Rhacodactylus
|
publication ID |
https://doi.org/ 10.5281/zenodo.211734 |
|
DOI |
https://doi.org/10.5281/zenodo.6166576 |
|
persistent identifier |
https://treatment.plazi.org/id/5C4F87E1-FFAF-FFB0-088F-F8ADD51FB423 |
|
treatment provided by |
Plazi |
|
scientific name |
Rhacodactylus |
| status |
|
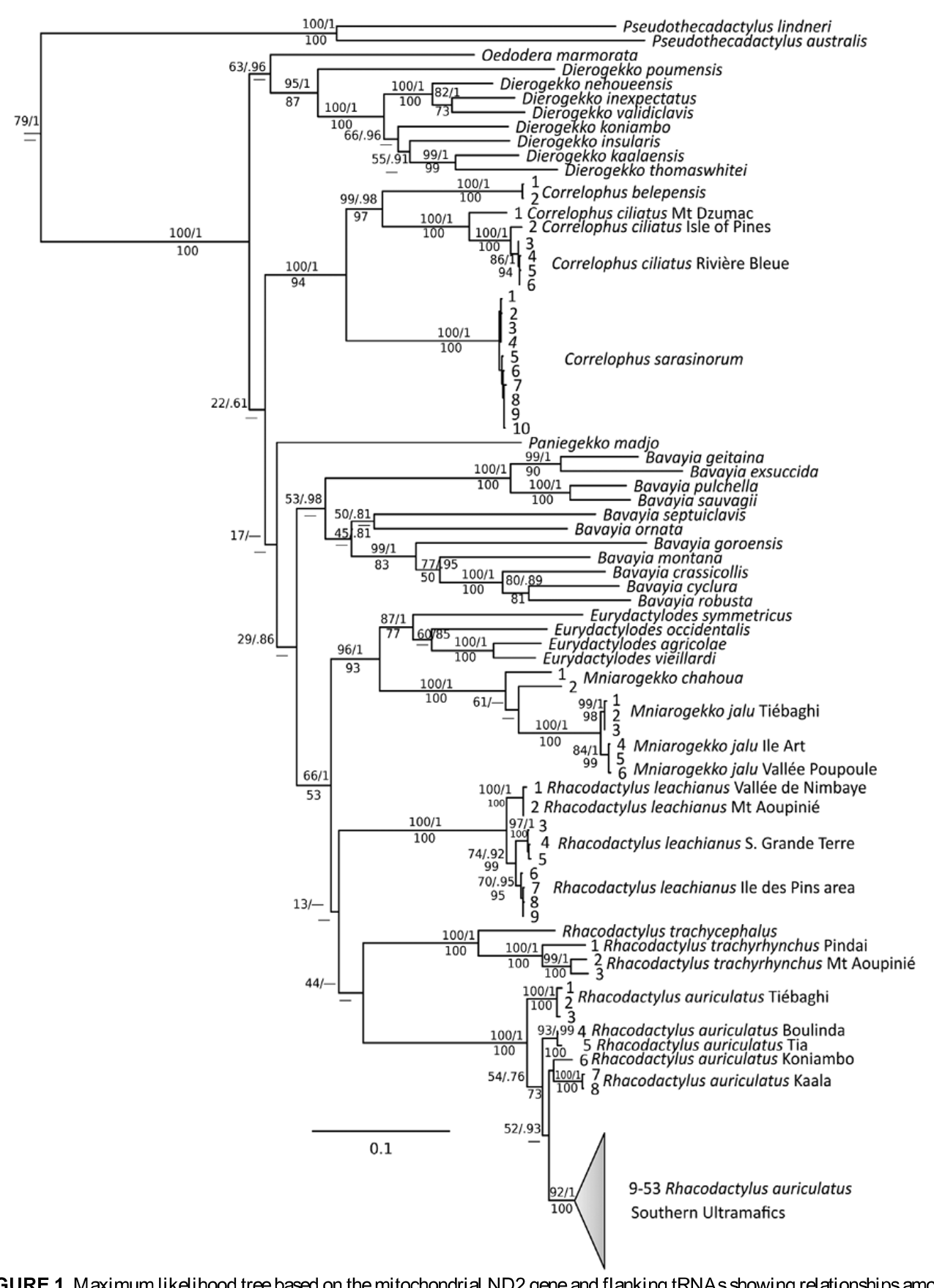
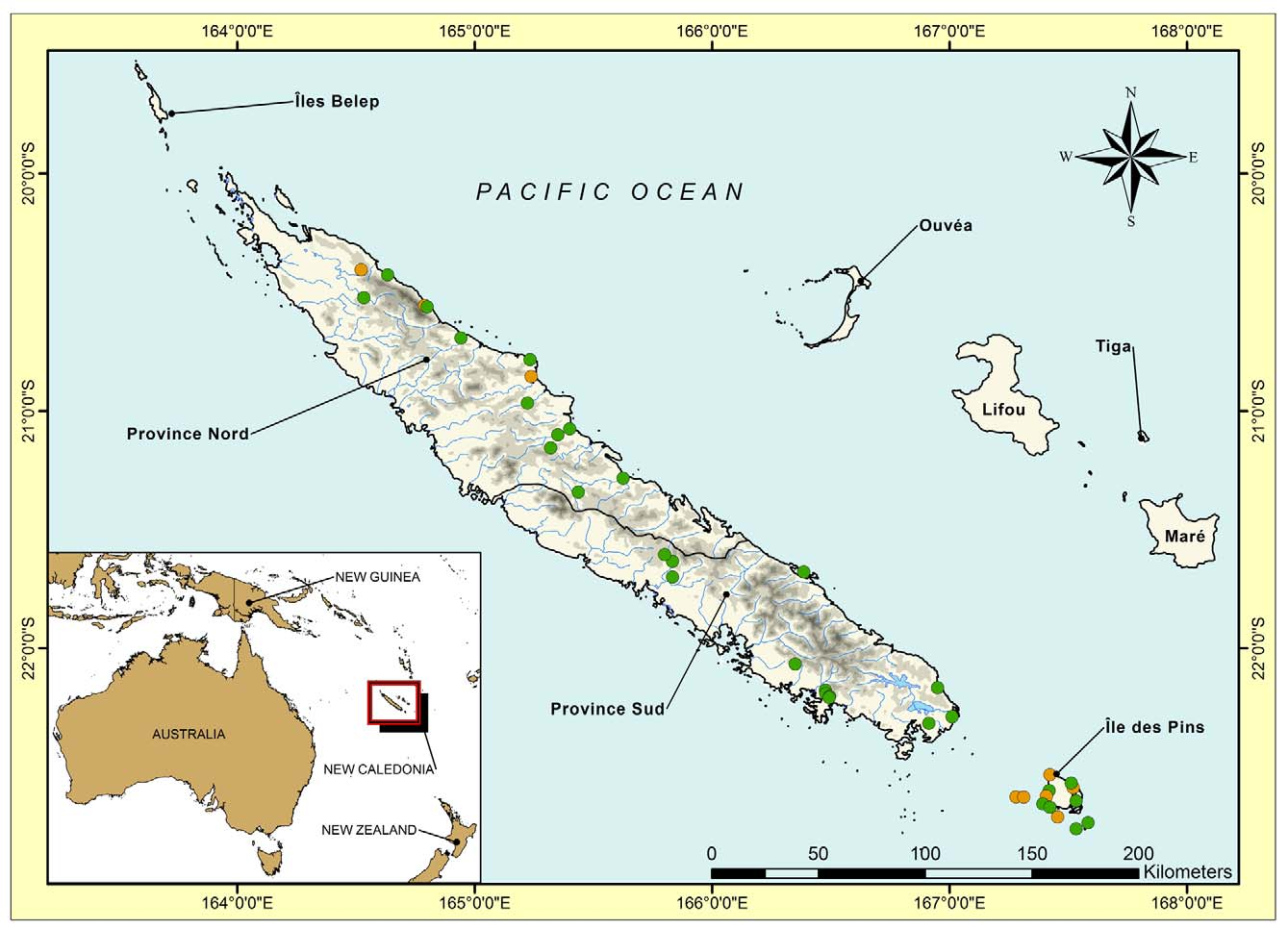
Rhacodactylus auriculatus —Variation is limited in R. auriculatus ( Figs. 1–2 View FIGURE 1 View FIGURE 2 ). Until recently this species was believed to be restricted to the southern ultramafic block of the Grande Terre (Bauer & Sadlier 2000, 2001). There is little divergence or substructure within the clade from this region. This is consistent with Bauer’s (1990) observation that R. auriculatus is polymorphic in color throughout this range and shows no geographically-related trends in character variation, and with the lack of allozyme variation reported by Good et al. (1997). However, extensive field surveys in the northern ultramafic ranges of the Grande Terre undertaken by the authors beginning in 2001, have revealed that R. auriculatus also occurs as far north as Dôme de Tiébaghi in the west and Poro in the east ( Whitaker et al. 2004; Bauer et al. 2006a, b; Fig. 4 View FIGURE 4 ). Our samples included specimens from the Dôme de Tiébaghi, Mt. Kaala, Massif de Koniambo , Plateau de Tia, Massif de Kopéto and Massif du Boulinda. Samples from the southernmost of these localities (Tia, Boulinda) are nearly genetically identical to one another, but each of the other localities, representing three isolated ultramafic blocks, are divergent, albeit at a low level (3.1–4.1%). The northernmost locality of Dôme de Tiébaghi is the most deeply divergent lineage. However, this divergence is less than between well-diagnosed species of Dierogekko or other giant geckos and we interpret the pattern seen as the result of isolation by distance within a lineage now known to have an almost island-wide distribution on ultramafic surfaces. The lack of variation within the southern ultramafic block or the Boulinda-Kopéto block probably reflects the continuity of gene flow between largely continuous blocks of maquis habitat or possibly recent rapid expansion. Unlike its congeners, R. auriculatus readily moves on the ground ( Bauer and Vindum, 1990) and occurs in maquis vegetation and at least on the periphery of humid forest habitat ( Snyder et al. 2010). Given that the northern populations of R. auriculatus escaped detection for nearly 150 years, it is possible that the species is even more widely distributed on ultramafic surfaces than now indicated.
Rhacodactylus leachianus —Morphological variation in Rhacodactylus leachianus , at least with respect to size, body proportions, and color pattern, has been remarked upon by numerous authors (e.g., Henkel 1991, 1993; Seipp & Obst 1994; Seipp & Henkel 2000, 2011; de Vosjoli et al. 2003; Cemelli 2009; Schönecker & Schönecker 2009b). In particular, R. leachianus from the offshore islands surrounding the Île des Pins have been recognized as R. l. henkeli Seipp and Obst, 2004 . Geckos from these populations are generally characterized by smaller size, stouter body, shorter snouts and tails, lower scale counts, and heavier patterning than most individuals from the Grande Terre. They have also been regarded as being more diurnal and less wary than individuals from the Grande Terre ( Seipp & Obst 1994; de Vosjoli 1995). Further, many “varieties” or “morphs” from different southern islands have been identified and are marketed as discrete entities in the pet trade (de Vosjoli et al. 2003; Cemelli, 2009). Good et al. (1997) reviewed the evidence for the recognition of R. l. henkeli and concluded that the scale counts and color patterns seen in the insular forms fell within the range of variation of the nominate form. They further argued that features such as smaller size and reduced wariness might be expected on islands, where resources are limited and predators absent. Lower scale counts may be a direct consequence of smaller body size ( Hecht 1952).
In fact, the level of genetic differentiation between populations of R. leachianus on the Grande Terre may be greater than that observed between populations on the southern islets and the main island. Vences et al. (2001) found no variation between specimens from four islands in the Île des Pins group and only a single base pair difference between these and a specimen from Nouméa in the southern Grande Terre. They did, however, find 18–19 base-pair differences between these southern forms and a specimen from Houaïlou on the central east coast. Our sampling within R. leachianus , which occurs throughout much of New Caledonia ( Fig. 5 View FIGURE 5 ), was limited (Table 1), but included specimens from two southern islands (Môrô and Bayonnaise), a far southern mainland locality (Kwa Néie), and two central localities (Mt. Aoupinié and Vallée de Nimbaye). The northernmost localities sampled were largely invariant and were sister to the southern ones, including the islands ( Fig. 1 View FIGURE 1 ), but the level of divergence was minimal, about half of that between northern and southern R. auriculatus , and the divergence between the southern mainland and islands was only 1.4%.
Although we do not doubt the observed phenotypic differences between the mainland and insular forms, we believe that most of these differences represent either phenotypically plastic traits or traits that have become fixed in very recent times. Indeed sea level minima of 100 m or more would have connected the Grande Terre to the Île des Pins as recently as 16,000–20,000 years ago ( Holloway 1979; Balouet & Olson 1989), although the presence or extent of suitable habitat on the land exposed by lower sea levels is unknown. Further, cyclones in the region are known to overwash and denude some of the small islands upon which R. leachianus lives (Geneva 2008). This suggests that existing populations may reflect not simply lizards isolated by rising sea levels, but the result of many recolonizations from either the Île des Pins proper or the southern Grande Terre. We therefore echo Good et al. (1997) in regarding “ henkeli ” as a morph of typical R. leachianus peculiar to the southern islets, rather than as a valid taxon.
The status of R. aubrianus Bocage, 1873 , which has been recognized subspecifically by some authors ( Roux 1913; Kluge 1967) cannot be evaluated. The syntypes of this form were destroyed by fire in 1978 ( Almaça & Neves 1987) and are without specific locality. Putatively diagnostic features of snout scalation given by Bocage (1873) as diagnostic, in fact, also occur in some typical R. leachianus . Thus, we also regard this form as strictly synonymous with R. leachianus , which is therefore, monotypic. Seipp and Henkel (2011) suggested that R. leachianus “dark morph” was distinct in coloration and biology from the typical form and that these two occurred in sympatry in some areas.
Rhacodactylus trachyrhynchus and R. trachycephalus — Boulenger (1878) described Chameleonurus trachycephalus from the Île des Pins, but later synonymized his new genus and species with Rhacodactylus trachyrhynchus ( Boulenger 1883) . The name remained largely unused for more than a century, until used in a subspecific context by Seipp and Obst (1994), Kluge (2001), and Seipp and Henkel (2000, 2011) and it has lately been used with some consistency in the herpetocultural literature (Henkel 2009; Kaverkin 2009). Vences et al. (2001) found a small difference (4 bp) between Île des Pins and Grande Terre (Mt. Koghis) samples. We sampled specimens from three locations: Mt. Aoupinié in central New Caledonia, an apparently isolated population from sclerophyll forest at Presqu’ïle de Pindaï on the west coast, and Îlot Môrô, off the Île des Pins. Divergence between the two mainland populations was nearly as great as that between the most divergent populations of R. auriculatus but the divergence from these to the Îlot Môrô sample are twice as great. Although not included by us, Good et al. (1997) studied a specimen from Mt. Gouémba (= Wô Bwa Wîwâ) in the far southeast of the Grande Terre and found that it differed by one fixed allozyme difference from Mt. Aoupinié specimens.
De Vosjoli et al. (2003) had suggested that the mainland and southern insular populations were distinct species based on differences in size, morphology and behavior. As noted by Seipp and Henkel (2000, 2011) the population from the region of the Île des Pins, the type locality of R. t. trachycephalus , differs in a number of ways from typical R. t. trachyrhynchus , which are known from scattered localities across the Grande Terre ( Fig. 6 View FIGURE 6 ). These include smaller size, a lower average number of scales in various counts, and a difference in the configuration of the head scalation. Myers and Pether (1998) suggested that Grande Terre animals were sometimes more yellowish than Île des Pins specimens, and differences in snout length have also been suggested ( Henkel 1991, 1993 Myers & Pether 1998). Although the same arguments regarding the recency and transiency of gecko populations on the lowlying satellite islands around the Île des Pins applies to this form as to R. l. henkeli , the Île des Pins itself is a high island (maximum elevation 262 m) that would have remained above water since the Miocene and which would have been isolated from the Grande Terre sporadically since that time ( Hope 1996). Issues of size aside, we believe that the deep genetic divergence and distinctive morphological features seen in the insular specimens are reflective of a meaningful evolutionary split and we recognize R. trachycephalus ( Boulenger, 1878) ( Figs. 7–8 View FIGURE 7 View FIGURE 8 ) as a valid species.
Boulenger’s (1878) description is relatively detailed and is accompanied by a well-executed plate. His synonymization (1883) with R. trachyrhynchus is understandable given that the small number of individuals of both species then known did not allow a distinction between individual or population variation and specific differences. Boulenger’s description was based on two specimens in the Institut Royal des Sciences Naturelles de Belgique in Brussels ( Fig 7 View FIGURE 7 ).
Diagnosis. Rhacodactylus trachycephalus may be distinguished from R. trachyrhynchus , the only other New Caledonian giant gecko with a rugose snout by its smaller size (maximum SVL 140 mm versus 190 mm), larger eye size relative to snout length and eye-ear distance, lower number of midbody scale rows (maximum 111 versus minimum 119) (fide Seipp & Henkel 2011), exclusion of the rostral from the nostril (versus rostral contacts the nostril or very narrowly excluded in R. trachyrhynchus ), and smaller, less rugose scales in the loreal region ( Fig. 8 View FIGURE 8 ).
Distribution. Seipp and Henkel (2001, 2011) gave the distribution of this species as the Île des Pins and “Koutouma” [sic] (= Kûtomo), de Vosjoli (1997) reported it from “ Island E,” and Vences et al. (2001) sequenced an individual supposedly from the Île des Pins. However, limited field investigations by Bauer and Sadlier (1994) and de Vosjoli (1995) could not verify its presence on the Île des Pins proper, although Bauer and Sadlier (1994) did identify appropriate habitat for the species on the island. We have encountered it only on Môrô, an island of ~ 0.1 km 2, where its biology has been studied by Cunkelman (2005).
Conservation. de Vosjoli et al. (2003) highlighted R. trachycephalus as the only endangered Rhacodactylus . This species certainly has the most restricted range of any member of the genus. On Môrô they are especially vulnerable because of the easy access from the Île des Pins. Rhacodactylus trachycephalus is at very high risk due to potential demands from the pet trade. Most live-bearing Rhacodactylus offered for sale are members of this species ( Kaverkin 2009). In comparison to most oviparous species, live-bearing Rhacodactylus have proven difficult to breed in captivity ( Myers & Pether 1998) and they remain very expensive in the pet trade, with online prices of US $3000 or more as of March 2012. This suggests there may remain a market for wild-caught individuals, a scenario which may no longer be true for some of the other giant geckos, which are now bred in great numbers in captivity and are available at relatively low prices. In addition to illegal collecting for the pet trade, this species is also likely to be highly vulnerable to introduced mammals, including rats and feral cats ( Cunkelman 2005). Based on its extremely small area of occupancy and extent of occurrence as well as observed decline in habitat quality, as well as threats from introduced predators and the pet trade R. trachycephalus is assessed as Critically Endangered (B1b; B2b).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Rhacodactylus
| Bauer, Aaron M., Jackman, Todd R., Sadlier, Ross A. & Whitaker, Anthony H. 2012 |
R. l. henkeli
| Seipp and Obst 2004 |
Rhacodactylus trachyrhynchus (
| Boulenger 1883 |
R. trachycephalus (
| Boulenger 1878 |
R. aubrianus
| Bocage 1873 |