Tripolydora spinosa Woodwick, 1964
|
publication ID |
https://doi.org/10.11646/zootaxa.4019.1.22 |
|
publication LSID |
lsid:zoobank.org:pub:88F2DB05-58C4-4726-89D5-99302FABB908 |
|
DOI |
https://doi.org/10.5281/zenodo.4658224 |
|
persistent identifier |
https://treatment.plazi.org/id/5E51D737-FFE9-FF94-FF4A-A0241DFBFBCB |
|
treatment provided by |
Plazi |
|
scientific name |
Tripolydora spinosa Woodwick, 1964 |
| status |
|
Tripolydora spinosa Woodwick, 1964 View in CoL
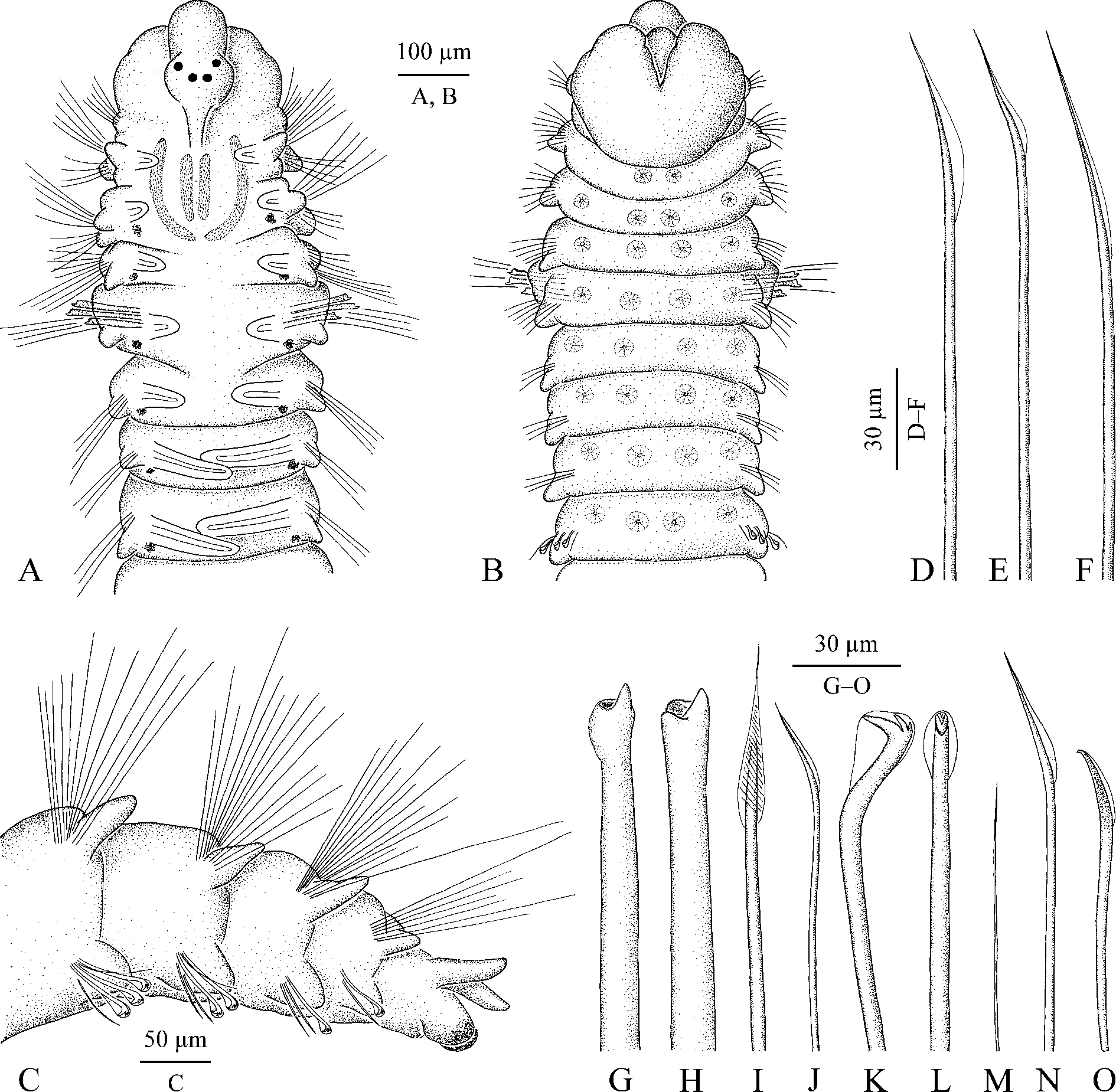
( Fig. 35 View FIGURE 35 )
Tripolydora spinosa Woodwick, 1964: 155 View in CoL –156, fig. 4 (6–9); Reish 1968: 222; Kohn & Lloyd 1973: 700; Blake & Woodwick 1981: 352 –362, figs 1–5; Ward 1981: 730; 1987: 368–369, fig. 3.II. I48; Hartmann-Schröder 1992: 70 –71, figs 72–75; Williams 2001: 457 –459, fig. 15.
Material examined. Queensland: AM W.47872, MI QLD 2379 (1).
Adult morphology. Single specimen in two fragments, comprising 16 anterior chaetigers 1.75 mm long, 0.3 mm wide and 15 posterior chaetigers about 1.5 mm long. Dark paired spots (possibly remains of larval melanophores) present on dorso-lateral sides from chaetiger 3 to end of anterior fragment; ochre pigment present in distal part of ventral pygidial cirri. Prostomium rounded anteriorly, extending posteriorly to end of chaetiger 3 as a low indistinct caruncle. Two pairs of dark eyes (appearing dark reddish in formalin-fixed specimen) arranged trapezoidally. Occipital antenna absent. Nuchal organs two pairs of wide ciliary bands comprising inner pair of almost straight longitudinal bands situated on sides of low indistinct caruncle, and longer outer pair with anterior part oriented longitudinally and posterior part curved inwards ( Fig. 35 View FIGURE 35 A). Palps missing.
Chaetiger 1 with short capillaries and low postchaetal lamellae in neuropodia; notopodia lacking. Chaetigers 2 and 3 with slender capillaries in both rami. Chaetigers 4 and 6 with dorsal superior and posterior-row notochaetae and neurochaetae slender capillaries ( Fig. 35 View FIGURE 35 E, F). Chaetiger 4 anterior-row notochaetae capillaries with swollen wing ( Fig. 35 View FIGURE 35 D); similar capillaries with less swollen wing also present in anterior row in notopodia on chaetiger 6. Posterior-row capillary notochaetae on chaetigers 7 and 8 with ribbed structures oriented obliquely on wing surface ( Fig. 35 View FIGURE 35 I). Notopodia on middle chaetigers with 5–10 slender capillaries and small postchaetal lamellae; notopodia on posterior chaetigers with bundles of 15–20 separate long needle-like spines and small postchaetal lamellae ( Fig. 35 View FIGURE 35 C).
Chaetiger 5 slightly larger than chaetigers 4 and 6, with noto- and neuropodial postchaetal lamellae same as those on chaetigers 4 and 6. Notochaetae comprising 2–3 long dorsal superior capillaries, four shorter posteriorrow capillaries, both kinds of chaetae same but fewer than those on chaetigers 4 and 6, and four anterior-row heavy acicular spines with terminal tooth flanked on one side by two wide knobs ( Fig. 35 View FIGURE 35 G, H). Neurochaetae comprising two vertical rows of capillaries (four capillaries in each row) and two shorter inferior capillary chaetae, all capillaries same as those on chaetigers 4 and 6.
Hooks in neuropodia from chaetiger 9, up to five in a series, accompanied by 2–4 short winged capillaries arranged in a vertical row in front of hook row ( Fig. 35 View FIGURE 35 J), 1–4 hair-like capillaries alternating with hooks ( Fig. 35 View FIGURE 35 M), and 1–2 inferior chaetae situated below hook row. Inferior chaetae in anterior hook-bearing segments winged capillaries ( Fig. 35 View FIGURE 35 N), on chaetigers 14–15 gradually transformed into sabre chaetae with narrow wing and fine granulation on distal part ( Fig. 35 View FIGURE 35 O). Hooks tridentate, with two upper teeth arranged in line above main fang, with only outer hood, shaft slightly curved, without constriction ( Fig. 35 View FIGURE 35 K, L).
Branchiae from chaetiger 2 to almost end of body, short on anterior chaetigers, full-sized from chaetiger 7 ( Fig. 35 View FIGURE 35 A). Branchiae free from notopodial postchaetal lamellae, flattened, with surfaces oriented parallel to body axis, with longitudinal ciliation on inner surface. Afferent and efferent arms of branchial blood loop not interconnected by radial capillaries.
Pygidium small, with one pair of thin dorsal cirri and one pair of thicker and shorter ventral bulbs ( Fig. 35 View FIGURE 35 C). Paired glandular organs arranged in transverse lines on ventral side of anterior chaetigers ( Fig. 35 View FIGURE 35 B). Apparently one pair of organs present on each of chaetigers 2 and 3; two pairs of organs present on each chaetiger from chaetiger 4 to about chaetiger 10; organs indistinct on succeeding chaetigers. Each organ appearing as a round pore about 3 µm in diameter, sided by 10–15 cells forming a rosette-like structure up to 25 µm in diameter.
Glandular pouches in neuropodia from chaetiger 1, very small in anterior chaetigers.
Digestive tract without gizzard-like structure.
Main dorsal blood vessel without heart body.
Nephridia from chaetiger 4 onwards.
Reproduction. Unknown.
Remarks. Tripolydora spinosa was originally described from the rocky intertidal on Eniwetok Atoll in the Marshall Islands by Woodwick (1964). It was later recorded from the tropical Pacific and western Indian Ocean ( Reish 1968; Kohn & Lloyd 1973; Ward 1981; 1987; Hartmann-Schröder 1992; Williams 2001). Blake & Woodwick (1981) recorded material from the Cook Islands and for the first time described the unique ribbed capillaries in the notopodia of chaetigers 7–10 and established homology of the modified chaetae of chaetigers 4 and 5 with corresponding groups of chaetae on chaetigers 2–3 and 6–10. Williams (2001) examined a single living specimen from the Philippines and described black pigmentation on the palps, prostomium, and dorsal side of the anterior and posterior chaetigers. He noted that this pigmentation disappeared after fixation. This is probably why this kind of pigmentation was not reported by other authors and also not observed in the present study. Reproductive biology and larval development of T. spinosa remain unknown.
The single specimen from Yonge Reef examined in the present study after fixation appears similar to T. spinosa described by Woodwick (1964) and other authors, and is referred to this species. Glandular pouches in neuropodia, ventral glands on anterior chaetigers, and nephridia are described herein for the first time for this spionid. The nuchal organs described in the present study, however, differ from those described by previous authors. Remarkably, fine epithelium of fragile worms is easy to destroy and dorsal ciliation of worms often appears indistinct, especially after fixation. Woodwick (1964) noted the caruncle extending to end of chaetiger 3 and did not provide any comment on the morphology of the nuchal organs. Blake & Woodwick (1981: fig. 1A) did not illustrate nuchal organs with SEM but depicted them as a pair of longitudinal bands of cilia on the sides of a wide caruncle extending to middle (until nototroch) of chaetiger 3. Williams (2001: 457) noted broad caruncle reaching end of chaetiger 2 but depicted nuchal organs as a pair of longitudinal bands of cilia on sides of caruncle extending to end of chaetiger 3 or even middle of chaetiger 4 ( Williams 2001: fig. 15A). This kind of nuchal organs is typical for polydorins and might have been in mind of the authors during interpretation of an indistinct structure. Nuchal organs as two pairs of wide ciliary bands comprising inner pair of almost straight longitudinal bands situated on sides of low and narrow indistinct caruncle, and a longer outer pair with anterior part oriented longitudinally and posterior part curved inwards are described in T. spinosa for the first time in the present study ( Fig. 35 View FIGURE 35 A). Same organs were also observed in other specimens of T. spinosa from other regions (Radashevsky unpublished). These nuchal organs appear similar to J-shaped organs typical for adult Microspio and Spio (see Jelsing 2002: fig. 2A, 2003: 2G; Bick et al. 2010: fig. 9; Radashevsky 2012: figs 2E, 4B).
Adult T. spinosa View in CoL are unusual among polydorins in having tridentate instead of bidentate hooks, anterior-row capillaries and alternating capillaries accompanying hooks, and sabre chaetae in neuropodia. The heavy acicular spines of chaetiger 5 positioned in the anterior row in notopodia of T. spinosa View in CoL are not homologs of the falcate spines positioned in the posterior-row in notopodia of Polydorini members (see Radashevsky & Fauchald 2000: fig. 4; Radashevsky 2012). The nuchal organs and ventral glands described herein for T. spinosa View in CoL are typical for Microspio View in CoL and Spio View in CoL (for ventral glands see Rößger et al. 2015). All these characters support the hypothesis of closer relationship of T. spinosa View in CoL to Microspio View in CoL and Spio View in CoL than to polydorins. This hypothesis will be estimated in a phylogenetic analysis of spiomorph polychaetes elsewhere.
Habitat. In this study, a single individual of T. spinosa View in CoL was found in coral sand at 10 m depth.
Distribution. Tropical Pacific. This is the first record of the species for the Great Barrier Reef and Australia.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Tripolydora spinosa Woodwick, 1964
| Radashevsky, Vasily I. 2015 |
Tripolydora spinosa
| Williams 2001: 457 |
| Hartmann-Schroder 1992: 70 |
| Blake 1981: 352 |
| Ward 1981: 730 |
| Kohn 1973: 700 |
| Reish 1968: 222 |
| Woodwick 1964: 155 |