio 12.3:32.5:19.5:27.1:8.7; propodus with 11 spinules on flexor margin; dactylus ends in a sharp curved spine and with 4–5 spinules on flexor margin, merus longest ( Fig. 2E, F View FIGURE 2 ). Fourth pereopods with merus and propodus of almost same size, propodus with 10 minute spinules on flexor margin and dactylus ends in a sharp curved spine and with 7–8 spinules on flexor margin ( Fig. 2H View FIGURE 2 ). Fifth pereopods with ischium, merus, carpus, propodus, dactylus in the ratio 8.4:28.9:16.9:35.0:10.8; merus with three, propodus with seven spinules on flexor margins and carpus with one distal spine; dactylus longer than ischium and ends in a sharp curved spine and with 42–43 bristle setae, propodus longest ( Fig. 2G View FIGURE 2 ).
Pleopods. Female: first pleopods more pediform, basis concave on the inner side, with long stiff setae along margins, exopod flat with scattered stiff setae on free border and endopod modified as in figure ( Fig. 2I View FIGURE 2 ); male: basis flat, exopod flattened with setae more towards tip, endopod with characteristic finger-shaped appendix interna and tip with stiff setae ( Fig. 2J View FIGURE 2 ). Second pleopod male: basis slightly broader and bears appendix masculina and appendix interna, appendix interna 1/3rd of appendix masculina ( Fig. 2K View FIGURE 2 ); female: basis leg like with appendix interna ( Fig. 2L View FIGURE 2 ); remaining pleopods pediform with exopod and endopod and appendix interna; basis as long as or slightly longer than rami and with scattered setae ( Fig. 2M View FIGURE 2 ). Uropods highly developed, exopod with a sharp spine at 2/3 distance, diaeresis with 18 prominent strong spines ( Fig. 1C, I View FIGURE 1 ).
Paratypes also possess the same characters described above.
The present species can be identified from the closely related species by the key provided below. 1 5–7 5 th 44–77 setae; Dorsal surface of telson with pairs of spines; dactylus of pereopod with prominent dieresis with 16–23 spines.............................................................................................. 2 th 41–44 17–19 - Dorsal surface of telson with 4–5 pairs of spines; dactylus of 5 pereopod with prominent setae; dieresis with spines.............................................................................................. 3 st
2 Disto-median region of telson pointed, outer lateral spines short and in-curved; carpus of 1 cheliped equals merus and fingers th shorter than palm; dactylus of 5 pereopod with 56–77 spinous setae; rostrum short, upper margin edentulous and ventral margin with 1–6 teeth............................................................ typus H. M. Edwards, 1837 st - Disto-median region of telson pointed, outer lateral spines short; carpus of 1 cheliped shorter than merus and fingers longer th 44 2–8 than palm; dactylus of 5 pereopod with spinous setae; rostrum long, upper margin edentulous and ventral margin with teeth............................................................................ villadolidi Blanco, 1939 View in CoL st 3 Disto-median region of telson pointed, outer lateral spines short-incurved; carpus of 1 cheliped shorter than merus and th 41–49 fingers longer than palm; dactylus of 5 pereopod with spinous setae; rostrum short, upper margin edentulous and ventral margin with 1–5 minute teeth situated at distal part; outer antennular flagellum without swollen basal segments............................................................................................. jeani Cai, 2010 View in CoL
1 st - Disto-median region of telson not pointed, outer lateral spines absent; carpus of cheliped longer than merus and fingers 5 th 42–43 setae; rostrum upper shorter than palm; dactylus of pereopod with spinous short, margin edentulous and ventral margin with 2 minute teeth situated at distal part; 16 segments at the basal part of outer antennular flagellum swollen......................................................................................... ravisankarani View in CoL sp. nov.
Coloration in life. Male and female both entirely translucent, with mild yellowish spots overall, more on carapace.
Notes on biology. Collection during June and December -January, the deep and shallow pools in the cave were dominated by berried females and probably the peak breeding period. A female collected in June 2018 had as many as 1300 eggs. The eggs (n=29) had an average length of 0.71± 0.03 mm (Mean±SD) and a width of 0.42± 0.03 mm.
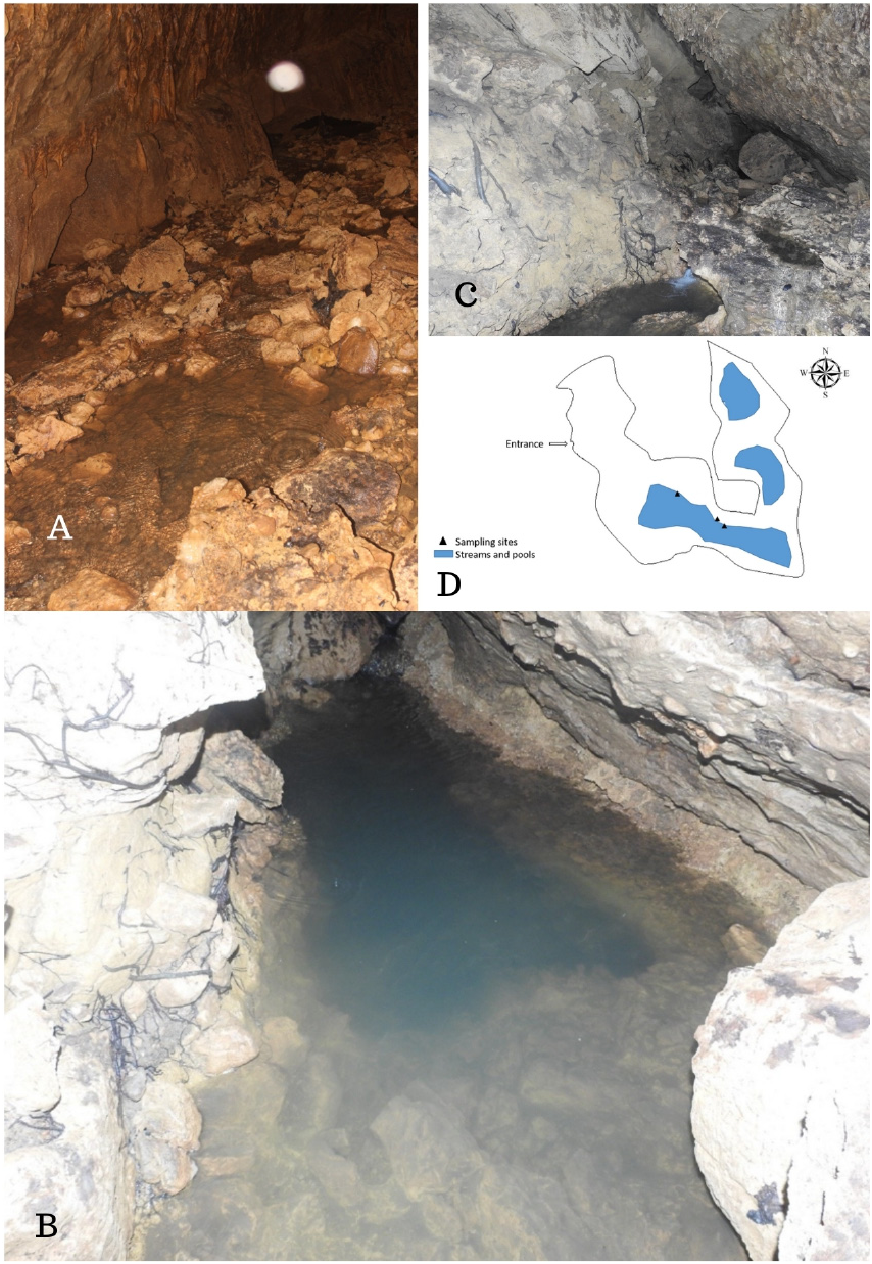
Distribution. A cave (CN2) on the Interview Island, Andaman and Nicobar Islands, India.
Habitat. CN2, a limestone cave around a kilometre inland from the west coast in the central part of Interview Island, holds underground water ( Fig. 3A, B, C View FIGURE 3 ). As the primary source of this water is rainwater and its runoff, the stream inside the cave flows during monsoon and post- monsoon periods leaving small pools during the dry season. The stream flows from one end of the cave (south-west) where the gushing water cascades into the deep pools, and towards the mid-section, it splits into streamlets and shallow pools ( 0.2 m depth) and ultimately goes underground at north-west where the cave is raised. The water in the cave travels approximately 20 m before emptying underground ( Figs. 3A, B, C View FIGURE 3 ). Except for a small portion near the opening, the entire length of the cave has no light. The water temperature in the cave’s sample collection zones was between 25–26˚C. The pH of the water was between 6.0–7.0. During the sample collection, the air temperature and relative humidity were 24˚C and 93.2%, respectively. This cave has seasonal deficient oxygen levels, especially during the pre-monsoon (dry) season between March and June. Geologically Interview Island has Archipelago series rock type ( Bandopadhyay & Carter 2017). The significant water source inside caves is through percolation during the monsoon and postmonsoon periods. This percolating water brings in organic matter as an external energy source to form a significant energy supply for the subterranean life in some caves ( Culver & Pipan 2009).
In CN2, shrimps were found under rocks, boulders, and crevices in the pools ( 0.2m to 8m deep) ( Fig. 3A, B, C View FIGURE 3 ). The presence of various sized individuals during the visits and no signs of their presence in the epigean water bodies indicate it being restricted to the hypogean habitats. Being photophobic, these shrimps quickly darted in the opposite direction when exposed to the light source. Some other shrimps and amphipods were also encountered in the same pools inside CN2 and are being identified.
Etymology. The species is named in honour of the renowned ornithologist and conservation biologist Dr. Ravi Sankaran, who pioneered cave studies in the Andaman and Nicobar Islands. He had first systematically documented the caves in the Andaman and Nicobar Islands while searching for populations of the cave-dwelling Ediblenest Swiftlet, A. fuciphagus Thunberg, 1812 and his dream and motivation led to the cave faunal study initiated in the Andaman Islands today.
Remarks. The present new species is closely related to Caridina typus H. Milne Edwards, 1837, Caridina villadolidi Blanco, 1939 and Caridina jeani Cai, 2010 . It can be at once separated from others based on the characters given in Table 1. C View TABLE 1 . typus has been extensively studied by H. Milne Edwards (1837), Bouvier (1904; 1905; 1913; 1925), De Man (1892), Edmondson (1935), Holthuis (1960), Cai (2010). In C. typus and C. villadolidi the rostrum extends as far as the tip of 2nd and 3rd segments of the antennular peduncle, respectively. The present new species rostrum is shorter and extends only up to the distal end of the basal segment of the antennular peduncle. Moreover, the position of adrostral carina and disposition of teeth are characteristic to the species. Disto-median part of telson ends in a sharp point and with 4–5 (up to 9) spines with setae and two curved-in short spines at the distolateral end in C. typus, C. jeani and C. villadolidi , whereas in the present species, the telson doesn’t end in a sharp point at disto-medial part and disto-lateral region with eight spines and without a pair of short spines; dorsal spines are distributed in the distal half in all the three species whereas in the present new species the proximal-most pair is at 1/3 rd distance from the base.
The middle segment of the antennular peduncle is longer than the distal segment in C. typus, whereas these segments are almost equal-sized in the present new species. The specimens of the new species possess a swollen basal part of the outer flagellum with 16 segments in the specimens studied and is characteristic.
The endopod of the second maxilliped has propodus and dactylus atrophied, and the nature of setation on it are characteristics to the species. In many species of Caridina reported from caves possess atrophied nature as above ( Guo et al. 1992, Liang 1993, Liang and Zhou 1993, Cai and Li 1997, 1999, Cai and Ng 2018). First pair of chelate legs in which merus and carpus are equal-sized in C. typus, merus longer than carpus in C. villadolidi whereas in the present new species carpus is longer than merus. Fingers are nearly equal to palm or slightly shorter and bear very long bushy setae and are characteristic to the new species. In all the other three species, fingers are slightly longer with normal setae on fingers. Second chelate legs are slender in all species. Ischium is slightly longer than fingers in the present species, whereas it is shorter in other species. The merus is longer than propodus in the present species, C. jeani and C. villadolidi , whereas it is slightly shorter in C. typus. The fingers possess characteristically very long setae in contrast to other species. The number of spinules on the flexor side of propodi, dactyli vary in all the species under study. There is a considerable difference in the proportion of different segments of 5th pereopod in all the species under study. In the present species, the dactylus possesses 42–43 comb-like bristles similar to C. villadolidi and C. jeani whereas in C. typus it is 56–77.
......continued on the next page
The finger-shaped appendix interna in the present new species differs from the other three species. The diaeresis of the new species is with 18 prominent erect spines with a broad base, is within the range reported for C. jeani (17–19) and C. villadolidi (16–22) whereas C. typus possesses more number (20–23). Moreover, the eyes with reduced pigmentation is an adaptation in the cave environment.
From the foregoing discussion, it is evident that the species described herein deserves the merit to be elevated to a new species based on the difference in the rostrum, antennules and flagellae, mandible, 1st maxilliped, 2nd maxilliped, 3rd maxilliped, bushy nature of setae on fingers and nature of different articles of the first pereopod, long nature of setae on fingers of the second pereopod, nature of spines on the flexor side of 3rd and 4th pereopods, number of bristles on the dactylus of 5th pereopod, appendix interna on male first pleopod, the position of dorsal spines, distal spines and setae of telson, reduced eyes and habitat. General nature of rostrum of this species tends to retain similarity with species, namely, C. typus H. M. Edwards, 1837, C. zebra Short, 1993 , C. confuse Choy & Marshall, 1997 , C. spinula Choy & Marshall, 1997 , C. nudirostris Choy, 1984 , C. singhalensis Ortmann, 1894 , C. imitatrix Holthuis, 1969 , C. villadolidi Blanco, 1939 and C. jeani Cai, 2010 .
Under the family Atyidae De Haan, 1849 (In De Haan, 1833 –1850) several species have been reported stygobionts and it would be appropriate to mention them: Atyoida pilipes ( Newport, 1847) ; Caridina cantonensis Yu, 1938 ; Caridina lovoensis Roth-Woltereck, 1955 ; Caridina troglophila Holthuis, 1965 ; Caridina troglodytes Holthuis, 1978 ; Caridina ablepsia Guo, Jiang and Zhang, 1992 ; Caridina guangxiensis Liang and Zhou, 1993 ; Caridina carvernicola Liang and Zhou, 1993 ; Caridina mengae Liang 1993 ; Caridina demenica Cai and Li, 1997 ; Caridina feixiana Cai and Liang, 1999 ; Caridina wumingensis Cai and NK Ng, 1999 ; Caridina caverna Liang, Chen and Li, 2005 ; Caridina acuta Liang, Chen and Li, 2005 ; Caridina alba Li and Li, 2010 ; Caridina longshan Cai and P K LNg, 2018; Caridina alu Cai and P K LNg, 2018; Caridina spinicrus Cai and P K LNg, 2018; Caridina beiliu Cai and P K LNg, 2018; Caridina jiangkou Cai and P K LNg, 2018; Caridina guilin Cai and P K LNg, 2018; Caridina laticarpalis Cai and P K LNg, 2018; Caridinopsis chevalieri Bouvier, 1912 ; Edoneus atheatus Holthuis, 1978 ; Edoneus erwini Cai & Husana, 2009 ; Edoneus marulas Cai & Husana, 2009 ; Edoneus sketi Cai & Husana, 2009 ; Mancicaris sinensis Liang, Guo, and Tang, 1999 ; Neocaridina brevidactyla Liang, Chen and Li, 2005 ; Palaemonias alabamae Smalley, 1961 ; Palaemonias ganteri Hay, 1902 ; Parisia deharvengi Cai & Ng, 2009 ; Parisia dentata Gurney, 1984 ; Parisia edentata Holthuis, 1956 ; Parisia gracilis Williams, 1964 ; Parisia macrophthalma Holthuis, 1956 ; Parisia microphthalma ( Fage, 1946) ; Parisia unguis Williams, 1964; Stygiocaris lancifera Holthuis, 1960 ; Stygiocaris stylifera Holthuis, 1960 ; Troglocaris anophthalmaanophthalma ( Kollar, 1848) ; Troglocaris anophthalma intermedia Babić, 1922 ; Troglocaris anophthalmalegovici Jugovic, Jalžić, Prevorčnik & Sket, 2012 ; Troglocaris anophthalma ocellata Jugovic, Jalžić, Prevorčnik & Sket, 2012 ; Troglocaris anophthalma periadriatica Jugovic, Jalžić, Prevorčnik & Sket, 2012 ; Troglocaris anophthalma sontica Jugovic, Jalžić, Prevorčnik&Sket, 2012 ; Troglocaris bosnica Sket & Zakšek, 2009 ; Troglocaris planinensis Birstein, 1948 ; Typhlatya arfeae Jaume & Bréhier, 2005 ; Typhlatya campecheae H. H. III Hobbs & H.H. Jr. Hobbs, 1976 ; Typhlatya consobrina Botoşăneanu & Holthuis, 1970 ; Typhlatya dzilamensis Alvarez, Iliffe & Villalobos, 2005 ; Typhlatya elenae Juarrero, 1994 ; Typhlatya galapagensis Monod & Cals, 1970 ; Typhlatya garciadebrasi Juarrero de Varona & Ortiz, 2000 ; Typhlatya garciai Chace, 1942 ; Typhlatya iliffei Hart & Manning, 1981 ; Typhlatya kakuki Alvarez, Iliffe & Villalobos, 2005 ; Typhlatya miravetensis Sanz &Platvoet, 1995 ; Typhlatya mitchelli H. H. III Hobbs & H. H. Jr. Hobbs, 1976 ; Typhlatya monae Chace, 1954 ; Typhlatya pearsei Creaser, 1936 ; Typhlaty arogersi Chace & Manning, 1972; Typhlatya taina Estrada & Gómez, 1987 ; Typhlatya utilaensis Alvarez, Iliffe & Villalobos, 2005 ; Typhlocaridina lanceifrons Liang and Yan, 1981 ; Typhlocaridina liui Liang and Zhou, 1993 ; Typhlocaridina semityhplata Cai, 1995 ; Xiphocaridinella ablaskiri Birstein, 1939 ; Xiphocaridinella dbari Marin, 2019 ; Xiphocaridinella fagei Birstein, 1939 ; Xiphocaridinella falcirostris Marin, 2020 ; Xiphocaridinella jusbaschjani Birstein, 1948 ; Xiphocaridinella kumistavi Marin, 2017 ; Xiphocaridinella kutaissiana Sadowsky, 1930 ; Xiphocaridinella motena Marin, 2019a ; Xiphocaridinella osterloffi ( Juzbaš’jan, 1941); Xiphocaridinella otapi Marin, 2018 ; Xiphocaridinella shurubumu Marin, 2018a ; Xiphocaridinella smirnovi Marin, 2020 . Caridina ravisankarani sp. nov. is a new addition to the species list.
Adaptations. Caridina ravisankarani sp. nov. possesses many adaptations in the cave environment, such as highly reduced pigmentation of eyes, atrophied propodus and dactylus of maxilliped 2, swollen segments of the outer antennular flagellum, more pediform pleopods, stronger ischium and merus of pereopods, and longer setae on all appendages as well as short closely set setae on mandibles. These adaptations help the animal to survive in the harsh environment of caves. These adaptations point towards it being a stygobite, a cave-dwelling aquatic species ( Sket 2008). However, further intensive surveys are recommended to understand more about the species and its habitat.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |