Mniarogekko jalu Bauer, Whitaker, Sadlier & Jackman
|
publication ID |
https://doi.org/ 10.5281/zenodo.211734 |
|
DOI |
https://doi.org/10.5281/zenodo.6166584 |
|
persistent identifier |
https://treatment.plazi.org/id/5C4F87E1-FFA1-FF8E-088F-FA27D7E5B22B |
|
treatment provided by |
Plazi |
|
scientific name |
Mniarogekko jalu Bauer, Whitaker, Sadlier & Jackman |
| status |
sp. nov. |
Mniarogekko jalu Bauer, Whitaker, Sadlier & Jackman sp. nov.
( Figs. 18–21 View FIGURE 18 View FIGURE 19 View FIGURE 20 View FIGURE 21 )
Holotype. MNHN 2012.0211 (formerly AMS R 161289), adult male ( Fig. 18 View FIGURE 18 ). New Caledonia, Province Nord, Îles Belep, Île Art, 2 km E Waala, Wênè Côgat, 19°42'46.9" S, 163°39'37.7" E, 230 m. Collected 28 May 2002 by A.H. Whitaker and V.A. Whitaker.
Paratypes. AMS 161285, subadult female, same data as holotype; AMS R 161286, adult male, R 161287 –288, adult females, data as for holotype, but collected 23 May 2002; AMS R 161224, adult male, New Caledonia, Province Nord, 24 km N Koumac, Forêt d’Ougne, Vallée Poupoule, 20°20’04.0”S, 164°17’07.1”E, 5 m, collected 15 October 2001 by A.H. Whitaker and V.A. Whitaker; AMS R 161237 –38, adult males, New Caledonia, Province Nord, 11 km NW Koumac, Dôme de Tiébaghi, 20°27'27.9"S 164°11' 22.8"E, 360 m, collected 17 October 2001 by A.H. Whitaker and V.A. Whitaker; CAS 250858 –59, adult females, New Caledonia, Province Nord, 15 km N Koumac, Rivière Néhoué, 20°25'09.7"S, 164°13'16.3"E, 8 m, collected 22 January 2003 by A.M. Bauer, R.A. Sadlier, T.R. Jackman, G. Watkins-Colwell, and S.A. Smith.
. Diagnosis. Mniarogekko jalu n. sp. is a large (to 140 mm SVL) diplodactylid. It may be distinguished from its sister taxon M. chahoua by its much lower number of precloacal pores in males (<95 [range 54–91] versus ~120) typically arranged in three ( Fig. 19 View FIGURE 19 ), rather than four rows. Color comparisons between the two species of Mniarogekko are difficult to make. There are relatively few wild caught M. chahoua in museum collections and captive animals have been bred for particular color patterns (de Vosjoli et al. 2003) so ‘wild type’ coloration, which is itself already quite variable ( Bauer 1985; Seipp & Henkel 2000, 2011; Stark 2006; Langner 2009), is difficult to characterize. Ventral body coloration in the new species seems to be uniformly a pale yellowish green ( Figs. 19–20 View FIGURE 19 View FIGURE 20 ), whereas M. chahoua is often white or cream, with a greenish tinge localized to some parts of the venter.
Description. (data from adult male holotype, MNHN 2012.0211). Specimen fixed with mouth open wide; abdominal incision for removal of liver sample for DNA. SVL 123.3 mm; TailL 76.2 mm (of which 19.0 mm are regenerated); TrunkL 51.9 mm; HeadL 32.4 mm; HeadW 22.8 mm; SnEye 12.6 mm; OrbD 7.0 mm; EyeEar 10.6 mm. Body moderately long (TrunkL = 42% SVL), robust, slightly depressed. Head oblong, large (HeadL = 26% SVL), wide (HeadW = 70% HeadL), well demarcated from neck ( Fig. 18 View FIGURE 18 ); nasofrontal region somewhat depressed; canthus prominent; snout relatively long (SnEye = 39% HeadL), much longer than eye diameter (OrbD = 56% SnEye). Scales on dorsum of snout approximately two times the diameter of those on occipital region. Eye relatively small (OrbD = 22% HeadL); pupil oval, margins crenellated. Ear opening approximately two times longer than high, canted posterodorsally to anteroventrally at <30° to the horizontal; eye to ear distance much longer than diameter of eyes (EyeEar = 150% OrbD). Rostral rectangular, more than twice as broad (5.3 mm) as high (2.3 mm), a very short rostral crease dorsally, contacted posteriorly by three small pentagonal internasals and two large supranasals, each approximately three times the size of larger (lateral) internasals; contacted posteroventrally by first supralabial. Nostrils oval to round, laterally oriented, surrounded by rostral, five (left) to seven (right) circumnarial scales, including enlarged supranasal, and narrowly contacted by first supralabial. Mental triangular, as deep as broad (3.2 mm). First infralabials somewhat elongate, narrowly separated from one another posterior to the mental by a small, irregular postmental scale. Scales in four to five rows posterior to anterior infralabials and three to five rows medial to posterior infralabials slightly enlarged and elongate (3–5 times size of throat granules. 12(right)–14(left) enlarged supralabial scales, posteriormost only about 3 times size of rictal scales, 10 supralabials to midpoint of orbit; 12 (right)–13 (left) enlarged infralabial scales; 44 scale rows between supraciliaries, 21 scale rows across frontal bones at midpoint of orbit. Supraciliary scales forming a denticulated row, posterior two thirds of scales distinctly spiny.
Dorsal scales small, weakly heterogeneous, domed to weakly conical, oval to rounded, highest point slightly posterior of center, each separated from one another by a rosette of six surrounding triangular scales; ventral scales ~1.5 times diameter of dorsals, smooth, flattened, subimbricate, enlarged in precloacal region. Posterior abdominal scales rounded, mid-abdominal scales slightly elongate. Approximately 189 scale rows around mid-body. Welldefined non-denticulate ventrolateral skin folds from just anterior to angle of jaw to anterior thigh. Distinct folds on anterior and posterior margins of forelimb almost to base of palm; postaxial margin of hindlimb with fold from base of thigh to ankle. Scales of fore limbs not differing from dorsals, although slightly subimbricate near limb insertion; scales of hind limbs subimbricate near limb insertion, distally, near ankle, granular and in regular rows, without rosettes of triangular interscales. Scales on palms and soles smooth, flattened. Fore- and hindlimbs short and thick (ForeaL/SVL ratio 0.12; CrusL/SVL ratio 0.15), axillary pocket well developed. Digits short, all bearing claws, those on digit I of both manus and pes reduced and partially sheathed, remaining claws long and strongly recurved; relative length of digits of manus: IV>III>V>II>I, and of pes: IV~V~III>II>I; digits moderately webbed; digits III and IV of pes tightly bound along length of elongate metatarsals. Subdigital lamellae all unpaired, somewhat bowed, with lateral margins gently angled distally (except for proximalmost ones which are straight, particularly in digit I). Claw of digit I, manus and pes, lies between a smaller lateral and a larger (twice size of lateral in manual digit, four times larger in pedal) medial apical scansor. Lamellar counts from right (and left) sides 13-16-19-21-14 (12-18-20-19 -17) manus and 12-18-16-18 -15 (13-16-19-19-15) pes (excludes apical scansors of digit I).
Large precloacal pores in a patch of somewhat enlarged scales, arranged in two rows (anterior to posterior) of 21 (L) + 20 (R), and 17 (L) + 18 (R), with left and right sides separated by a single poreless scale. Posterior row fragmented with some poreless scales separating pored scales on each side of ventral midline. Pores extend only on to very base of thighs. Hemipenial bulge large; cloacal spurs on raised base just posterior to hindlimb insertion, with a single very large, flattened, conical, posterodorsolaterally-directed scale subtended by a series of two (right) or 3 (left) smaller scales of similar form. Tail (approximately 25% regenerated in holotype) 62% of snout-vent length, thick, roughly round in cross-section, with a distinct longitudinal dorsal crease. Caudal scales small, flat, juxtaposed (proximally) to weakly subimbricate (distally), squarish to rectangular with rounded free margins, arranged in regular rows. Surface of tail weakly segmented, caudal scale rows forming whorls, each whorl 8 dorsal scale rows and 6 ventral scale rows long; ventral caudals 1.5–4.0 times larger than dorsals, midventral caudal scales not enlarged. Midventral scales of pygal region smaller than those of post-pygal region. Rows of scales on regenerated portion of tail not arranged in segments, with some irregular scales.
Color in preservative (based on holotype): Dorsum a mottled mid-brown with slightly lighter irregular transverse markings over shoulder and posterior abdomen ( Fig. 18 View FIGURE 18 ). A dark triangle over occiput and nape, with its apex directed posteriorly. Irregular darker brown patches over lumbar region and posterior sacrum. A small, oval reddish-brown spot on nape just left of center. Scattered white scales forming isolated patches of speckling from the posterior border of the orbit, above and below the ear, along neck and on to shoulder, where they are dense enough to form a broken shoulder patch. Additional scattered white scales forming scattered clusters along the trunk, mostly on flanks, across sacrum, and on pygal portion of tail.
Muzzle and occiput darker brown; crown grayish brown, similar to lighter transverse markings on trunk. A pale, diffuse, whitish line extending from posteriormost corner of orbit towards ear. Labial scales beige with slightly darker margins. Limbs similar to dorsum, with irregular, alternating lighter grayish and darker grayishbrown markings; a narrow band of white scales at junction between each set of alternating markings on forelimbs. Palms and soles grayish-cream. Tail roughly same color as dorsum, with mottling of brown and grayish-brown and several thin grayish-white bands.
Venter cream with incomplete, diffuse, pale brown transverse chevrons that are continuous laterally with markings on ventrolateral folds, forming a barred pattern along the fold between the limb insertions. Tail venter pale brown with some narrow pale bands. See Variation for a discussion of coloration in life.
Osteology. Vertebral counts are typical for diplodactylid geckos, with 26 presacral and 2 sacral vertebrae. The first three cervical vertebrae are without ribs, as is the last presacral (lumbar) vertebra. The caudal skeleton typically includes 5 pygal vertebrae, although only 4 are present in CAS 250859. Paratypes CAS 250858 –59, AMS R 161237 –38, 161224, 161286, and 161288 have the tail autotomized in the first post-pygal vertebra. In AMS R 161287 the tail is autotomized in the second post-pygal vertebra. MNHN 2012.0211, the holotype, has a nearly complete tail, whereas AMS R 161285 has a complete tail with 27 post-pygal vertebrae. The holotype and all paratypes have the tail broken within the first postpygal vertebra. The phalangeal formulae of the manus and pes are unreduced, 2-3-4-5-3 and 2-3-4-5-4, respectively. Total tooth loci in upper jaw of holotype 67, of which 9 in premaxilla; total mandibular tooth loci 62. A single pair of crescentic cloacal bones is present in the holotype and in all male paratypes. In all female paratypes the extent of the intracranial endolymphatic system is made visible in x-rays by its radio-opaque calcium content. The smallest specimen in the type series, AMS R 161285, has the epiphyses of the long bones unfused, indicating that is not yet skeletally mature.
Variation. Comparative mensural data for the holotype and paratypes are given in Table 3. Meristic characters of paratypes are mostly similar to those of the holotype, and are mentioned hereafter only if they differ. Postmentals and anterior chin shields highly heterogeneous in many specimens; first infralabials separated to broadly contacting behind the mental.
Female specimens lack precloacal pores. All four male paratypes with three rows of precloacal pores, posteriormost much shorter than anterior two.
Color pattern is highly variable across the type series ( Fig. 21 View FIGURE 21 ), from pale grayish to dark brown, but always with dorsal mottling. A darkish patch on occiput, nape, or shoulders usually present. Dorsal patterning diffuse to bold (AMS R 161238, 161285, 161288). White scales invariably present, scattered over body; variably expressed but most evident on posterior of head and nape and on shoulders or near forelimb insertions. AMS R16185, which has a largely original tail exhibits irregular caudal banding, with some bands incomplete. Venter variably marked but always with brown mottling, some with distinct, irregular transverse bars or chevrons (AMS R 161237, 161286, 161287).
In life the dorsal coloration is a complex and often irregular pattern of several different colors, including grayish brown, brick red, salmon, and mossy green ( Fig. 21 View FIGURE 21 ). There is typically a white or lichenous green nape patch and the mid-dorsum typically bears a series of dark blotches with lighter centers. The flanks and side of the head bear scattered small white flecks. A dark reddish brown, posteriorly-directed triangle is usually present on the fronto-parietal region of the head. The original tail bears irregular dark blotches on a reddish brown background. The venter from the posterior portion of the throat to the pygal portion of the tail is a pale yellowish-green with diffuse chevrons or transverse bands of brown ( Fig. 20 View FIGURE 20 ). The anterior portion of the throat is white with dark brown transverse markings, some fusing to form broad swaths of dark pigment, or it may be entirely brown. The iris is silvery and the tongue and mouth lining are unpigmented. Although it is unclear if there are consistent geographical differences in coloration, specimens from Forêt d’Ougne and Néhoué appear greener than those from Île Art and Tiébaghi.
Etymology. The specific epithet is derived from the word jâlu , which means spirit (a being from the spirit world) in the Nyêlâyu language which is used in the northern Province Nord from Balade, through Ouégoa, Baie de Harcourt to Arama, and on Balabio and the Îles Belep. The name is thus parallel in construction to that of its sister taxon M. chahoua , which according to Bavay (1869) meant “devil” in an unspecified Kanak language. Throughout New Caledonia giant geckos have an association with elements of the spirit world that are both feared and respected (Bauer & Sadlier 2000). The name is a noun in apposition.
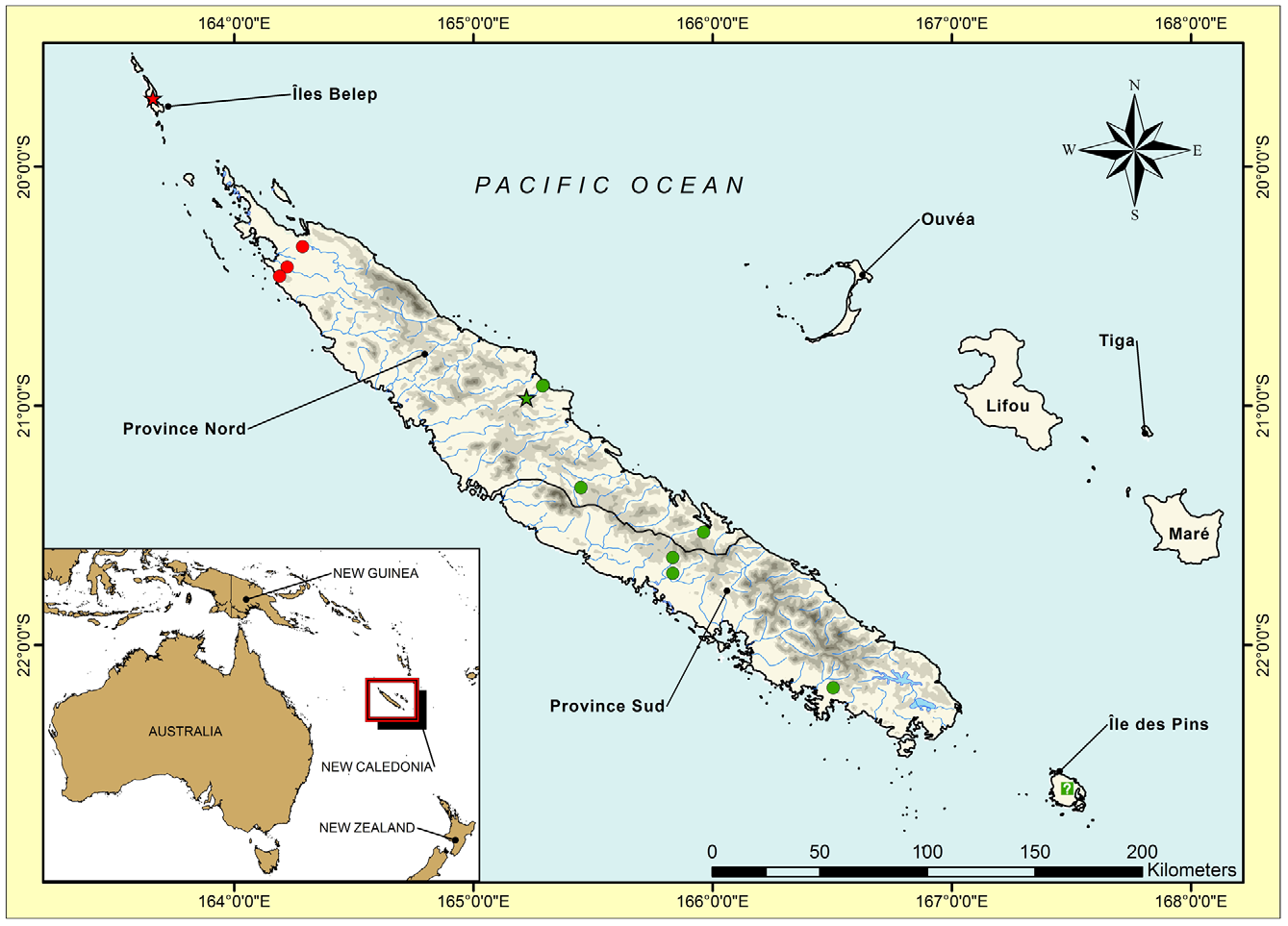
Distribution. The first confirmed record of Mniarogekko jalu was at Rivière Néhoué in 1998 (Siepp & Henkel 2000; Henkel & Böhme 2001) and it was subsequently illustrated by Watkins-Colwell (2003) and Langner (2009). Since then M. jalu has been recorded at two further locations in the extreme north of Grande Terre (Dôme de Tiébaghi and Forêt d’Ougne) and from Île Art in the Îles Belep, 40 km north of the Grande Terre, and its continued occurrence Rivière Nehoué has been confirmed ( Whitaker et al. 2004; Langner 2009; Fig. 17 View FIGURE 17 ).
Natural History. Mniarogekko jalu has been found only in old-growth, closed-forest habitat with large canopy trees. These typically have numerous holes, cracks and crevices which would provide an array of sheltering sites by day. The Rivière Néhoué ( Fig. 22 View FIGURE 22 D–E) and Forêt d’Ougne ( Fig. 22 View FIGURE 22 C) localities are at low elevation (<10 m) on schist substrates, with the geckos inhabiting tall gallery forests (to more than 20 m) on alluvial soils along the valley floors. The Dôme de Tiébaghi ( Fig. 22 View FIGURE 22 A–B) and Îles Belep ( Fig. 16 View FIGURE 16 ) localities are on ultramafic substrates—at Dôme de Tiébaghi one population is in mid-elevation (280–380 m) gully forest with a canopy height to about 12 m; on the summit plateau of Dôme de Tiébaghi (500–550 m) and the plateaux of Île Art (220–250 m) the geckos inhabit low (<8 m) closed forest on bouldery cuirasse surfaces.
Mniarogekko jalu appears to be exclusively arboreal. At night they have been observed foraging in the twigs and outer foliage of canopy trees or less often perched on branches and trunks in the upper vegetation strata (all observations of this species made at night were in the upper half of the vegetation). During the day they shelter in crevices and holes in branches and trunks, often descending close to the forest floor (<1 m) to such sites. Favored retreat sites are in the holes and crevices in the complex root structure of banyan trees ( Ficus prolixa G. Forst., Fam. Moraceae ). When in retreat crevices this species often rests near the entrance where it is clearly visible, only moving out of sight when disturbed. Eggs of M. jalu were found in a tree crevice 2.5 m above the ground on Dôme de Tiébaghi and in humus in epiphytic ferns 8 m above ground at Forêt d’Ougne (AMS R 161280). Paratype CAS 250858, collected in late January at Rivière Néhoué, has two large eggs visible in x-ray.
At Forêt d’Ougne this species was frequently observed at night in the emergent crowns of cerisier bleu trees ( Elaeocarpus angustifolius Blume, Fam. Elaeocarpaceae ) that were fruiting heavily and, as the sister species M. chahoua is known to be at least partially frugivorous ( Bauer 1985), were assumed to be feeding on the fleshy berries.
The defence behaviour of R. jalu when under immediate threat was to take evasive action by coiling into a tight ball and falling from the vegetation to the forest floor. This evasive behaviour has also been documented in captive M. chahoua ( Vosjoli et al. 2003) .
Trombiculid mites are present between lamellae and in one or both popliteal pockets in all of the specimens examined. In one specimen mite infestation of the cloacal sacs is extreme and mites have caused and/or infested a midventral cavity between the cloacal sac apertures.
On Île Art Mniarogekko jalu is syntopic with Correlophus belepensis , Eurydactylodes agricolae and Dierogekko insularis and at Dôme de Tiébaghi it is syntopic with Rhacodactylus auriculatus , Eurydactylodes agricolae and Dierogekko nehoueensis . At the other two locations it is the only giant gecko species present but is variously syntopic with Bavayia aff. exsuccida , B. aff. cyclura , Eurydactylodes agricolae and Dierogekko nehoueensis . Other sympatric lizards in its habitat include Hemidactylus frenatus , H. garnotii Duméril & Bibron, 1836 , Lepidodactylus lugubris , Caledoniscincus aquilonius Sadlier, Bauer & Colgan, 1999 , C. atropunctatus , C. austrocaledonicus , C. haplorhinus , Kanakysaurus viviparus , Lioscincus nigrofasciolatus , L. novaecaledoniae ( Parker, 1926) and Phoboscincus garnieri .
Conservation status. At present Mniarogekko jalu is known only from a small part of northern Grande Terre, north of Koumac, and on the Belep archipelago. Whether its actual range is so confined is unclear but the nearest known location for its sister species M. chahoua is at Vallée d’Amoa, on the east coast 105 km south-east of Koumac (Bauer & Sadlier 2000). Within the known extent of occurrence for M. jalu the old-growth closed forests that are its preferred habitat are now reduced to scarce and isolated remnants—largely as a result of repeated burning since the arrival of Melanesian colonists>3000 ybp but also including more recent clearance for cattle ranching and, in localized areas, for mining.
Mniarogekko jalu faces a number of direct and indirect threats. All remaining areas of forest habitat are under continued threat from wildfires that affect northern New Caledonia each dry season. Browsing ungulates are fortunately absent from the Îles Belep but the forests on the Grande Terre suffer the on-going depredations of Sunda Sambar, Rusa timorensis View in CoL (de Blainville, 1822), and feral pigs at all localities, with the addition of cattle at Rivière Néhoué and Forêt d’Ougne. Introduced rats ( Rattus View in CoL spp.), feral cats and little red fire ants ( Wasmannia auropunctata View in CoL ) are present at all localities where M. jalu has been found. Rats and cats are known to be serious predators of lizards but no direct evidence of predation on M. jalu was obtained; little red fire ants are known to have a severe impact on lizard populations, even resulting in local extirpation ( Jourdan et al. 2000, 2001).
The localities on Dôme de Tiébaghi are under immediate threat of total destruction resulting from expansion of the open-cast nickel mine on the massif. Although there appear to be no immediate plans to mine on Île Art, the ultramafic plateaux have had extensive prospecting for nickel in the past and the whole area is held under current mining licenses. The Forêt d’Ougne locality is on a cattle ranch and subject to on-going browsing pressure. Only the locality at Rivière Néhoué has reserve status. However, it is administered as a recreation reserve, is small in extent and has high human use.
There are no quantitative data on population size and trends available for Mniarogekko jalu . Surveys in 2001–2002 indicated that it was relatively numerous at each of the known sites, with encounter rates ranging from 0.19/hour on Dôme de Tiébaghi to 1.75/hour on Île Art, and at Forêt d’Ougne eight were observed on 190 m of forest margin ( Whitaker et al. 2004). However, two factors point to the species’ potential vulnerability. In 2001 at Forêt d’Ougne M. jalu was relatively common in one valley yet it was not detected in an immediately adjacent valley (<350 m away) with identical forest but where little red fire ants were exceptionally abundant. Also in 2001 M. jalu was moderately abundant in closed forest at a gully site on the slopes of Dôme de Tiébaghi but it could not be detected at this same location six years later after increased mining activity had led to the vegetation being blanketed in wind-blown dust from a nearby mining haul-road.
Because of its limited extent of occurrence, restricted area of occupation (<30 km ²), limited number of locations (four), the threats to its habitat (wildfires, browsing ungulates, mining), the presence of mammalian predators (rats, cats) and the impacts of fire ants, Mniarogekko jalu is assessed as Endangered (B1a, b[ii–iii, v]; B2a, b[ii–iii, v]) ( IUCN 2001).
Remarks. Correlophus ciliatus x Mniarogekko chahoua hybrids have been reported in captivity ( Seipp & Henkel 2011), thus, despite their genetic divergence, it is likely that there is also some degree of compatibility between members of the two genera. Indeed, levels of genetic differentiation between genera of New Caledonian diplodactylids are relatively low in comparison to many other gecko groups (Jackman & Bauer 2006), so it is not surprising that similarly-sized members of the clade can interbreed. Although the viability of F1 hybrids has been demonstrated, we are unaware of data on their fertility or the viability of subsequent generations.
Jouan (1863, 1864) noted the existence of a giant gecko on the Îles Belep, but collected no specimens. As Rhacodactylus spp. appear to be absent from this island group, it seems likely that Jouan’s reports refer to M. jalu , which is the largest gecko on the Belep islands and is relatively abundant in appropriate habitat ( Whitaker et al. 2004).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |