Geostiba Thomson, 1858
|
publication ID |
https://doi.org/ 10.5281/zenodo.155701 |
|
DOI |
https://doi.org/10.5281/zenodo.6277548 |
|
persistent identifier |
https://treatment.plazi.org/id/5B50E916-FF9A-392D-4D2D-FBD0FDF7FE74 |
|
treatment provided by |
Plazi |
|
scientific name |
Geostiba Thomson, 1858 |
| status |
|
Geostiba Thomson, 1858 View in CoL ( Figs. 1340 View FIGURES 1 7 View FIGURES 8 17 View FIGURES 18 20 View FIGURES 21 25 View FIGURES 26 28 View FIGURES 29 33 View FIGURES 34 37 View FIGURES 38 50 View FIGURES 51 56 View FIGURES 57 60 View FIGURES 61 71 View FIGURES 72 76 View FIGURES 77 80 View FIGURES 81 93 View FIGURES 94 99 View FIGURES 100 107 View FIGURES 108 119 View FIGURES 120 125 View FIGURES 126 133 View FIGURES 134 145 View FIGURES 146 151 View FIGURES 152 156 View FIGURES 157 168 View FIGURES 169 173 View FIGURES 174 177 View FIGURES 178 186 View FIGURES 187 192 View FIGURES 193 196 View FIGURES 197 203 View FIGURES 204 212 View FIGURES 213 218 View FIGURES 219 229 View FIGURES 230 246 View FIGURES 247 252 View FIGURES 253 261 View FIGURES 262 266 View FIGURES 267 270 View FIGURES 271 283 View FIGURES 284 288 View FIGURES 289 293 View FIGURES 294 305 View FIGURES 306 309 View FIGURES 310 315 View FIGURES 316 320 View FIGURES 321 326 View FIGURES 327 335 View FIGURE 336 View FIGURE 337 View FIGURE 338 View FIGURE 339 View FIGURE 340 )
Evanystes Gistel, 1856: 387 View in CoL (Type species: Aleochara circellaris Gravenhorst, 1806 , by subsequent designation (Blackwelder 1952)).
Geostiba Thomson, 1858: 33 View in CoL (Type species: Aleochara circellaris Gravenhorst, 1806 , by monotypy).
Sipalia: Fenyes, 1920: 249 View in CoL (non Mulsant & Rey, 1853; non Fauvel, 1902a) (as valid genus in tribe Myrmedoniini Thomson, 1867 ).
Geostiba: Fenyes, 1920: 249 View in CoL (as synonym of Sipalia ).
Sonomota: Fenyes, 1920: 249 (as subgenus of Sipalia ).
Sipalia: Bernhauer & Scheerpeltz, 1926: 599 View in CoL (as valid genus in subtribe Athetina Casey, 1910 View in CoL ).
Geostiba: Bernhauer & Scheerpeltz, 1926: 599 View in CoL (as synonym of Sipalia ).
Sonomota: Bernhauer & Scheerpeltz, 1926: 599 (as subgenus of Sipalia ).
Sipalia: Scheerpeltz, 1951: 166 View in CoL (as valid genus).
Evanystes: Blackwelder, 1952: 163 View in CoL (as valid genus).
Geostiba: Blackwelder, 1952: 163 View in CoL (as synonym of Evanystes View in CoL ).
Geostiba: Benick & Lohse, 1974: 111 View in CoL (as valid genus in tribe Callicerini Lohse, 1969 View in CoL ).
Geostiba: Seevers, 1978: 126 View in CoL (as valid genus in subtribe Geostibina Seevers, 1978).
Evanystes: Seevers, 1978: 126 View in CoL (as synonym of Geostiba View in CoL ).
Glossola: Seevers, 1978: 126 View in CoL (non Fowler 1888) (as synonym of Geostiba View in CoL ).
Sonomota: Seevers, 1978: 128 (as subgenus of Geostiba View in CoL ).
Geostiba: Lohse & Smetana, 1988: 270 View in CoL (as valid genus).
Geostiba: Ashe View in CoL in Newton, Thayer, Ashe & Chandler, 2000: 371 (as valid genus in subtribe Geostibina).
Evanystes: Ashe View in CoL in Newton, Thayer, Ashe & Chandler, 2000: 371 (as synonym of Geostiba View in CoL ). Glossola: Ashe View in CoL in Newton, Thayer, Ashe & Chandler, 2000: 371 (non Fowler 1888) (as synonym of Geostiba View in CoL ).
Sonomota: Ashe in Newton, Thayer, Ashe & Chandler, 2000: 371 (as subgenus of Geostiba View in CoL ). (Other references for Palaearctic Geostiba View in CoL are omitted)
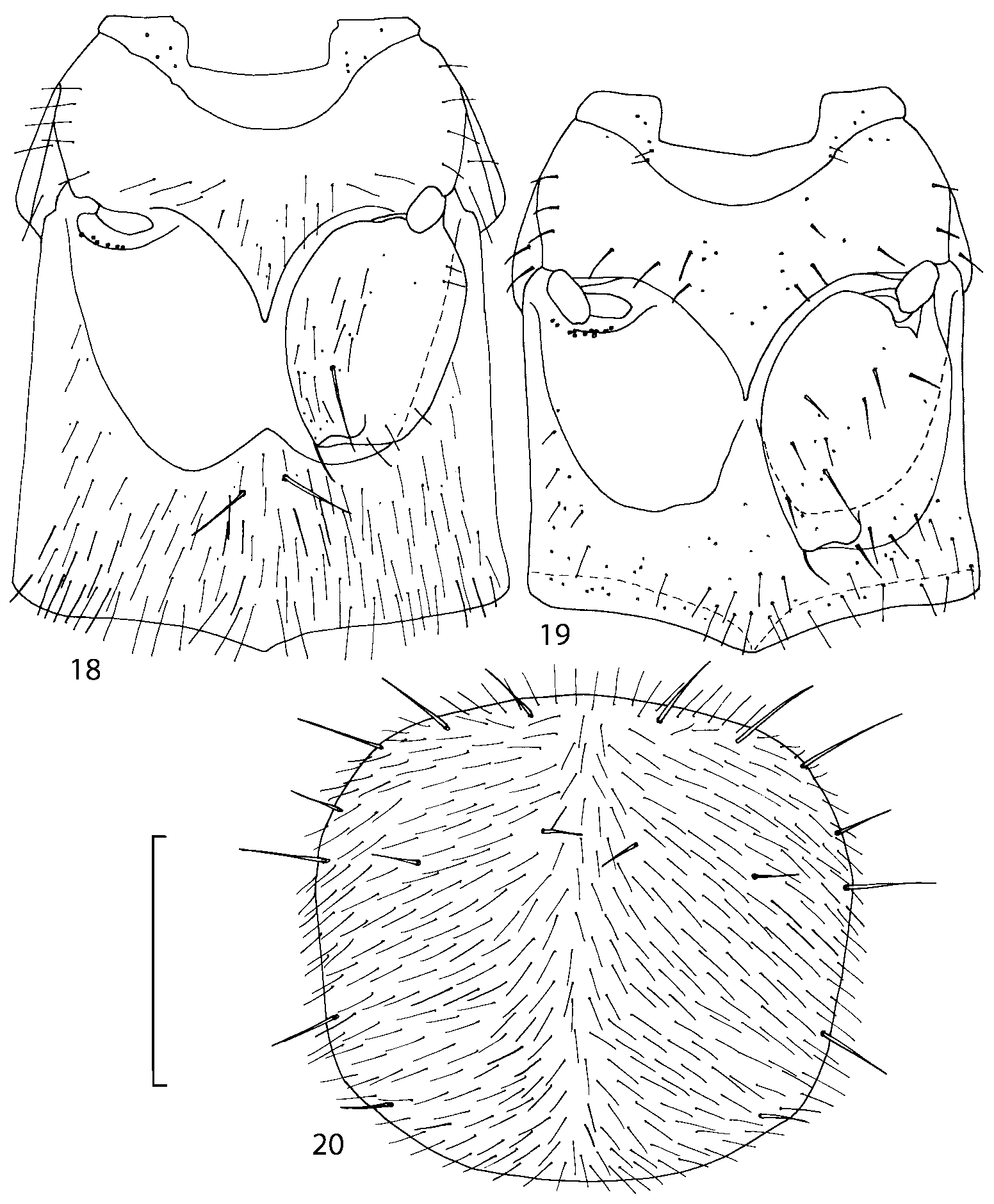
Diagnosis. Geostiba can be distinguished from other athetine genera by the combination of the following characters: parallelsided body; ligula divided into two separate but close lobes ( Figs. 810 View FIGURES 8 17 ); pronotum with microsetae directed posteriorly along the midline of the disc (Type VI or V, Benick & Lohse 1974) ( Fig. 20 View FIGURES 18 20 ); pronotal macrosetae short; pronotal hypomera fully visible in lateral view; mesotibia with short median macroseta; metatarsal segment 1 longer than segment 2; one empodial seta. Most species of Geostiba are wingless, have shortened elytra and reduced eyes.
Geostiba View in CoL differs from the species of Atheta Thomson, 1858 View in CoL with similar pronotal pubescence and short elytra in having the ligula divided into two separate lobes.
Geostiba View in CoL differs from Tropimenelytron Pace, 1983 View in CoL in having contiguous mesocoxae and the ligula divided into two separate lobes.
Geostiba differs from Alpinia Brundin, 1948 in having the ligula divided into two separate lobes.
Geostiba is similar to Ousipalia Gozis, 1886 in having the ligula divided into two separate lobes, but in Geostiba these lobes are contiguous while in Ousipalia they are widely separated.
Description. Length 1.73.2 mm. Body from dark brown to yellow, parallelsided.
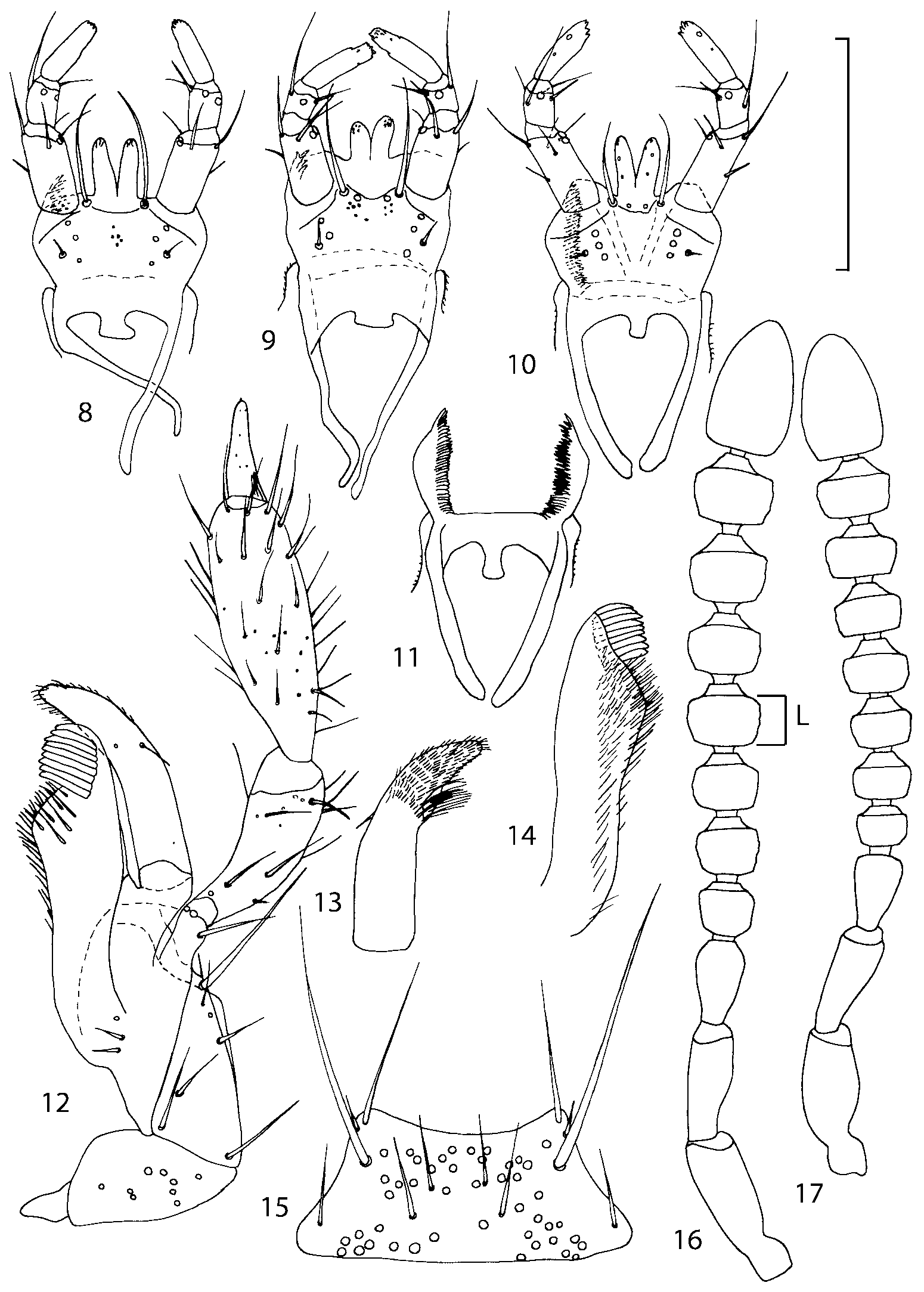
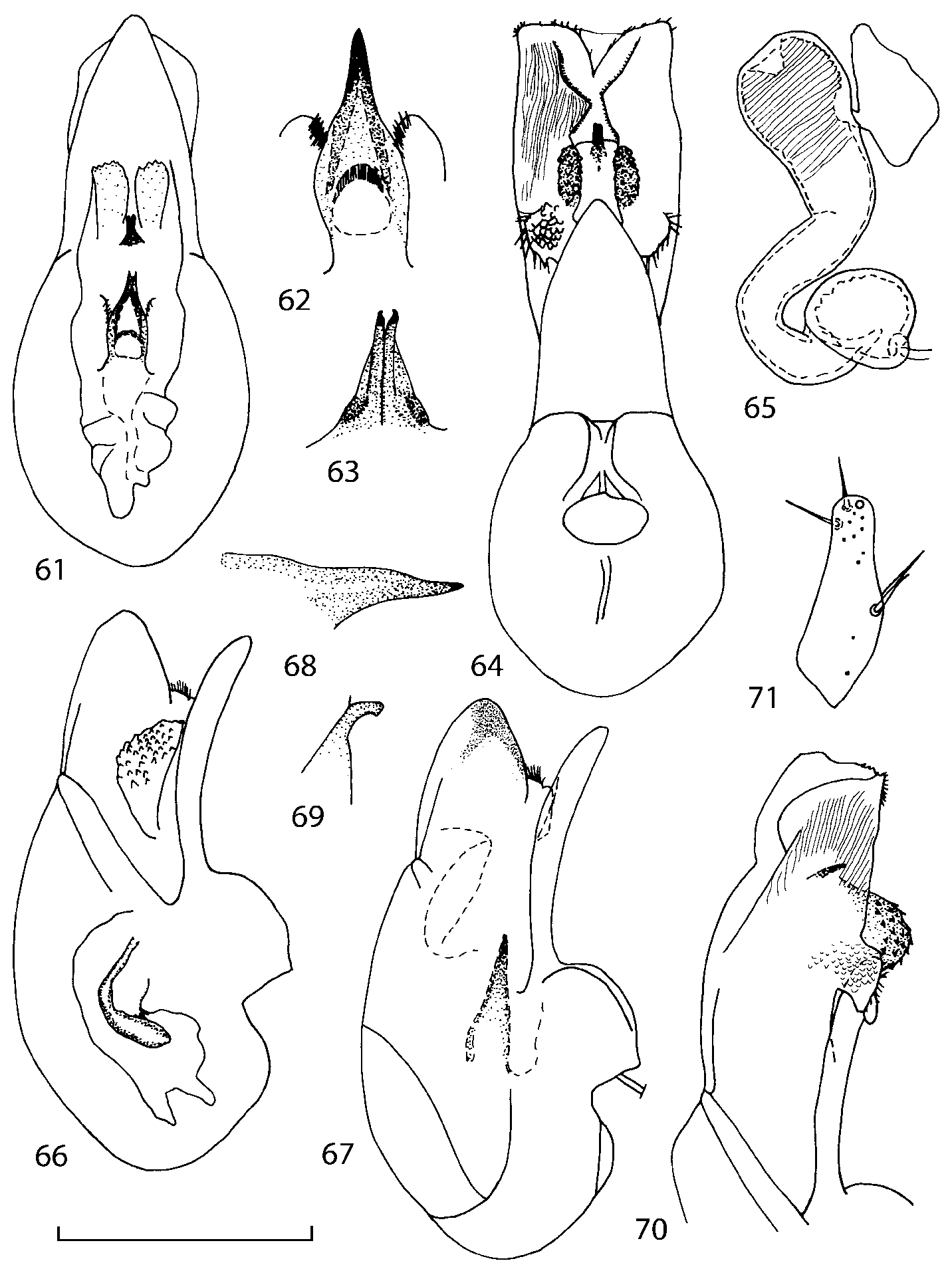
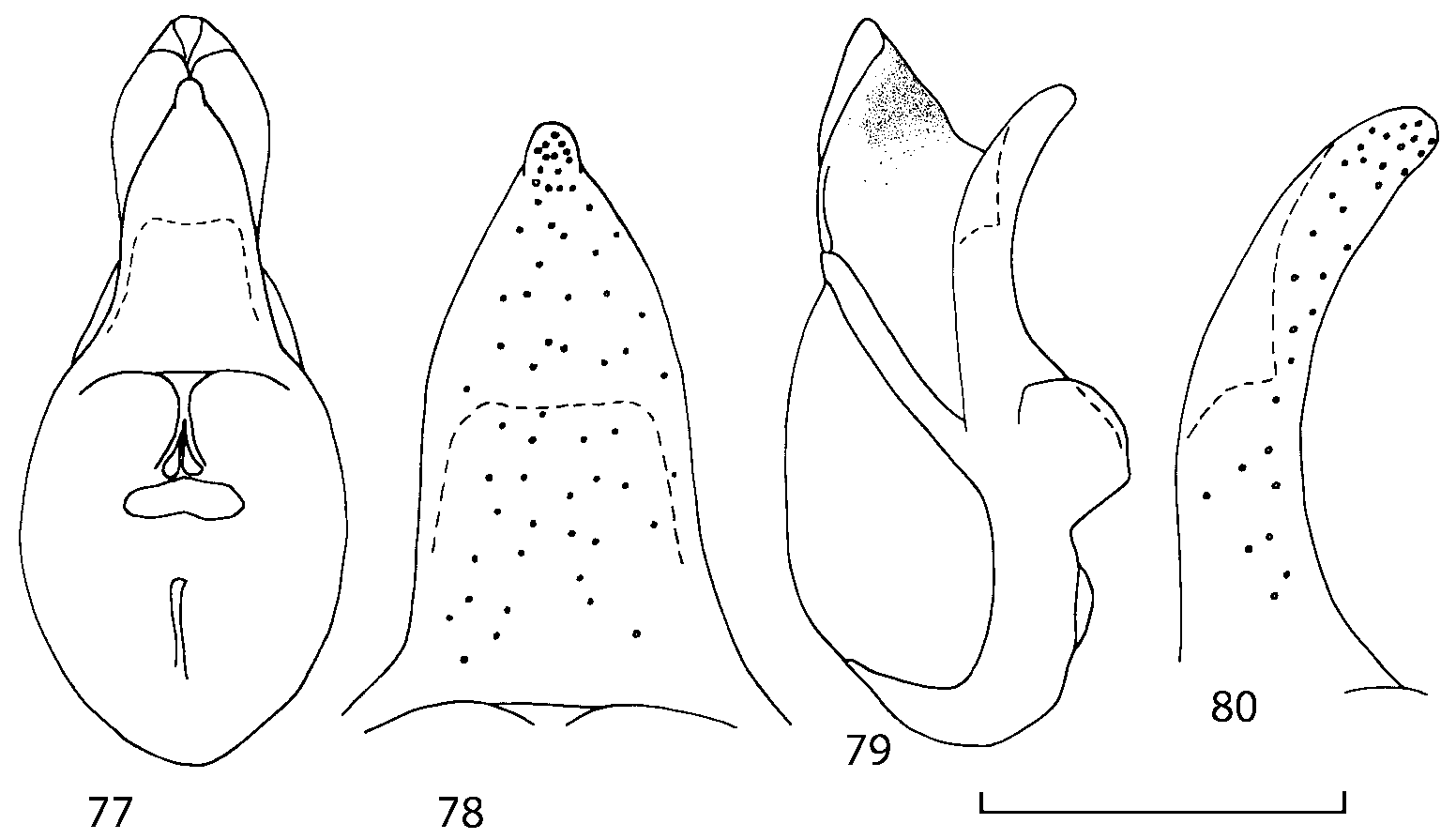
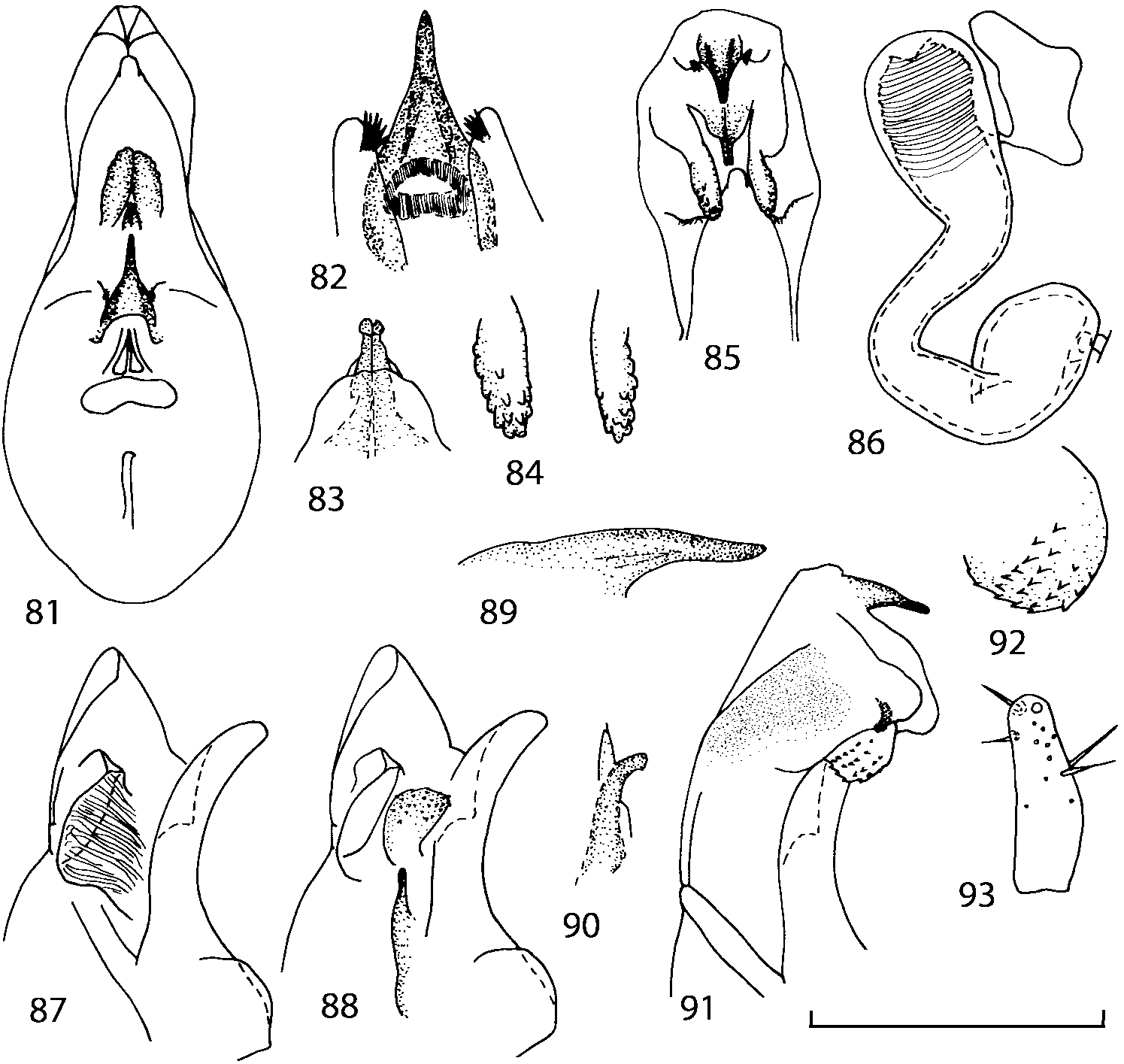
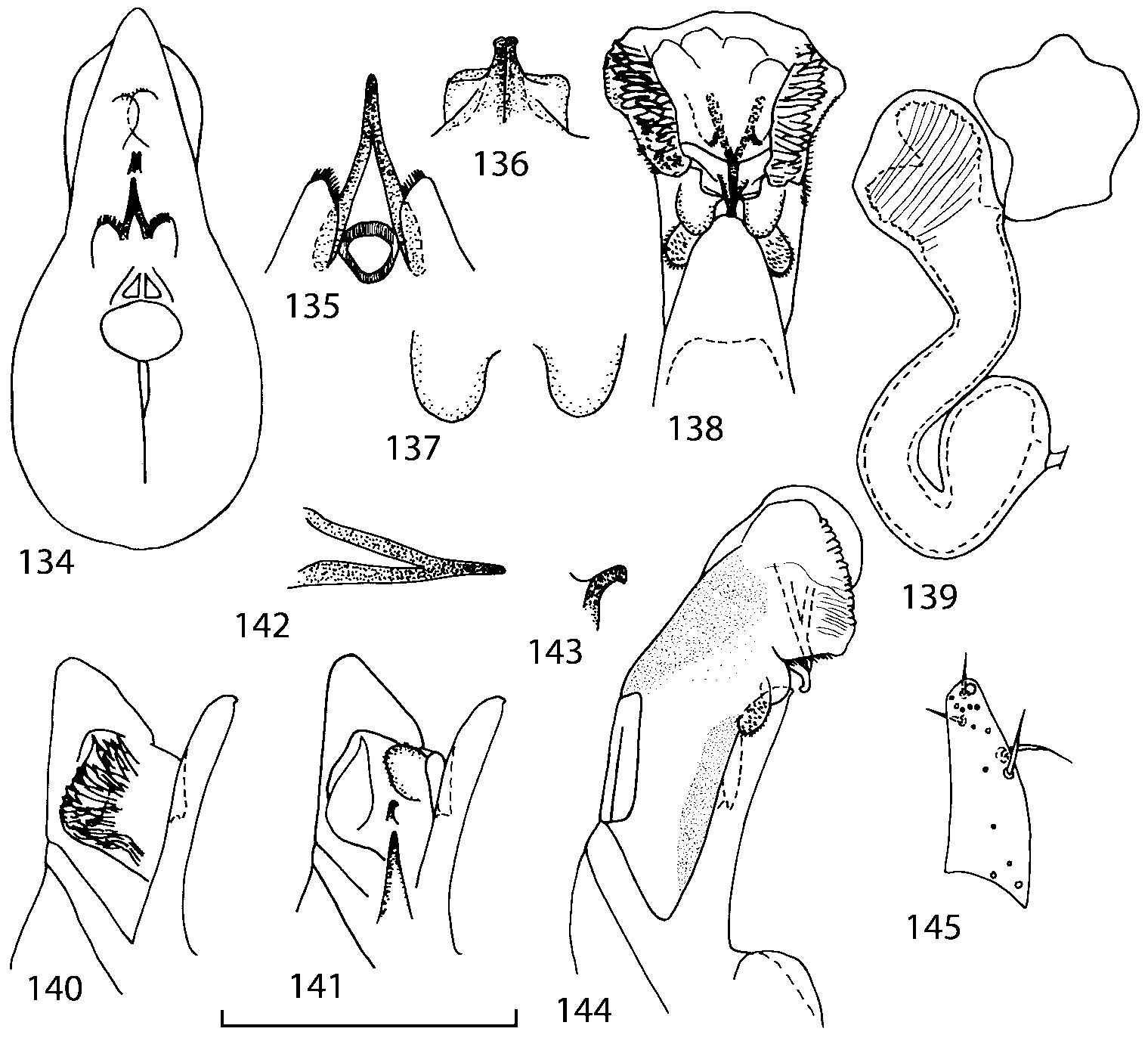
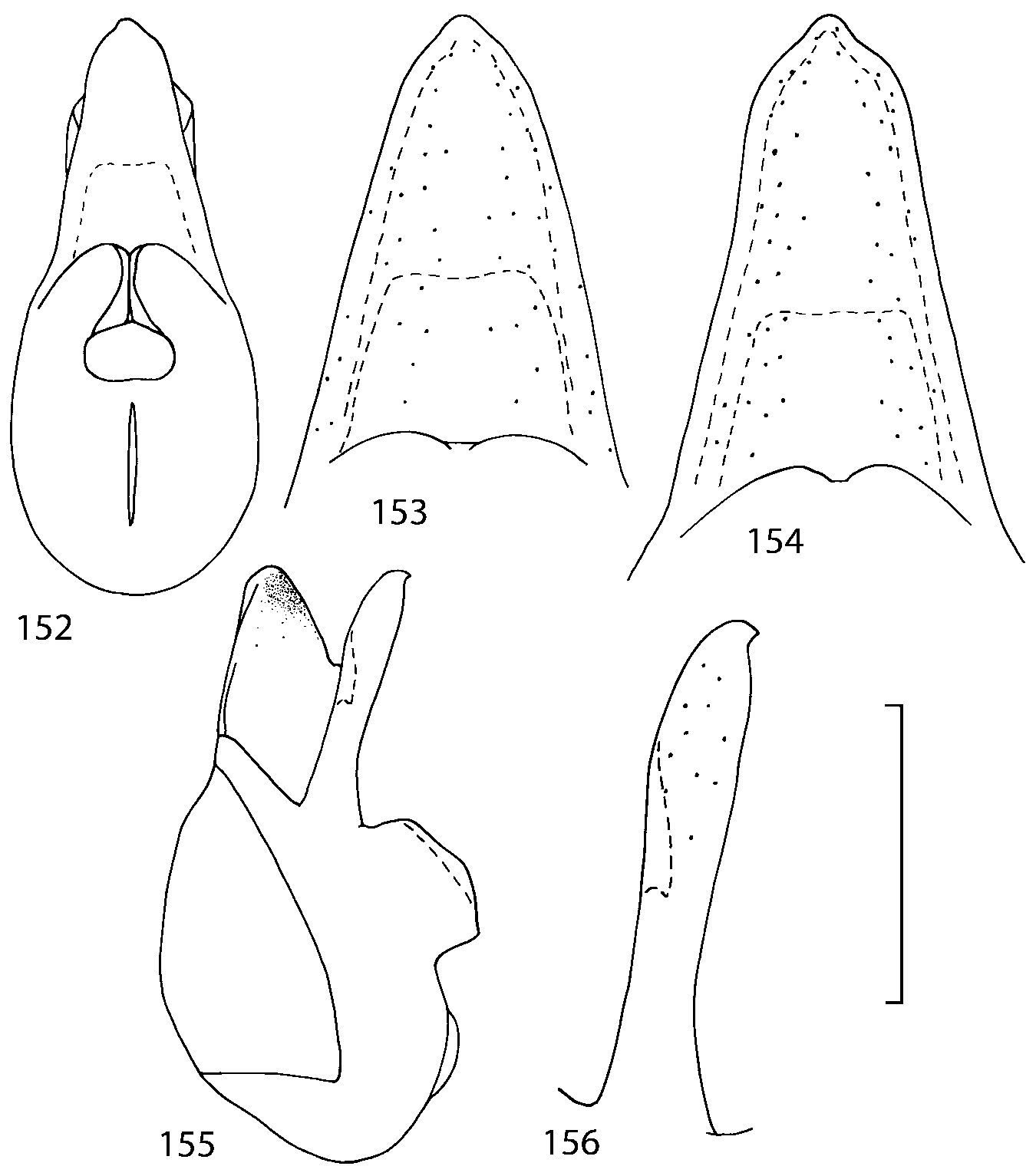
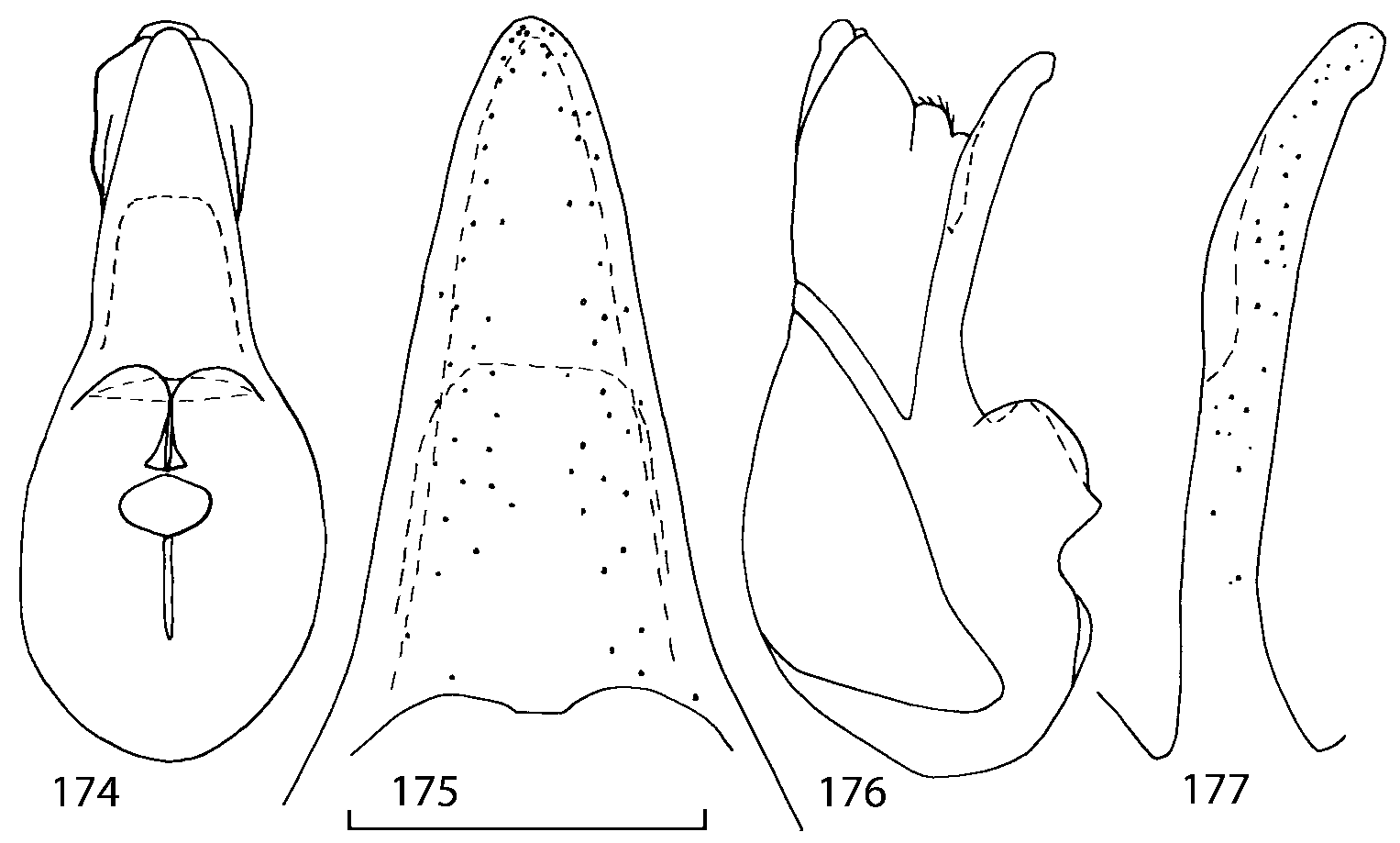
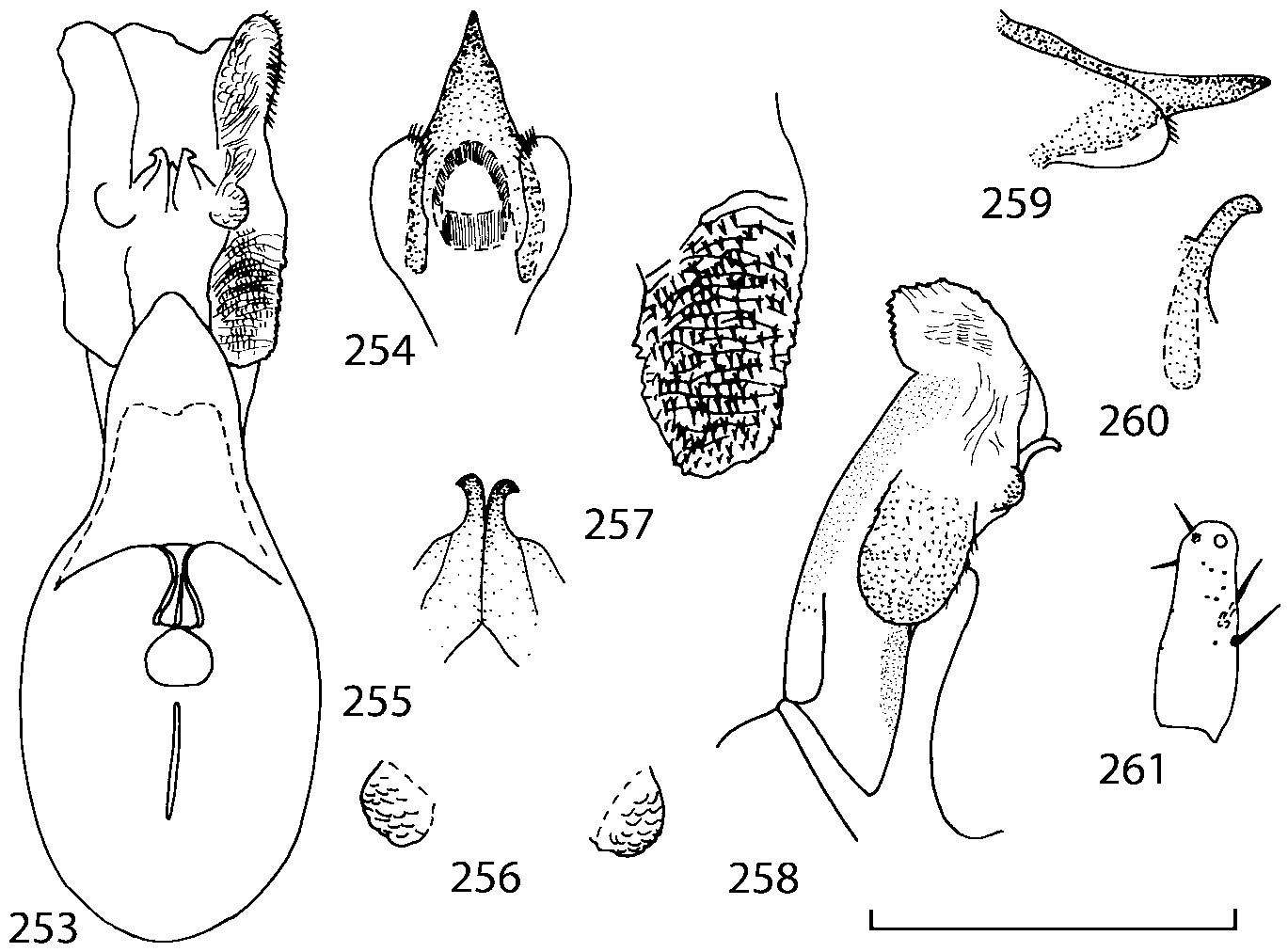
Head as wide as long; eyes 1/2 to 1/10 as long as temples or absent; infraorbital carina absent. Antennal article 2 longer than article 3, 4 subquadrate or transverse, 510 transverse, last article as long as 9 and 10 combined, without coeloconic sensilla ( Figs. 1617 View FIGURES 8 17 ). Labrum ( Figs. 1, 3 View FIGURES 1 7 ) transverse, with straight anterior margin. Adoral surface of labrum (epipharynx) as in Figs. 2, 4 View FIGURES 1 7 . Mandibles ( Figs. 57 View FIGURES 1 7 ) symmetrical and broad, right mandible with a small medial tooth; ventral molar area without patches of denticles (400x). Maxilla ( Figs. 1214 View FIGURES 8 17 ) with galea extending beyond apex of lacinia; apical lobe of galea covered with numerous fine and short setae; apical quarter of lacinia with row of closely spaced spines, middle portion produced medially and covered with numerous setae. Maxillary palpus as in Fig. 12 View FIGURES 8 17 . Labium as in Figs. 810, 15 View FIGURES 8 17 ; labial palpi with three articles; ligula divided into two separate but close lobes; medial area of prementum with 12 pores and 0 10 pseudopores, lateral areas with 23 pores and single spinose pore. Hypopharyngeal lobes as in Fig. 11 View FIGURES 8 17 . Mentum ( Fig. 15 View FIGURES 8 17 ) with anterior margin concave.
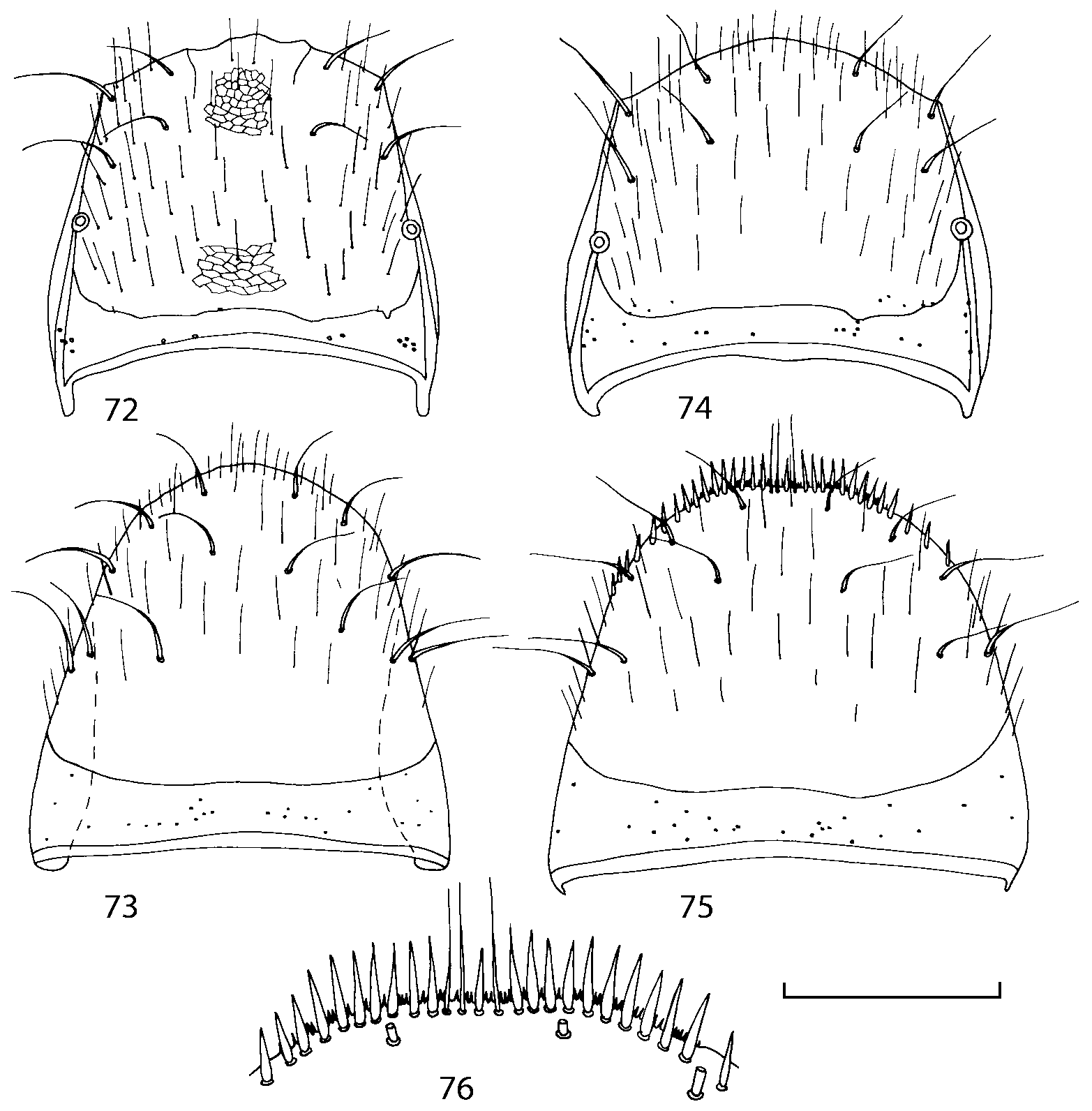
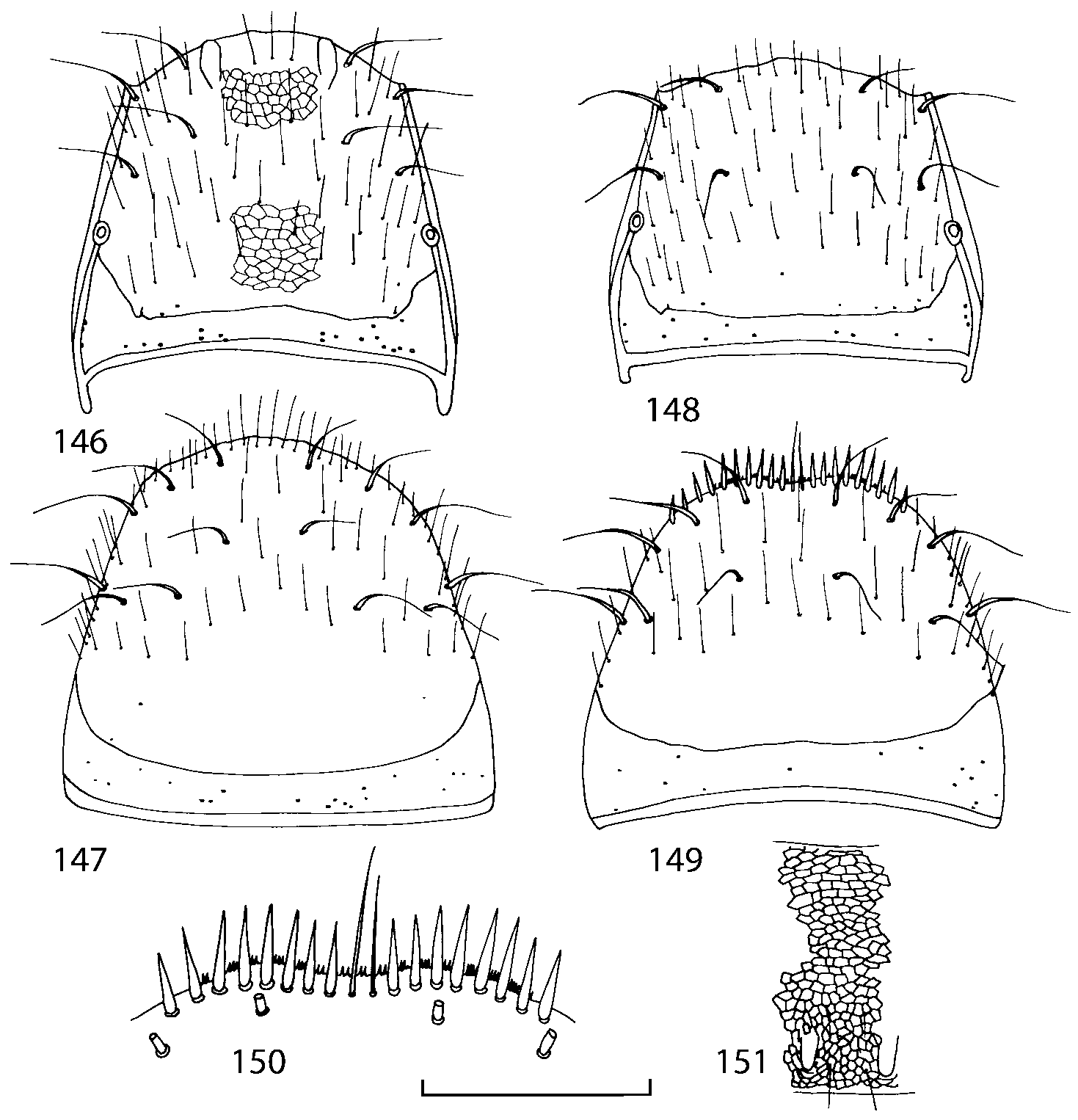
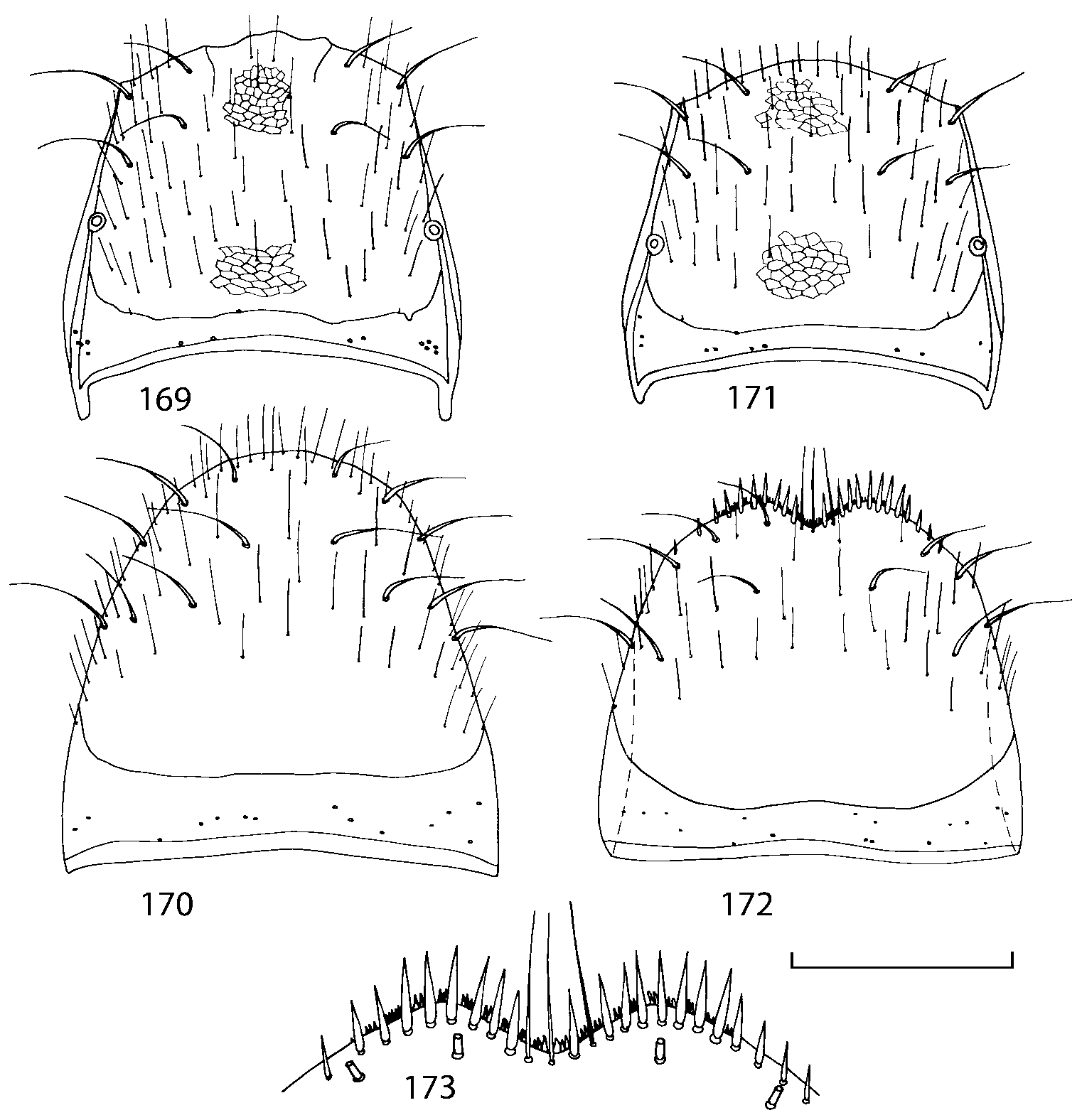
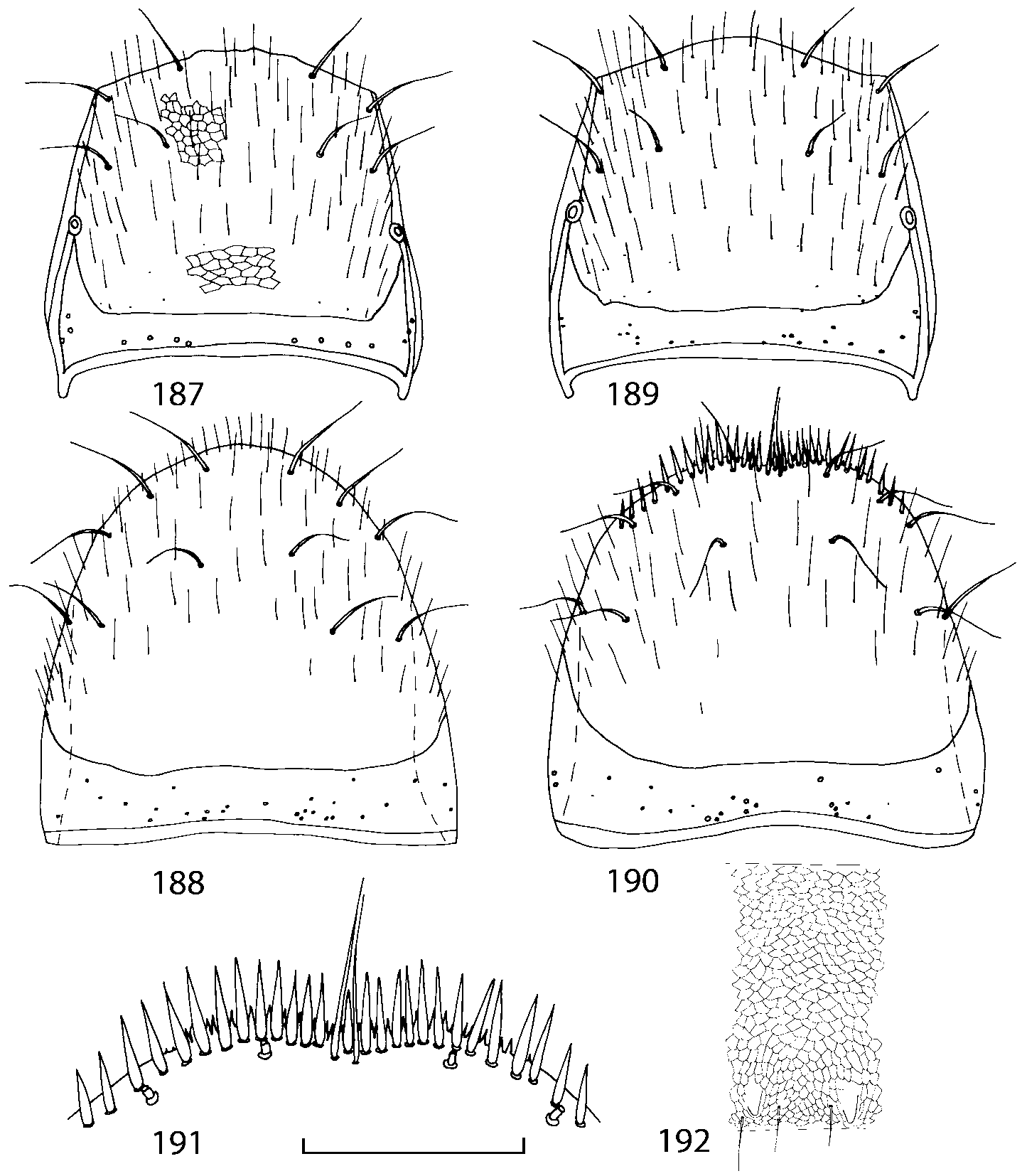
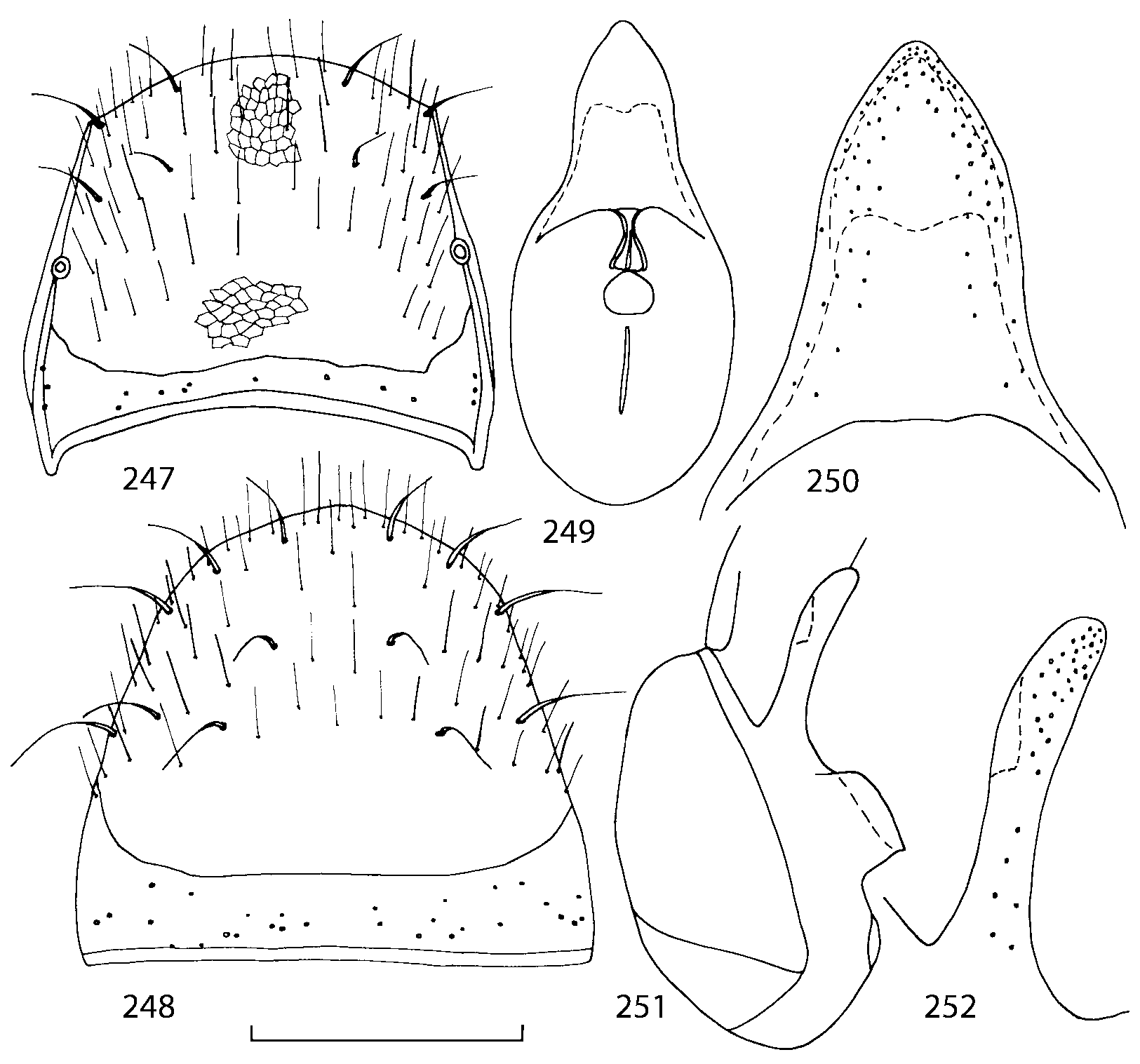
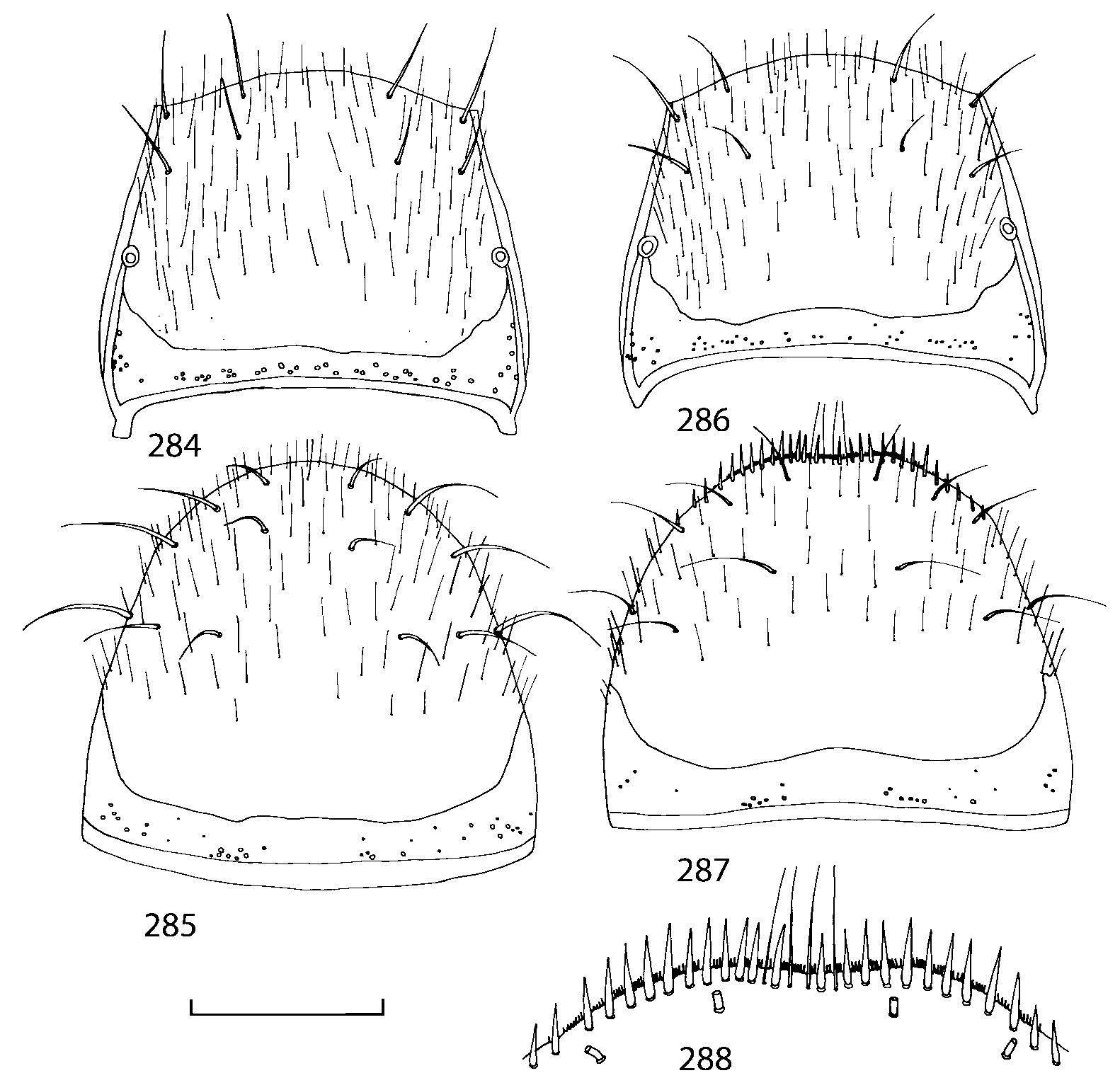
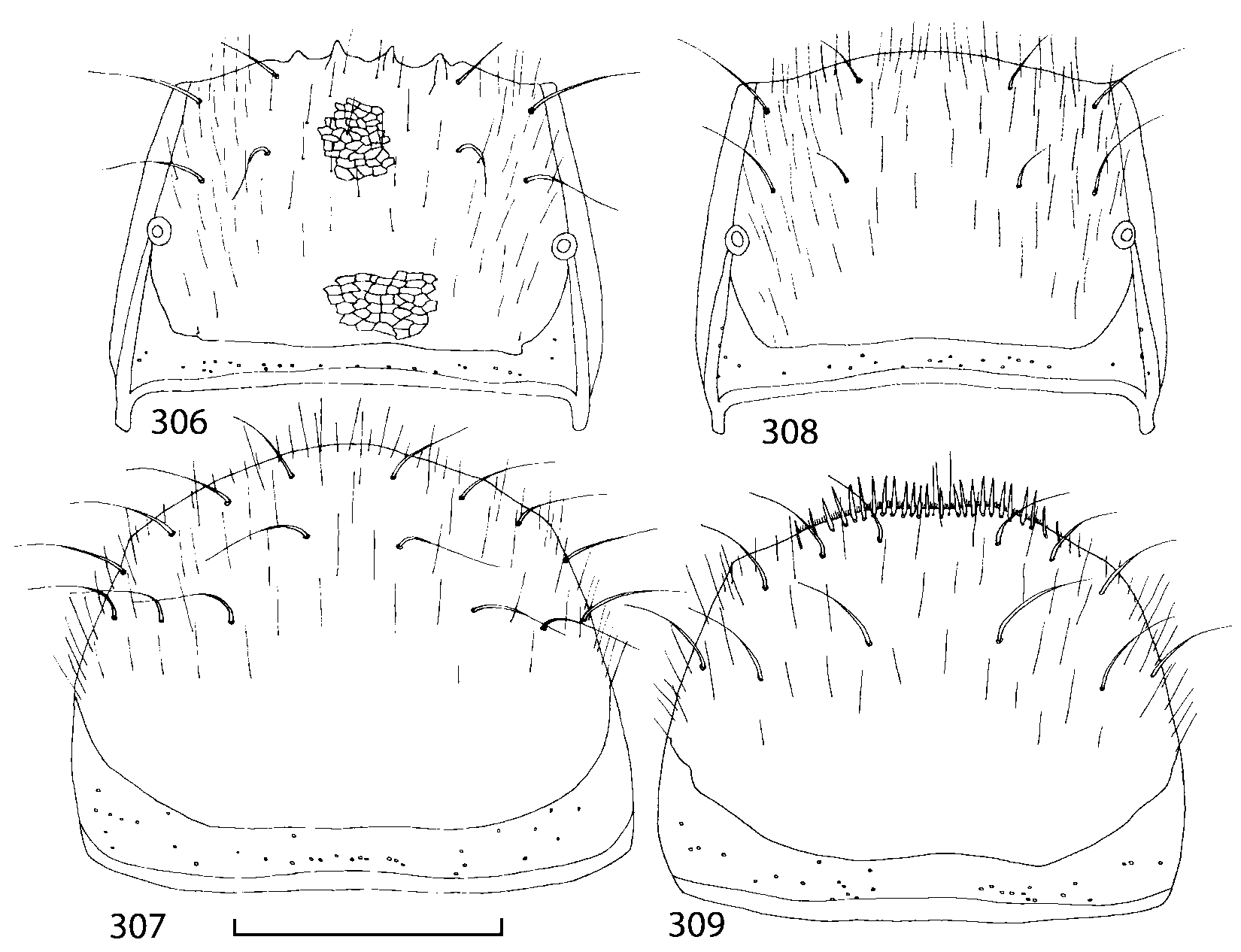
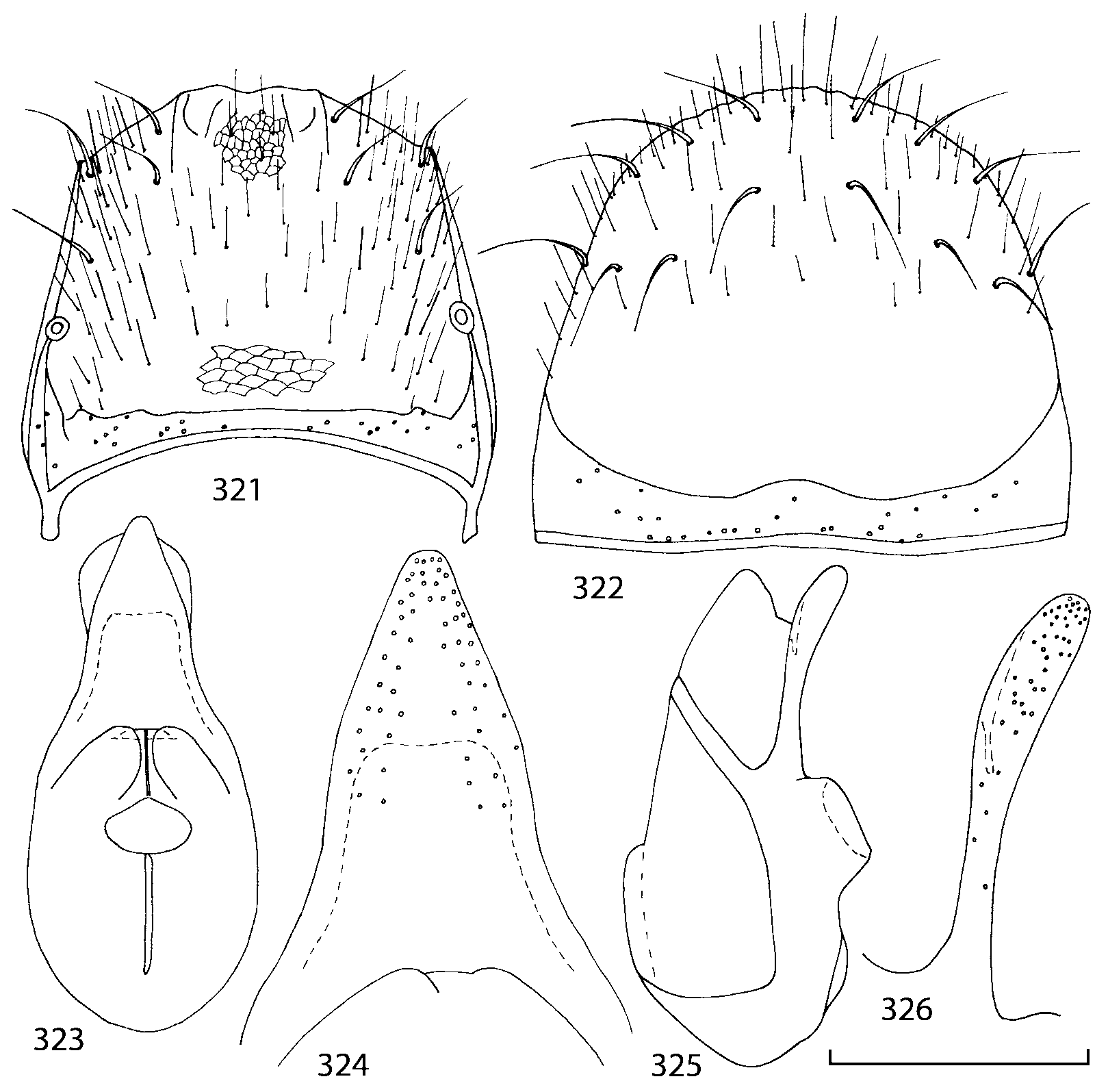
Pronotum subquadrate or slightly transverse, broadest slightly in front of middle, sides broadly rounded; anterior margin convex; anterior and posterior angles rounded; posterior margin convex; surface covered with microsetae directed posteriorly in midline, posteriorly or obliquely posteriorly in lateral areas ( Fig. 20 View FIGURES 18 20 ) (Type VI or V, Benick & Lohse 1974); macrosetae short; hypomera fully visible in lateral view. Posterior margin of elytra straight. In few species wings fully developed, in most species wings shorter than elytra or absent. Meso metasternum as in Figs. 1819 View FIGURES 18 20 , mesosternal process long and wide, extended about 1/2 length of mesocoxal cavities, metasternal process short or not outlined (in wingless species), posterior margin of mesocoxal cavities complete or interrupted; mesocoxae contiguous. Mesotibia with short median macroseta (not longer than tibial width). Tarsal segmentation 455; metatarsal segment 1 longer than segment 2. One empodial seta present.
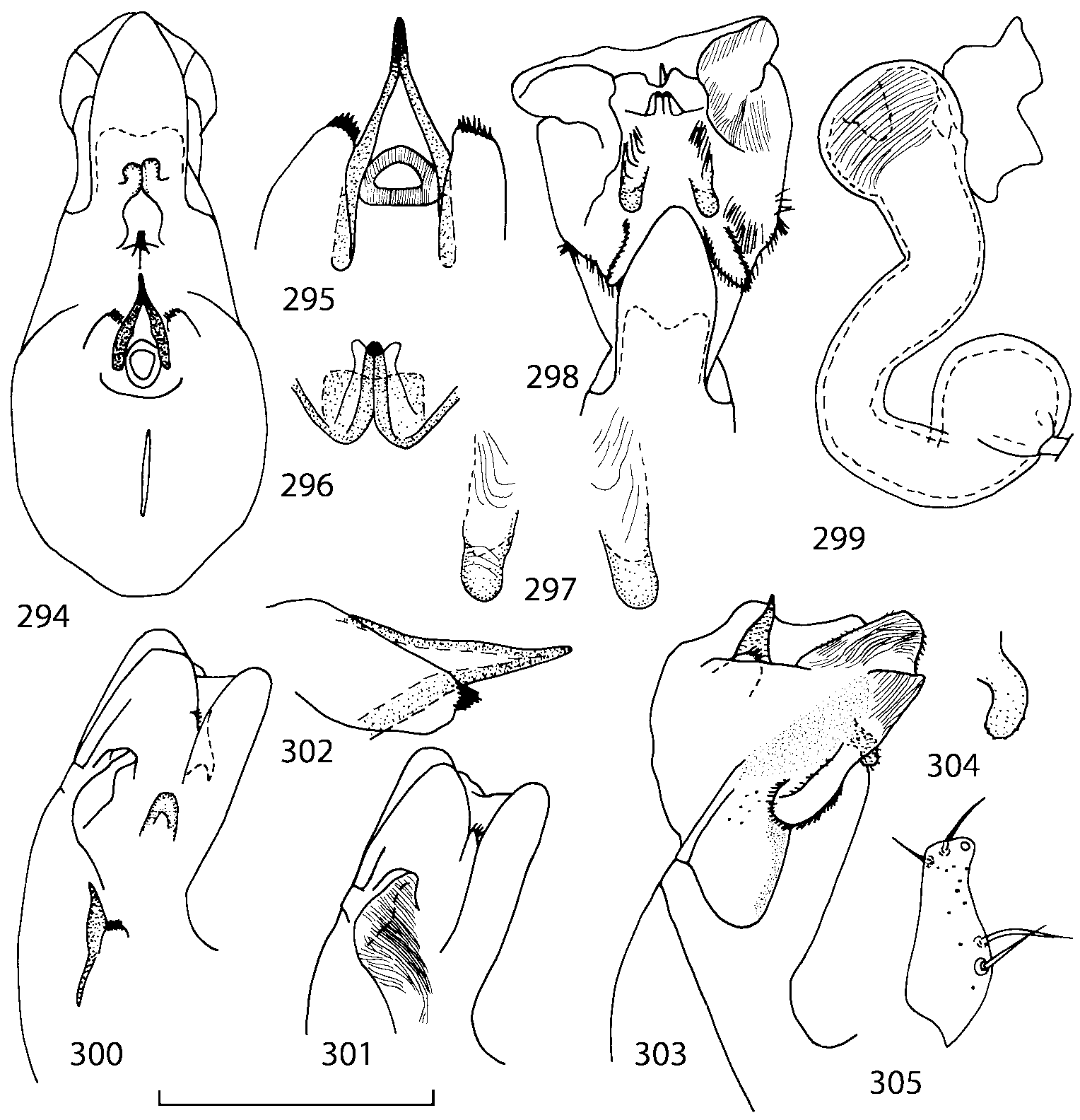
Abdominal terga 35 with moderate transverse basal impressions. Tergum 7 1.21.3 times as long as tergum 6. Puncturation on terga 67 sparser than on terga 35. Female sternum 8 ( Figs. 3233 View FIGURES 29 33 ) with row of apical microsetae (as in other Athetini ).
Male secondary characters absent or include some of the following: impression along pronotal midline, medial tubercle or extending lobe at posterior margin of pronotum, tubercle or carina near scutellum and/or impression on each elytron, 12 medial tubercles or carinae on tergum 7, tubercles or carinae on tergum 8.
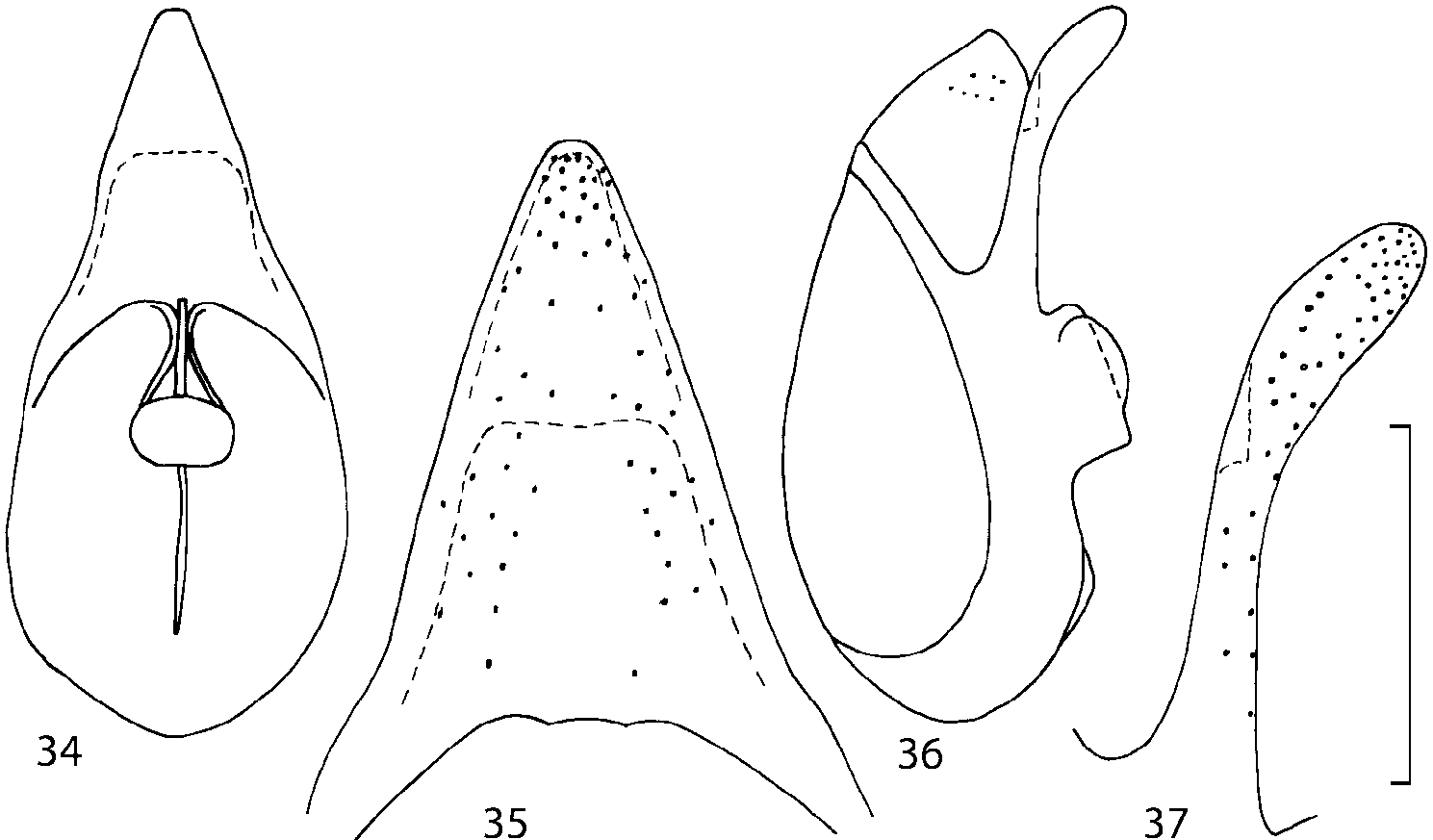
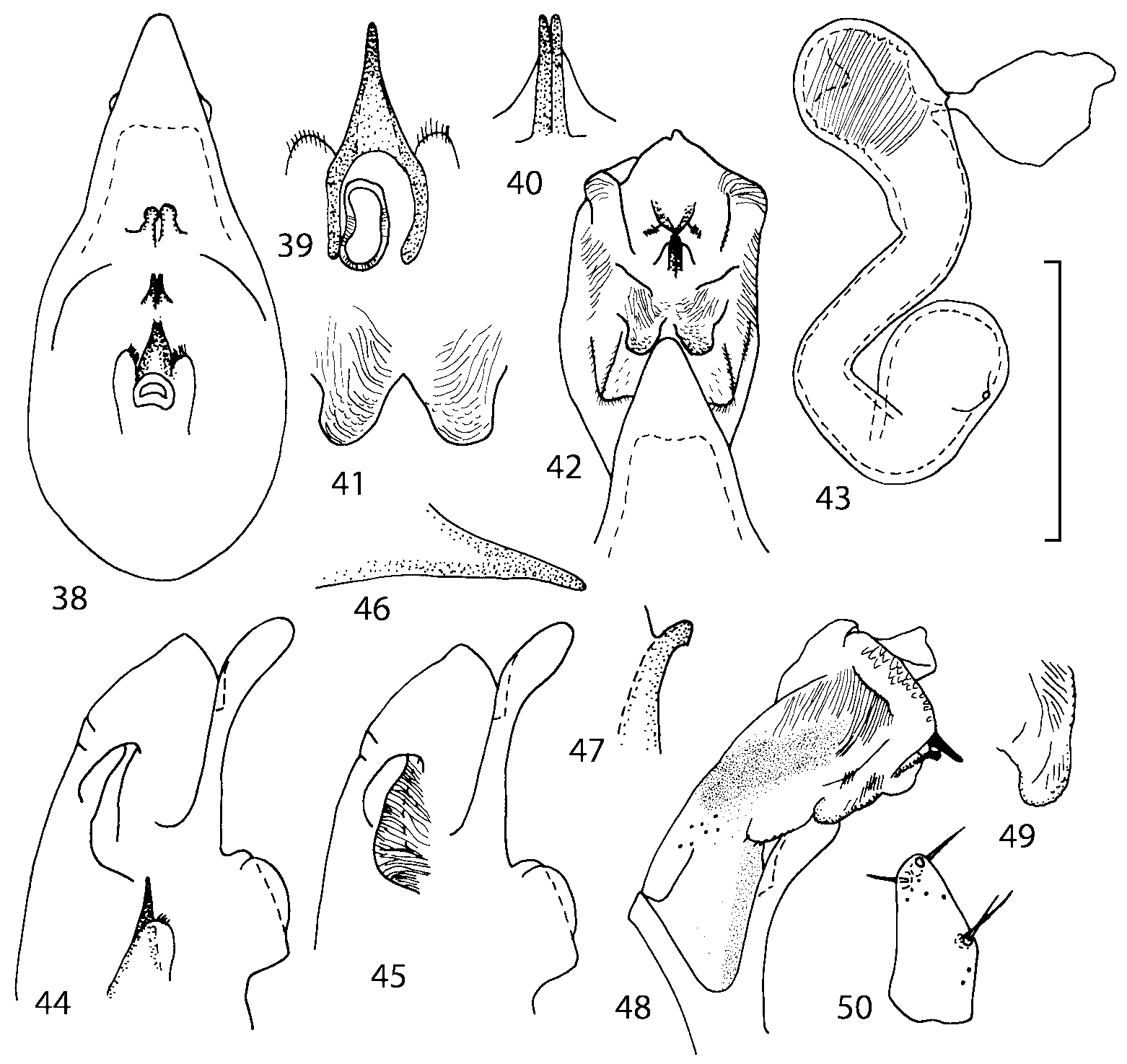
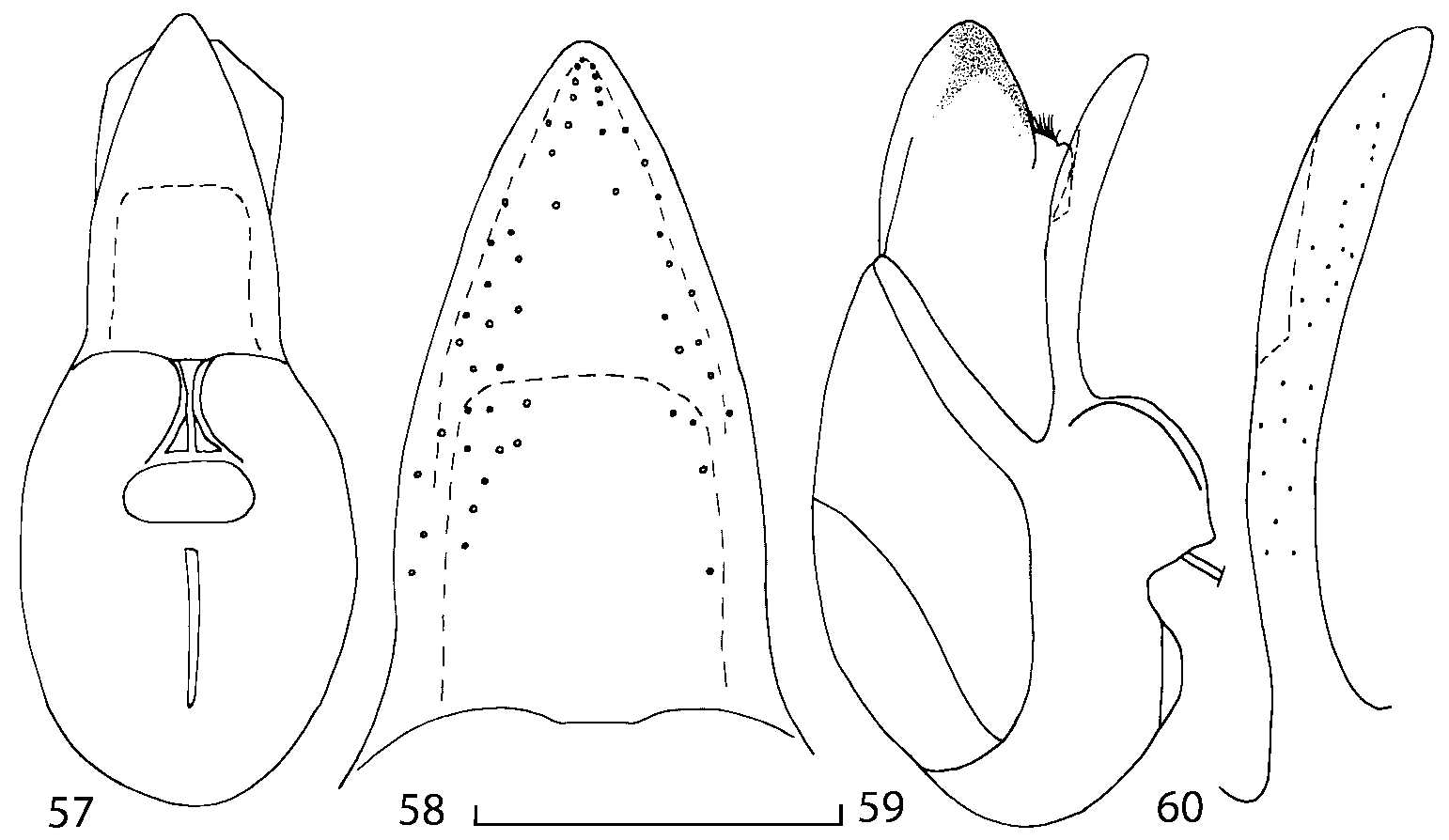
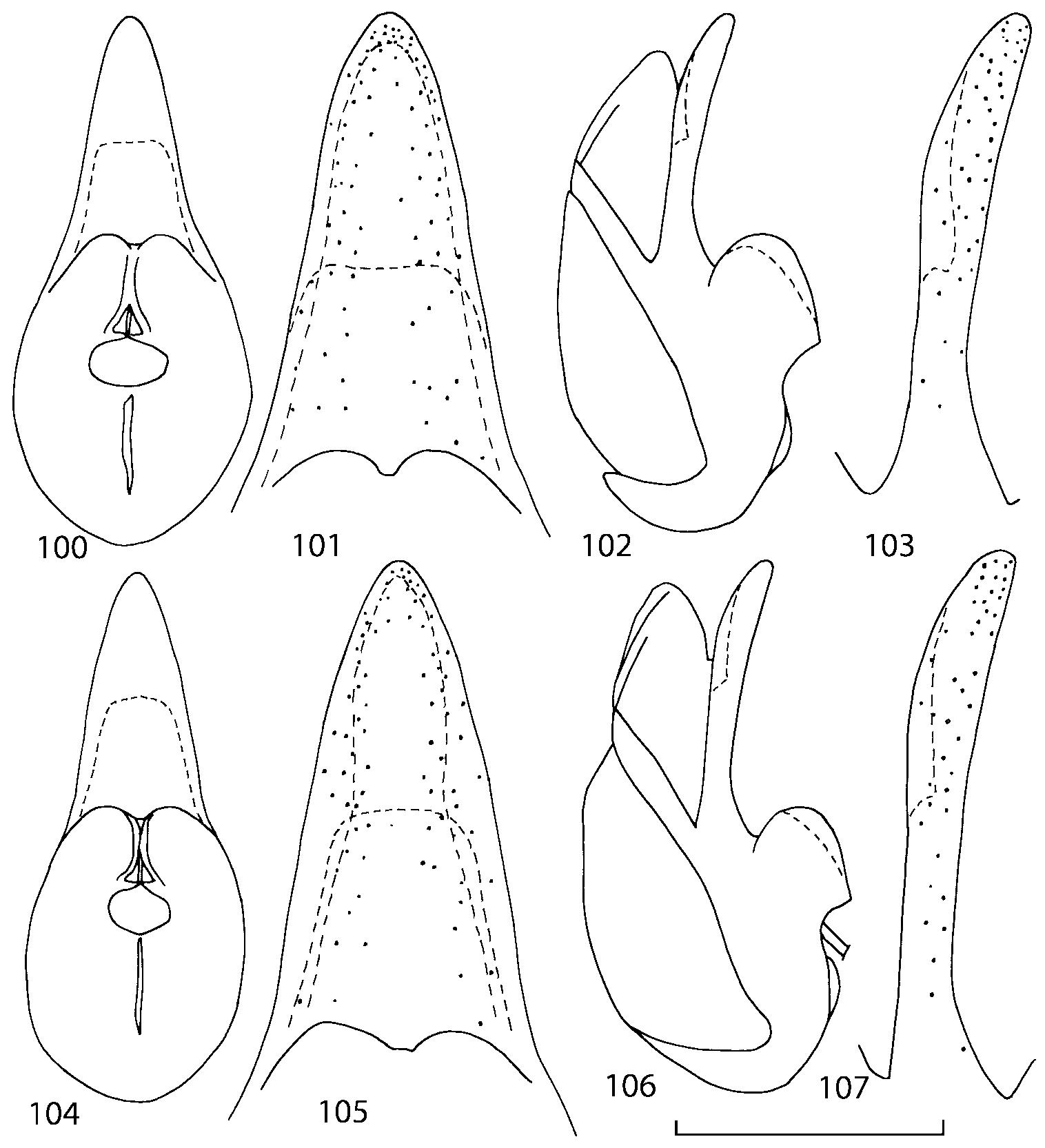
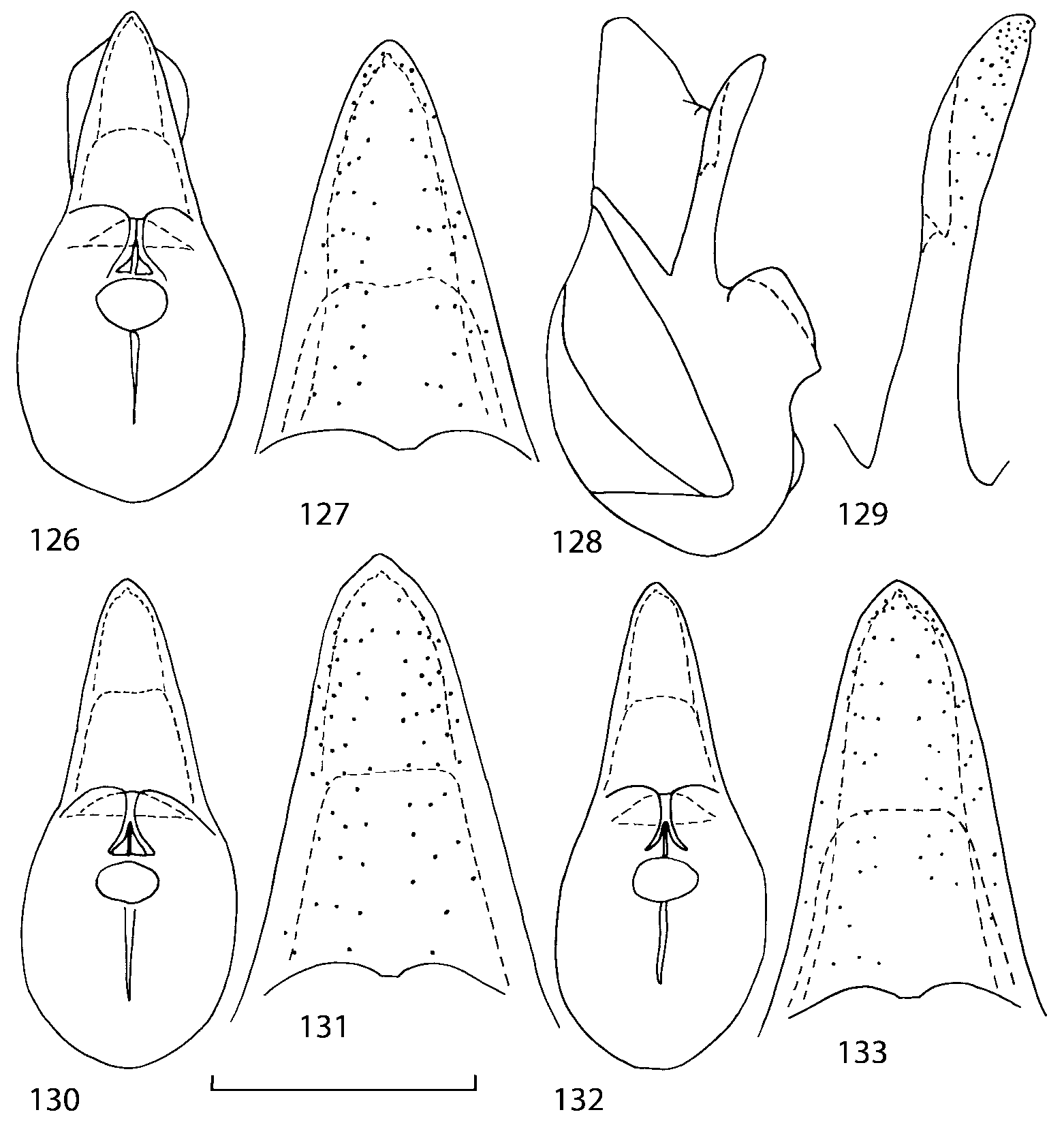
Median lobe of aedeagus in ventral view narrows apically ( Figs. 3435 View FIGURES 34 37 , 5758 View FIGURES 57 60 , 100 101 View FIGURES 100 107 ). Internal sac with 12 pairs of diverticula ( Figs. 2122, 2428 View FIGURES 21 25 View FIGURES 26 28 , 42 View FIGURES 38 50 , 64 View FIGURES 61 71 , 85 View FIGURES 81 93 , 112 View FIGURES 108 119 ). Copulatory piece with narrow apical process ( Figs. 23 View FIGURES 21 25 , 27 View FIGURES 26 28 , 39 View FIGURES 38 50 , 62 View FIGURES 61 71 ). Medial lamellae narrow ( Figs. 2122, 2428 View FIGURES 21 25 View FIGURES 26 28 , 40, 47 View FIGURES 38 50 ).
Synonyms. Gistel (1856) proposed the generic name Evanystes to include eight species but did not designate the type species or provide the description. For a long time the name Evanystes was ignored by staphylinid taxonomists who used the name Geostiba or Sipalia . The species included by Gistel in Evanystes made it a very broadly defined group, somewhat equivalent to Homalota of early staphylinid taxonomists. Blackwelder (1952) made a very unfortunate choice when he designated Aleochara circellaris as a type species of Evanystes . As a result the name Geostiba was made a junior objective synonym of Evanystes . To preserve the stability of nomenclature the majority of taxonomists working on the group continued to use the name Geostiba . Because Blackwelder (1952) and a few other entomologists used the name Evanystes as valid, the requirements of the Code (§23.9.1, ICZN 1999) for preservation of Geostiba as a valid name are not met. An application to preserve the use of Geostiba and suppress the use of Evanystes has been submitted to the Commission on Zoological Nomenclature (Case 3239) by Gusarov.
Mulsant and Rey (1853) proposed Sipalia as a subgenus of Homalota Mannerheim, 1830 , and included six species (three of them are now placed in Leptusa Kraatz, 1856 , two in Geostiba , and one in Octavius Fauvel, 1873 ). Fauvel (1902a) validly designated Homalota difformis Mulsant & Rey, 1853 as the type species of Sipalia Mulsant & Rey, 1853 . Because at that time Homalota difformis was already a member of Leptusa ( Kraatz 1856; Bernhauer 1900), Sipalia replaced Leptusa ( Fauvel 1902b) . Evidently Fauvel’s designation was overlooked by most workers. Leptusa continued to be used for the genus that included the type species of Sipalia ( Bernhauer 1905; Reitter 1909; Bernhauer & Scheerpeltz 1926; Scheerpeltz 1966, etc.) while Sipalia was used for Geostiba ( SainteClaire Deville 1906; Reitter 1909; Bernhauer & Scheerpeltz 1926; Scheerpeltz 1934; etc.). Lohse (1974) and Benick and Lohse (1974) brought attention to the synonymy of Sipalia and Leptusa and since then the name Geostiba was almost universally used as valid. A more detailed discussion of the name Sipalia can be found in the application to preserve the use of Leptusa and suppress the use of Sipalia , submitted to the Commission on Zoological Nomenclature (Case 3256) by Gusarov and Herman.
The generic name Glossola Fowler, 1888 is listed by Seevers (1978) and Newton et al. (2000) as a synonym of Geostiba . This is incorrect: Glossola should be listed as a synonym of the genus Aloconota Thomson, 1858 because the type species of Glossola ( Homalota gregaria Erichson, 1839 ; by monotypy) is a synonym of the type species of Aloconota ( Tachyusa immunita Erichson, 1840 ; by monotypy) which is regarded by Seevers (1978, p. 110) and Newton et al. (2000, p. 368) as a valid genus.
Lohse and Smetana (1988) placed the name Sonomota Casey, 1911 (described as a subgenus of Sipalia ) in synonymy with Microdota Mulsant & Rey, 1873 , a subgenus of Atheta Thomson, 1858 . This synonymy was overlooked by Newton et al. (2000) who list Sonomota as a subgenus of Geostiba . The type of Sipalia lippa Casey 1911 (the type species of Sonomota , by original designation) has the ligula divided into two lobes only at the apex as in other species of Atheta . Atheta lippa is similar to some other species of Atheta with short elytra ( A. turpicula ( Casey, 1910) described from Colorado, A. pacifica ( Casey, 1910) from California and British Columbia, A. hesperica ( Casey, 1910) from California and A. cornelli Pace, 1997 described from Oregon) and may represent a distinct lineage within Atheta . This group will be revised elsewhere.
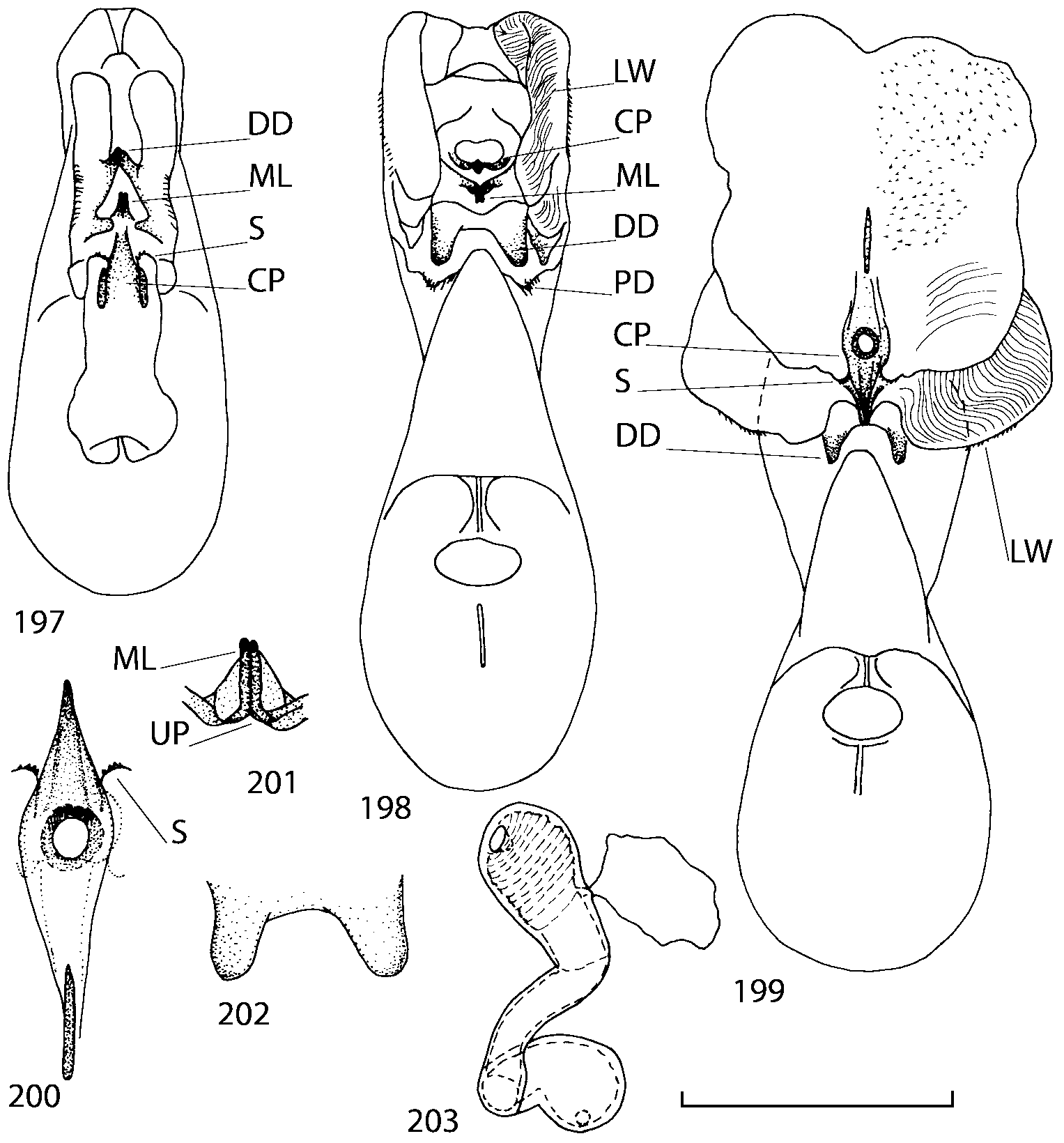
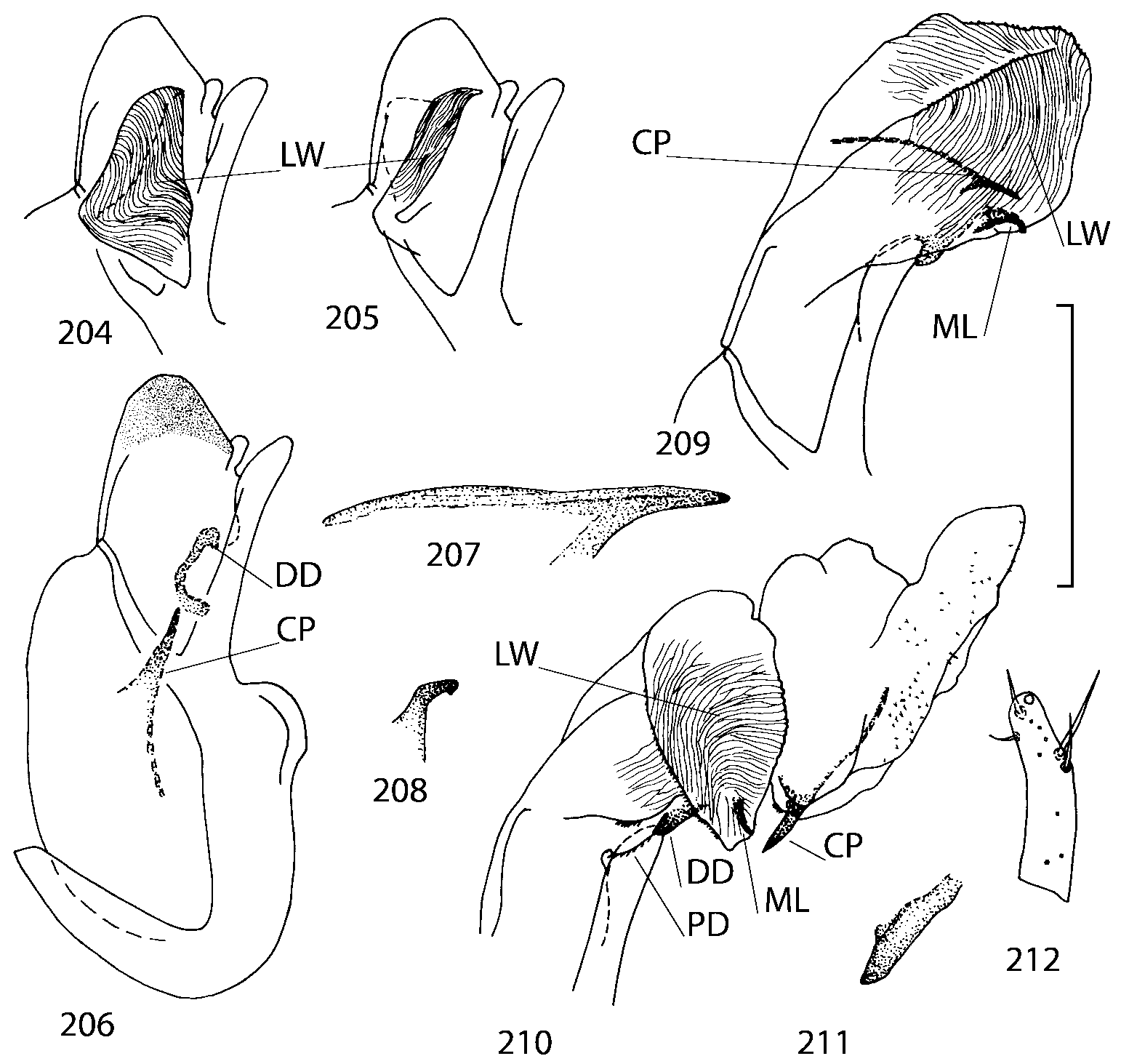
Discussion. To find additional characters for species identification and to establish the relationships of Geostiba to the other genera of Athetini a special study of the internal sac of the aedeagus has been undertaken. The details of the internal sac of Nearctic species of Geostiba have not been illustrated adequately in published drawings ( Lohse & Smetana 1988; Pace 1997). The internal sac has been studied both in retracted and everted position in some Palaearctic species and in all Nearctic species described in this paper. Comparison of the details of the internal sac in Geostiba with those in some species of Atheta and Philhygra ( Brundin 1944; Sawada 1972; Gusarov unpublished) demonstrates that in all three genera the internal sac has the same parts. The terms used by Brundin and Sawada can be applied to the homologous parts in the internal sac of Geostiba . The illustrations of the internal sac in retracted, partially and completely everted state show position of these parts ( Figs. 197202 View FIGURES 197 203 , 204211 View FIGURES 204 212 ).
To evert the internal sac, the aedeagus was placed in potassium hydroxide solution in which the soft tissue was partially dissolved. After that, the aedeagus was transferred into a drop of water. While the water was diffusing into the aedeagus it was often possible to evert the internal sac by gently pressing (dorsally) with an insect pin at the basal part of the median lobe. This apparently simulates the natural evertion of the internal sac during copulation when the muscles connecting the ventral and dorsal sides of the base of the aedeagus contract. To complete the evertion a thin but blunt minuten can be introduced inside the median lobe basally and then the partially everted internal sac can be pushed further out. After the internal sac has been everted, the aedeagus can be transferred back into potassium hydroxide solution for complete removal of soft tissue.
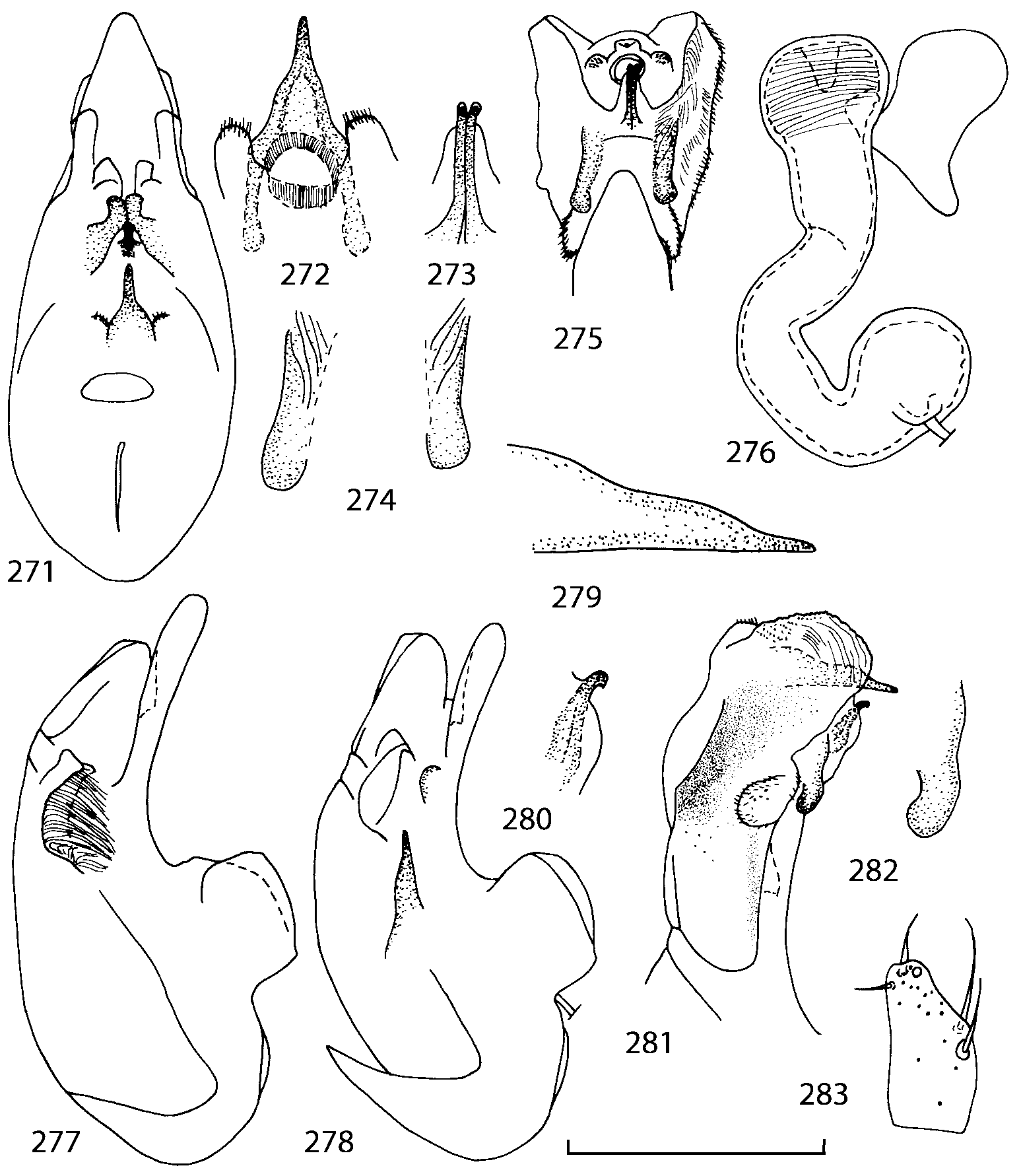
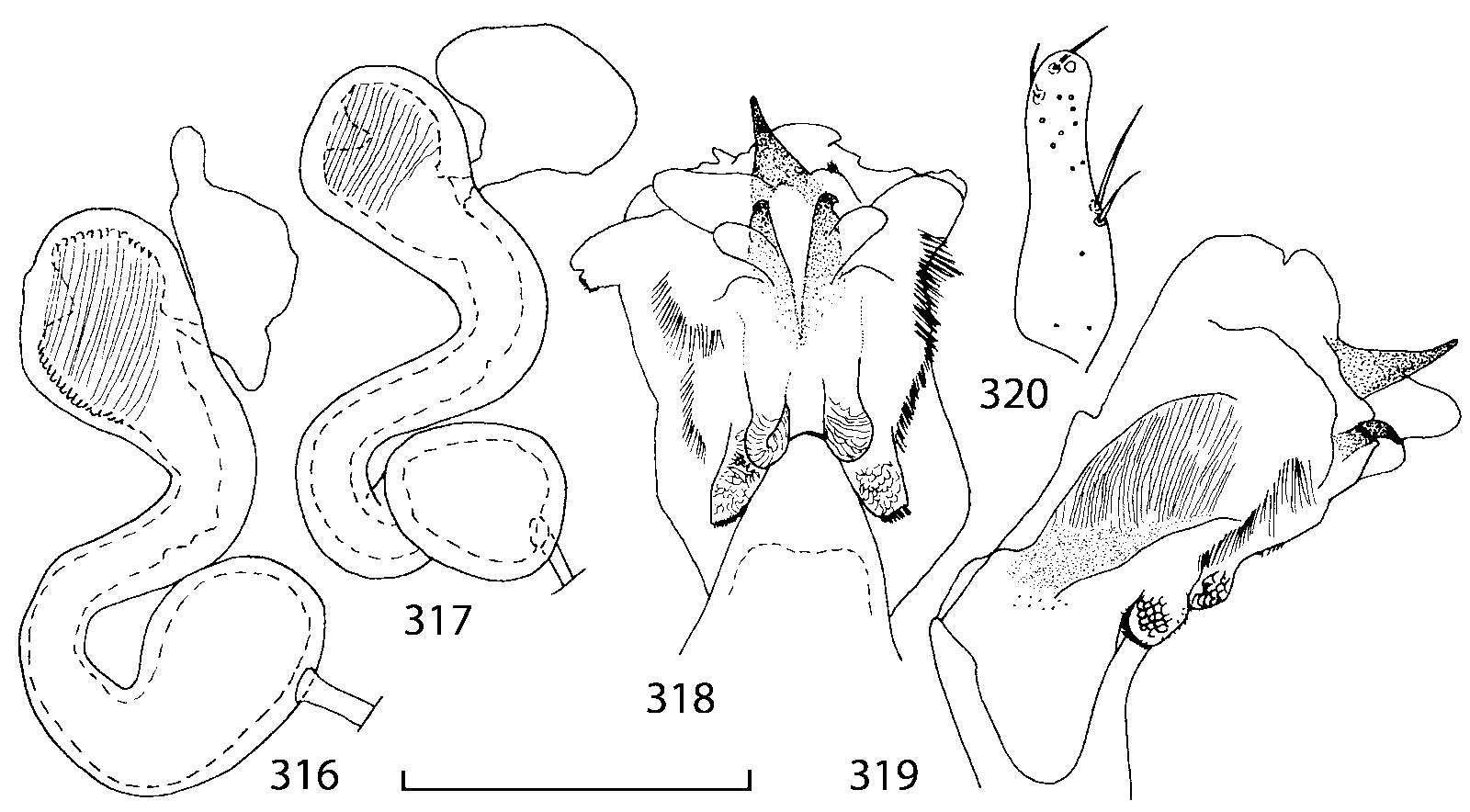
The copulatory piece (the term used by Sawada (1972); equivalent to the Ductuslamelle in Brundin (1944)) is the internal sac sclerite with the opening of ejaculatory duct in it (CP, Figs. 197200 View FIGURES 197 203 , 206207, 209210 View FIGURES 204 212 ). This sclerite is present in many tribes of the Aleocharinae , including the tribe Athetini . In Geostiba the copulatory piece narrows apically into a pointed process ( Figs. 23 View FIGURES 21 25 , 39 View FIGURES 38 50 , 62 View FIGURES 61 71 ). The suspensoria (the term used by Sawada (1972); equivalent to the Sförmigen Stäbe or hakenförmigen Bildungen of Brundin (1944)) are two structures connected laterally to the basis of the copulatory piece (S, Figs. 197, 199200 View FIGURES 197 203 ). In Geostiba the suspensoria are poorly sclerotized, with a group of tiny denticles at the apex.
The medial lamellae (Mediallamellen of Brundin (1944)) are two sclerotized structures attached to the Ushaped plate (U förmige Platte, Brundin 1944). When the sac is everted these structures are located on the ventral surface of the sac, proximal of the copulatory piece. In some groups of Athetini , the medial lamellae and the Ushaped plate are large and act to guide the copulatory piece while the internal sac is being everted. In Geostiba the medial lamellae are thin and narrow (ML, Figs. 197198, 201 View FIGURES 197 203 , 208210 View FIGURES 204 212 , 2122, 2428 View FIGURES 21 25 View FIGURES 26 28 ).
In Geostiba the ventral side of the everted internal sac proximal of the medial lamellae has one or two pairs of diverticula which may be an autapomorphy of the genus. In Palaearctic G. (s. str.) circellaris , G. (Sipalotricha) infirma (Weise, 1878) , G. (Sibiota) padana (Weise, 1878) and G. (Sibiota) oertzeni (Eppelsheim, 1888) there is one pair ( Figs. 2122, 2428 View FIGURES 21 25 View FIGURES 26 28 ), in all native Nearctic species of Geostiba there are two pairs of diverticula (DD, PD, Figs. 197199, 202 View FIGURES 197 203 , 206, 210 View FIGURES 204 212 ). The distal pair is more sclerotized than the proximal pair. A study of different Palaearctic lineages of Geostiba is required to confirm the presence of diverticula in all Geostiba and to establish homology between one of the two pairs of diverticula in the Nearctic species and the single pair in the few examined Palaearctic species.
While trying to evert the internal sac in different specimens of Geostiba , it was relatively easy to achieve what may be just a partially everted state. In this state, the lateral wall of the sac, which in many species has striate microsculpture (LW, Figs. 198 View FIGURES 197 203 , 209 View FIGURES 204 212 ), forms a flange encircling the hollow from which the copulatory piece and medial lamellae protrude. When the internal sac is retracted into the median lobe, the striate lateral wall of the sac is clearly visible (LW, Figs. 204205 View FIGURES 204 212 ). Because the lateral wall is folded into three layers it often looks like a sclerite. This structure is shown schematically in some drawings by Lohse and Smetana (1988: Figs. 4 View FIGURES 1 7 , 12 View FIGURES 8 17 , 19 View FIGURES 18 20 , 26 View FIGURES 26 28 ) and in one drawing by Pace (1997: Fig. 13 View FIGURES 8 17 ). The shape of the folds and the microsculpture of the internal sac wall are stable within species and can be used for identification, although it is important to bear in mind that when internal sac starts to evert the position and the shape of the folds may change. In a few specimens I succeeded in everting the internal sac completely ( Figs. 199 View FIGURES 197 203 , 210 View FIGURES 204 212 , 234, 242 View FIGURES 230 246 ) and revealed the apical membranous portion of the sac which have tiny scattered denticles in some species. Additional study is necessary to determine whether the sac is completely or partially everted during copulation.
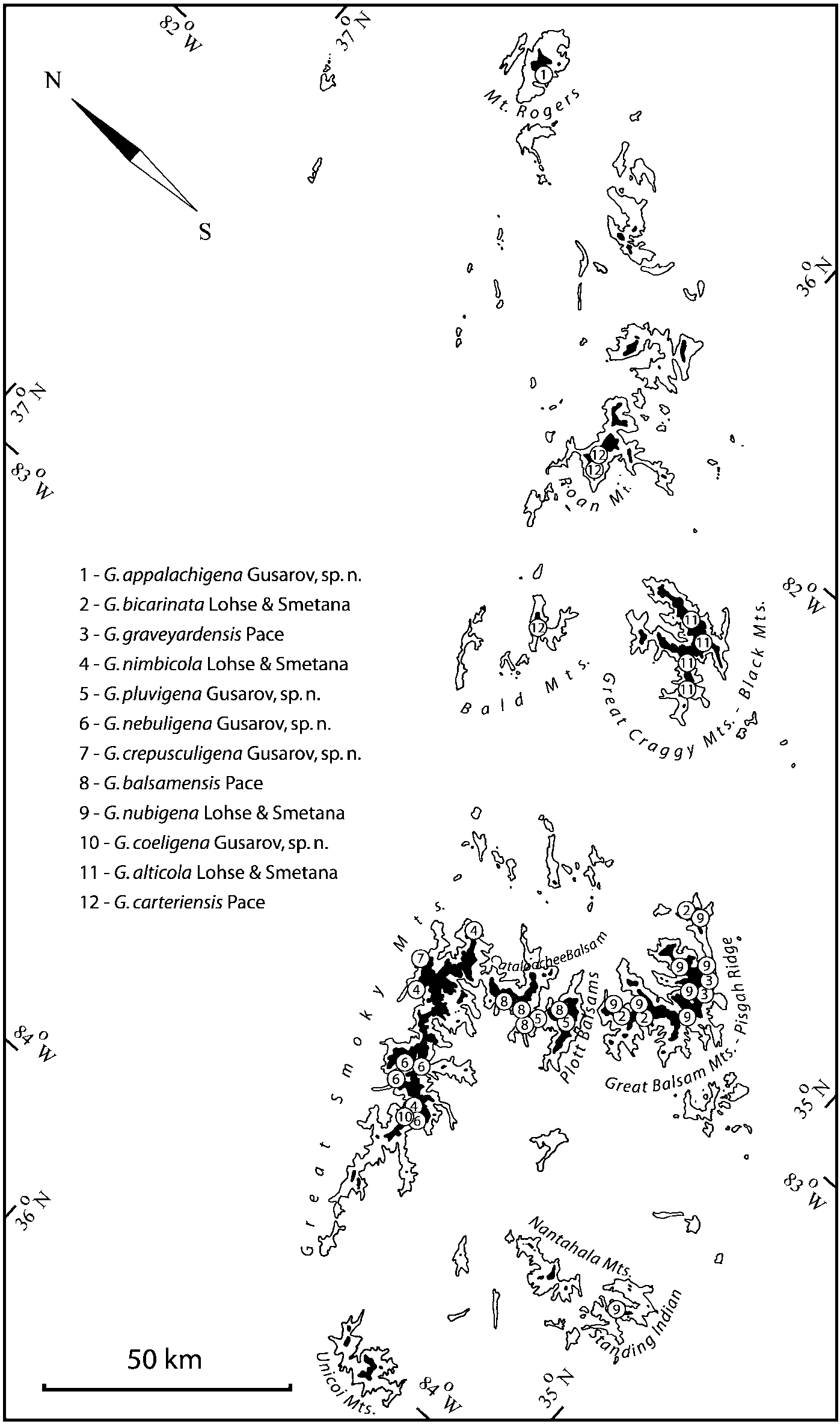
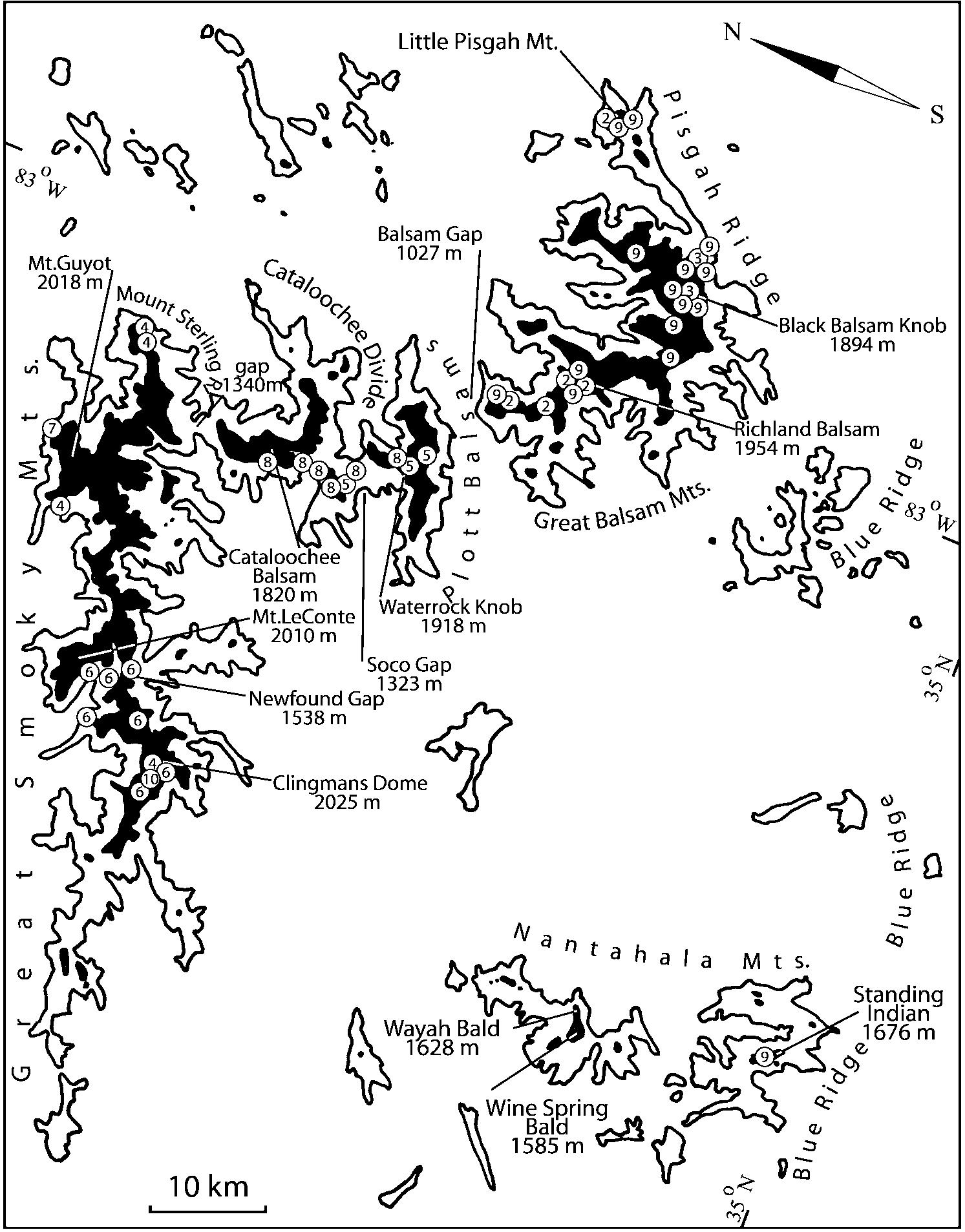
Some populations of Southern Appalachian Geostiba are very similar and it is difficult to decide whether they represent distinct species or forms of the same species. When two forms are sympatric and found in the same sample they are considered to represent different species. The situation with allopatric forms is more difficult. Even when a certain gap between two allopatric forms exists, additional consideration is needed to decide how to reflect this difference taxonomically. In this paper the difference between G. nimbicola and G. nebuligena is used as a criterion for assigning species rank to closely related allopatric forms. Both species are known from the Great Smoky Mountains massif and both were collected on Clingmans Dome, however never in the same sample or on the same date. The two species can be distinguished only by the shape of the apex of the median lobe of aedeagus ( Figs. 100107 View FIGURES 100 107 , 152156 View FIGURES 152 156 ). Whenever I encountered the gap on the same scale between two allopatric forms, I assigned to them species rank.
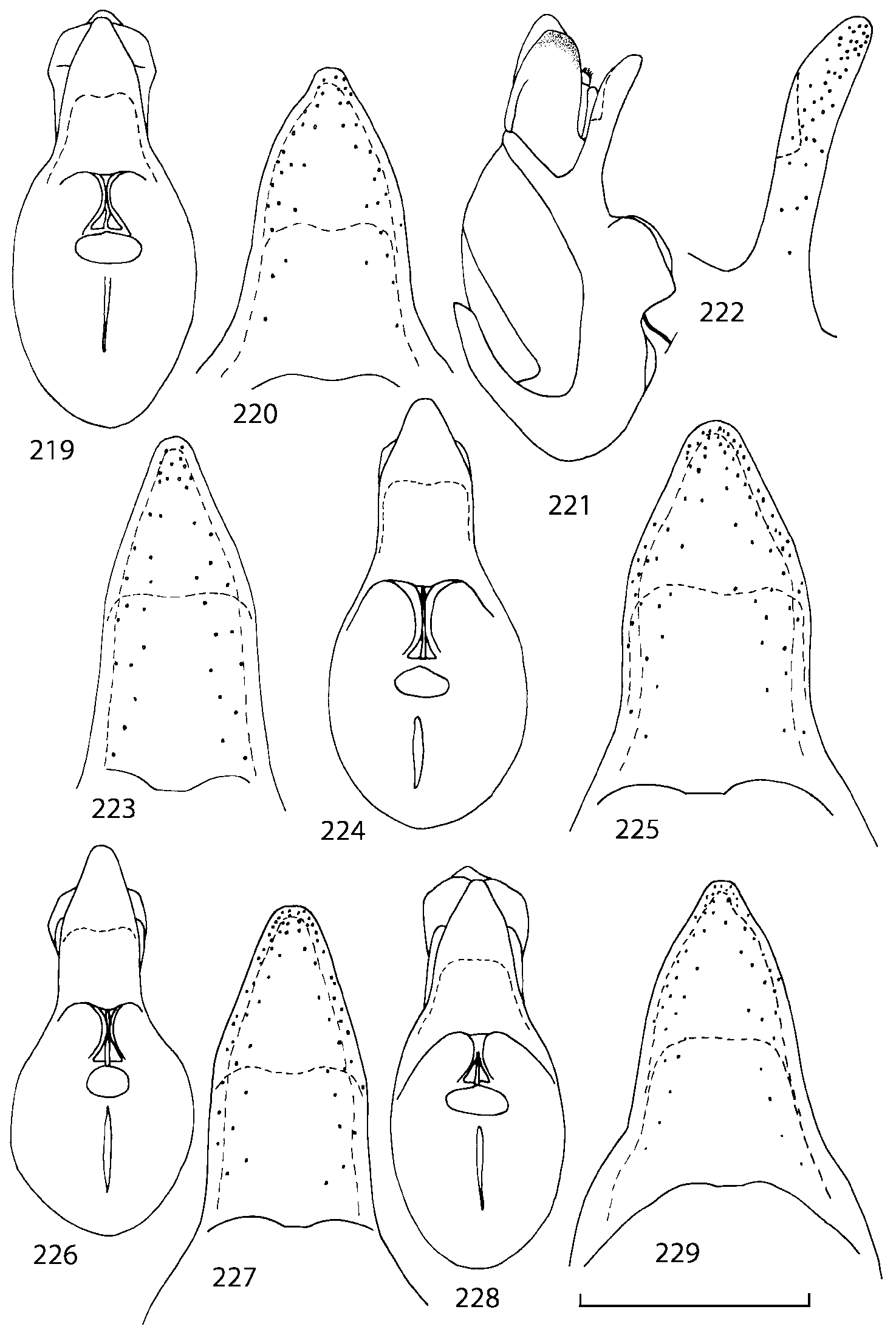
Some characters previously used in keys to distinguish species of Geostiba ( Lohse & Smetana 1988; Pace 1997) are found to be of limited utility. For example, the microsculpture of the abdominal tergum 7 is similar in all native Nearctic species. In the basal portion of the tergum the microsculpture consists of transverse meshes, while in the apical portion it consists of isodiametric or even elongate meshes ( Figs. 56 View FIGURES 51 56 , 99 View FIGURES 94 99 , 125 View FIGURES 120 125 , 151 View FIGURES 146 151 , 192 View FIGURES 187 192 , 218 View FIGURES 213 218 ). I did not count the number of ommatidia in the eyes but instead compared eye length to temple length.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Aleocharinae |
Geostiba Thomson, 1858
| Gusarov, Vladimir I. 2002 |
Geostiba:
| Newton 2000: 371 |
Evanystes:
| Newton 2000: 371 |
| Newton 2000: 371 |
Sonomota:
| Newton 2000: 371 |
Geostiba:
| Lohse 1988: 270 |
Geostiba:
| Seevers 1978: 126 |
Evanystes:
| Seevers 1978: 126 |
Glossola:
| Seevers 1978: 126 |
Sonomota:
| Seevers 1978: 128 |
Geostiba:
| Benick 1974: 111 |
Evanystes:
| Blackwelder 1952: 163 |
Geostiba:
| Blackwelder 1952: 163 |
Sipalia:
| Scheerpeltz 1951: 166 |
Sipalia:
| Bernhauer 1926: 599 |
Geostiba:
| Bernhauer 1926: 599 |
Sonomota:
| Bernhauer 1926: 599 |
Sipalia:
| Fenyes 1920: 249 |
Geostiba:
| Fenyes 1920: 249 |
Sonomota:
| Fenyes 1920: 249 |
Geostiba
| Thomson 1858: 33 |
Evanystes
| Gistel 1856: 387 |