Isoperla borisi, Beaty & Holland & Lenat, 2017
|
publication ID |
https://doi.org/10.5281/zenodo.4752853 |
|
publication LSID |
lsid:zoobank.org:pub:8EFAB403-E49D-489A-A9BA-F237880142C6 |
|
DOI |
https://doi.org/10.5281/zenodo.4758914 |
|
persistent identifier |
https://treatment.plazi.org/id/5F11AD51-286B-FFBA-4DDF-F933D99CFB50 |
|
treatment provided by |
Felipe |
|
scientific name |
Isoperla borisi |
| status |
sp. nov. |
Isoperla borisi View in CoL sp. nov.
http://lsid.speciesfile.org/urn:lsid: Plecoptera .speciesfile.org: TaxonName:501184
Slate Belt Stripetail
( Figs. 20–44 View Fig View Fig View Figs View Figs View Figs View Figs View Figs View Fig )
Material examined. USA – Holotype ♂ and larval exuvia, NORTH CAROLINA, Orange Co., Collins Creek, NC 54, 35.93139, -79.20590, 30/ IV/2014, D. R. Lenat ( NMNH). GoogleMaps Paratypes: same locality as holotype, Chatham Co., Georges Creek, SR 2142, 35.60250, -79.25833, 18/IV/2015, S. R. Beaty, D. R. Lenat GoogleMaps , 1♀ ( NCDWR); Terrells Creek, NC 87, 35.82166, -79.25555, 30/III/2012, S. R. Beaty GoogleMaps , 1♀ (reared) and exuvia ( NCDWR). Moore Co., Killets Creek GoogleMaps , Bethlehem Church Rd., 35.35431, -79.45444, 13/IV/2015, D. R. Lenat GoogleMaps , 1♀ (reared) ( NCDWR). Orange Co., Collins Creek, NC 54, 35.9314, -79.2059, 23/IV/2014, D. R. Lenat , 3♂ (reared) and exuvia, 3♀ (reared) and exuvia ( NCDWR), 9 larvae ( NMNH); 25/IV/2014, D. R. Lenat , 3♀ (reared) and exuvia ( NCDWR); 30/IV/2014, D. R. Lenat , 12 larvae, 2♂ (reared), 21♀ (reared) and exuvia ( NCDWR); 6/ V /2014, D. R. Lenat , 1♀ (reared) and exuvia ( NCDWR); 13/IV/2015, V.B. Holland, 1♂ (reared) and exuvia ( CSUIC); 13/IV/2015, V.B. Holland, 2♂ (reared) and exuvia, 10♀ (reared) and exuvia ( NCDWR); 25/IV/2015, S. R. Beaty, V.B. Holland, 5 ♀ (reared) and exuvia ( CSUIC); 25/IV/2015, S. R. Beaty, V.B. Holland, 20♀ (reared) and exuvia, 2 larvae ( NCDWR); 30/IV/2015, D. R. Lenat , 2♂ (reared) and exuvia ( NCDWR); 5/ V /2015, D. R. Lenat , 1♂ (reared) and exuvia ( NCDWR); 9/ V /2015, S. R. Beaty, V.B. Holland, 1♀ ( NMNH), 1♀ w/eggs ( NCDWR); 16/ V /2015, V.B. Holland, 1♂ (reared), 1♀ (reared) and exuvia ( NCDWR).
Additional material: NORTH CAROLINA, Chatham Co., Bear Creek GoogleMaps , SR 2155, 35.63194, -79.2367, 23/III/2009, 2 larvae ( NCDWR); Georges Creek GoogleMaps , SR 2142, 35.60250, -79.25833, 25/II/1993, 4 larvae ( NCDWR); 10/III/2003, 4 larvae; Terrells Creek GoogleMaps , NC 87, 35.82166, -79.25555, 19/III/2009, E.D. Fleek, V.B. Holland, T. L. Morman, 13 larvae ( NCDWR); White Oak Creek GoogleMaps , NC 751, 35.75444, -78.95944, 8/II/1993, D. R. Lenat, L.E. Eaton, G. Coleman, 9 larvae ( NCDWR). Orange Co., Collins Creek GoogleMaps , NC 54, 5.9314, -79.2059, 15/III/2015, S.R. Beaty , D. R. Lenat, 27 larvae ( NCDWR); Jones Creek GoogleMaps , Hillsborough Rd., 35.9639, -79.1071, 15/IV/2013, L. E. Eaton, C. Gregory, L. Montgomery, 2 larvae ( NCDWR); 1/III/2009, D. R. Lenat, 4 larvae ( NCDWR); Old Field Creek , off Milhouse Rd, 22/IV/2015, D. R. Lenat, 1 larva ( NCDWR). Person Co., Crooked Creek GoogleMaps , SR 1558, 36.4878, -78.8069, 19/IV/2006, E.D. Fleek, T. F. McPherson, C Tyndall, 5 larvae ( NCDWR). Union Co., Richardson Creek GoogleMaps , SR 1751, 34.98972, -80.51027, 13/III/1989, T.F. McPherson , L.E. Eaton, D. Penrose, 8 larvae; location unknown, 1/II/1977,11 larvae ( NCDWR).
Distribution. USA – NC.
Adult Male — Macropterous. Forewing length 8.3–9.7 mm (n=11), body length 7.5–9.4 mm (n=9). General pattern on head approximately “A”- shaped, or “H”-shaped on lighter specimens. General body color brown to dark brown in life, yellow-brown in in alcohol, with darker brown markings.
Head: Palpi brown to medium brown. Labrum with stiff setae of medium length. Dorsum of head ( Fig. 20 View Fig ) with wide, dark brown to black bands connecting median ocellus with lateral ocelli; interocellar area yellow-brown and narrowly open posteriorly, ocellar spot may appear closed at epicranial suture in some specimens; tentorial callosities distinct and darkly connected to lateral edge of ocellar bands; pale brown spot anterior to median ocellus oval to subtriangular, rugosities lateral to pale spot usually as dark as ocellar bands and connected to frontoclypeal wrinkles by brown to dark brown pigment; anterior frontoclypeal area brown with posteriorly directed bands on either side of anterior pale spot connected to ocellar bands, paler anteromedially; frontoclypeus pale anterolaterally and anterior to eyes; numerous wrinkles on frontoclypeus near antennal bases; subtriangular pale yellow area on occiput along vague epicranial stem widest at hind margin of head and congruent with pale pronotal stripe; occiput mostly brown with area near eyes lighter and with brown rugosities and short setae. Antennae medium brown with scape darker brown, basal few segments slightly lighter than remainder of flagellum.
Thorax: Pronotum with median pale hourglassshaped stripe ( Fig. 20 View Fig ); a few small slightly darkened rugosities within posterior half of pronotal stripe; anterior pronotal margin dark brown, narrowly interrupted medially by pale area; posterior pronotum margined with brown, pale medially; lateral pronotal margins barely lighter; mid-dorsal pronotal suture with thin brown line; pronotal disks brown with rugosities darker; rugosities coalesced medially, more isolated laterally, raised. Meso- and metanota brown with dark brown scutal humps, pale yellow-brown stripe medially. Wings dusky with dark brown veins. Meso- and metabasisterna brown; furcal sternites pale with sutures and pits darker. Wings dusky with dark brown venation. Legs: Brown overall. Inner and outer faces of femora pale brown, darker dorsally with a thin dark longitudinal sub-dorsal band, with dark brown subapical vertical band and pale at extreme apical margin ( Fig. 21 View Fig ); tibiae brown with dark brown band on basal fourth and slightly darker apically; tarsi dark brown.
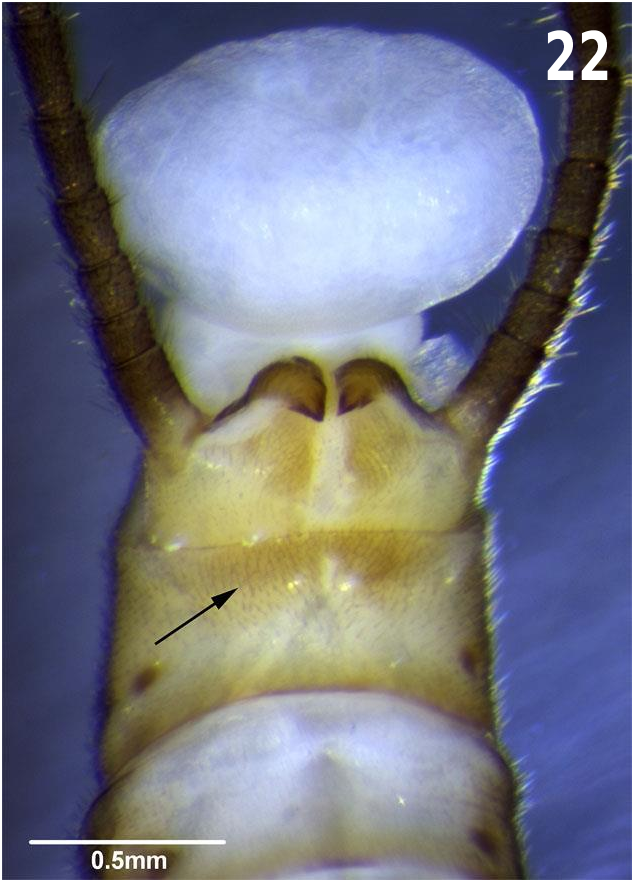
Abdomen: Overall yellow-brown with a broad longitudinal dark brown stripe along each side of the abdomen and dorsal to pleural folds, stripe with an irregular dorsal border and narrowing on posterior segments. Terga 2–9 pale yellow-brown, each with a lateral transverse series of 3 small brown dots, the lateral most dot often obscured by brown longitudinal pleural stripe; each tergum with a mesally divided dense patch of long stout setae, patches on middle segments laterally removed and sparse; tergum 9–10 brown, 9 elevated and with transverse patch of stout spinulae on posterior half, patch divided anteromesally but complete posteromesally ( Fig. 22 View Fig ); tergum 10 with a pair of small darkly sclerotized posterior elevations divided by a pale mesal furrow, with stout spinulae on raised areas. Paraprocts stout, heavily sclerotized, apically bifid with a large subapical ventral tooth and a dorsal tooth ( Figs. 23, 26 View Figs , 32 View Figs ), dorsal tooth may appear worn and jagged; dorsum of paraprocts rugose and with spinous setae; paraprocts curve upwards and medially and recurved over the apex of tergum 10. Anterior sterna pale but gradually darkening to brown towards abdominal apex; sterna 2–7 with faint sublateral brown dots; sternum 7 with posterior margin darker; sternum 8 with a large, evenly rounded, and darkly sclerotized brown vesicle, slightly wider than long and continuous with dark posterior margin of sternum 8 ( Fig. 24 View Figs ); vesicle with stout marginal hairs and overlapping sternum 9 by up to one-fifth its length, the posterolateral corners of the vesicle usually weakly sclerotized. Cerci medium brown to dark brown, as long as abdomen, each segment with one long ventral seta at posterior margin, an occasional middle segment with 2 posteroventral setae.
Aedeagus: with paired glabrous basolateral lobes ( Figs. 25a, 26a View Figs ); a heavily spiculate ventrobasal lobe ( Figs. 25b, 26b View Figs ), stout golden spines on ventrobasal lobe decrease in size distally toward terminal lobe and transition dorsally to a densely covered area of golden scale-like spinules and short, sharp spines, most spinules with a fine apical filament ( Fig. 31 View Figs ); large bulbous terminal lobe ( Figs. 22 View Fig , 25c, 26c View Figs ) with a posterodorsal invagination ( Fig. 27 View Figs ) and a small posteromedial lobe below invagination divided by a shallow medial furrow; terminal lobe with a medial longitudinal furrow dorsad of invagination creating a pair of small tuberculate nipple-like points near apex of terminal lobe; lobe with a dense golden brown arrow-shaped patch of spines ventromedially ( Fig. 25 View Figs ), long bi- and trifurcate spines within patch decrease in size and transition to short, flat palmate and pectinate spines towards the edges of the patch ( Figs. 28, 29 View Figs ); entire anterior half of terminal lobe covered with short spinules with subapical and apical hair-like filaments, some spinules bifurcate or trifurcate ( Fig. 30 View Figs ); sensilla basiconica scattered on the ventral surface of the terminal lobe and surrounding the arrow-like spine patch ( Fig. 30 View Figs - arrows); posterior half of terminal lobe glabrous with some sensilla basiconica near the ventromedial spine patch.
Adult Female — Macropterous. Forewing length 9.5–11.5 mm (n=9), body length 8.5–10.5 (n=9). Head pattern and body color similar to that of the male. Sternum 9 with two posterolateral dark areas, sometimes reduced to dark dots.
Subgenital plate: generally triangular, originating at the distal third of sternum 8 and produced posteriorly approximately half the length of sternum 9; plate basally broad, originating near pleural fold, receding to a somewhat truncate apex, usually with a shallow posterolateral emargination near apex giving the apex large nipple-like appearance ( Fig. 33 View Figs ), median emargination may be larger and deeper in some specimens ( Fig. 34 View Figs ); plate brown medially, apex usually darker brown, weakly sclerotized medially, and sometimes reflected ventrally.
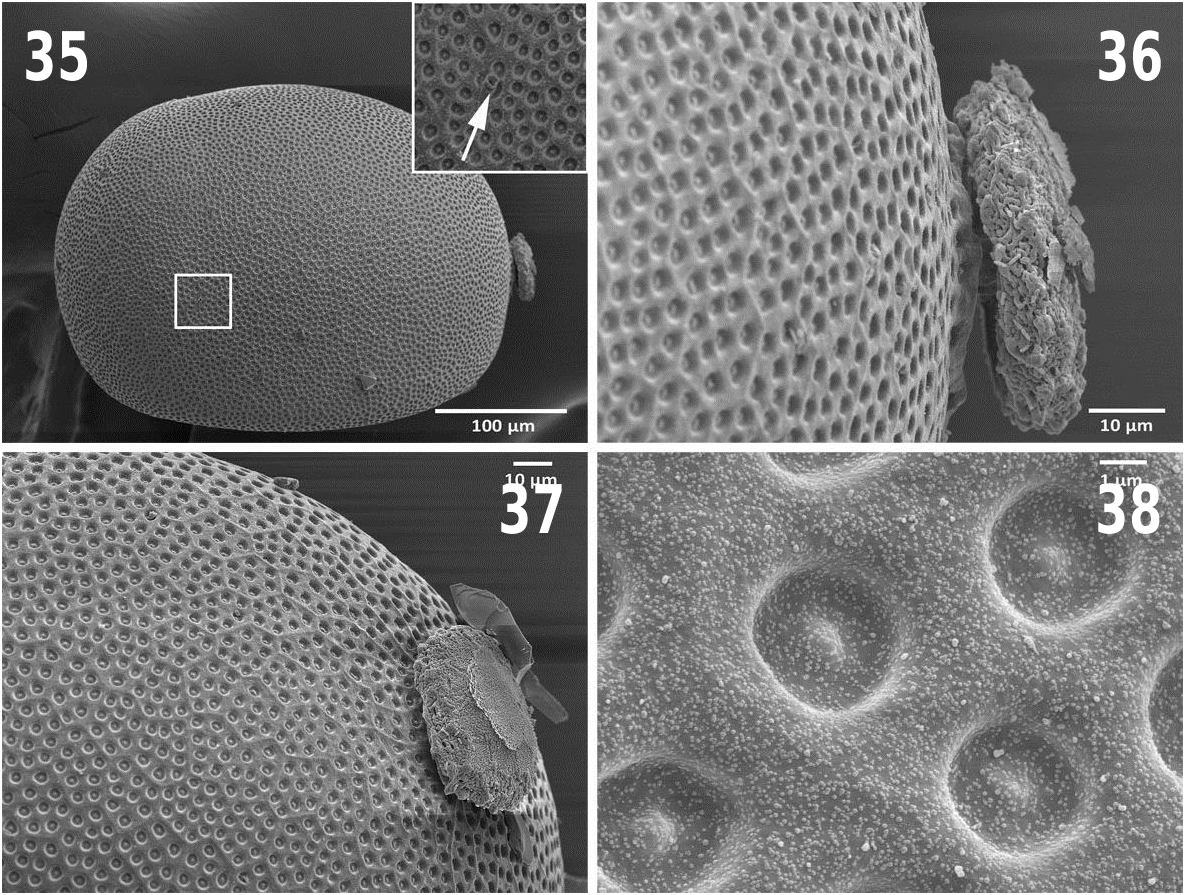
Ovum — General shape oblong, cross section concave. Color brown and opaque. Length 363 µm; width 263 µm ( Fig. 35 View Figs ). Collar low, disc-like, central stalk with ridges, apex of collar unknown ( Fig. 36 View Figs ). Chorionic surface covered with numerous shallow circular punctations, each with a small central spire ( Fig. 38 View Figs ), and approximately 3 µm in width; punctations within faint hexagonal follicle cell impressions most visible near poles ( Fig. 37 View Figs ). Eclosion line absent. Micropyles single, located near anterior third; orifices small and flanged, located on chorionic ridges ( Fig. 35 View Figs inset).
Mature Larva — Pre-emergent larvae 8.2–12.2 mm (n=10). Body slender with contrasting body pattern ( Fig. 39 View Figs ). Clothing hairs present but inconspicuous, clear.
Maxilla: Lacinia bidentate; narrowing evenly from base to subapical tooth ( Fig. 40a View Figs ); a thin, long marginal seta present between apical and subapical teeth; 6–9 evenly-spaced, striated, stout marginal setae set below subapical tooth, distal half of marginal setae progressively smaller toward base; an additional 2–9 thin, unevenly-spaced marginal setae along palm edge; a closely-set submarginal row of 7–9 striated, stout setae below apical tooth, an occasional extra thin submarginal seta interrupting row ( Fig. 40b View Figs ); 6–30 short, thin, sometimes bifid setae on ventral surface near palm edge and approaching base of lacinia; dorsal setae absent. Length of striated apical tooth of lacinia between 0.35–0.42X palm length and slightly shorter than palm width. Subapical tooth 0.50– 0.63X the length of the apical tooth. Galea 0.8–0.95X the lacinial palm length, with a ventral row of 8–34 setae and tipped with 2–4 apical spinous setae. Maxillary palp setose, 1.3–1.6X length of lacinia; segments 1–4 successively longer, segments 3 and 5 subequal; segments 1–3 with transverse row of apical spinous setae ( Fig. 40a View Figs ) segment 5 tipped with 4–5 setae.
Mandibles: Left mandible bicuspid; outer cusp with 3 teeth, ventral tooth largest and serrated basally, dorsal tooth smallest; a band of unorganized, long spinous setae on ventral surface from base of teeth to base of mandible; outer cusp with 2–3 teeth, a small tooth may be present at base of dorsal tooth (frequently worn or broken); a brush of dense setae basal to cusp near molar ridge; a series of hispid spine-like setae along molar ridge, setae longer and thinner towards base of mandible; a band of unorganized serrated setae on dorsal surface from base of inner cusp towards base of mandible, setae shorter and thinner towards base. Right mandible similar to left mandible except dense brush of setae at base of inner cusp replaced by a small patch of denticlelike acanthae.
Dorsum of head: Frons with two enclosed pale spots, an oval to diamond shaped interocellar spot and a large subtriangular median pale area anterior to median ocellus; median pale area almost always trilobed ( Fig. 41a View Figs ), with small dark extensions from the surrounding brown pigment toward but not reaching median ocellus; frons with extensive blackish markings within brown pigmented areas; anterolateral dark spots near anterior frontoclypeal pale area ( Fig. 41 View Figs ); occiput with brown areas along epicranial stem somewhat removed; strong oblique dark stripes originating behind eyes and extending to post-occipital margin. Occiput with a transverse row of closely set spicules, spicule origins darkened. Antennae brown, darker apically; scape slightly darker than anterior flagellar segments.
Thorax: Pronotum with pale median stripe; lateral edges pale; pronotal disk with wide, dark brown curvilinear stripes from anterior to posterior margin; irregular brown rugosities on disk, median suture thinly brown; anterior and posterior pronotal flanges brown, interrupted mesally. Meso- and metanota with extensive brown markings, wing pads with longitudinal submedial brown stripes ( Fig. 42 View Figs ). Thoracic sterna pale, without distinctive markings.
Legs: Pale brown overall; ventral face with a brown medial obfuscation on distal half; anterior face with a thin brown, longitudinal subdorsal band; femora and tibiae with a dorsal fringe of long silky setae; spinous setae on femora with dark origins giving a speckled appearance; femora with distal fifth pale, lacking dark setal origins and contrasting with a slightly darker subapical area; tibiae with proximal fourth slightly darker; with two longitudinal dorsal rows of short spines; tibiae with a ventral longitudinal row of spines. Tarsi pale brown with ventral row of stiff setae and sparse dorsal row of silky setae.
Abdomen: Three longitudinal stripes, two lateral and one median; median stripe weak, often interrupted; each segment with an anterior transverse row of 8 small dark dots ( Fig. 43 View Figs ); terga with stout, socketed spines with dark origins giving abdomen an overall speckled appearance; posterior edges of terga bearing a closely transverse row of short, clear spines. Abdominal sterna pale, freckled with dark spine origins; small, dark spots visible on sterna 9 and 10; posterior edges of sterna bearing a transverse row of short, socketed spines, row incomplete medially on anterior segments. Cerci brown with sparse dorsal fringe of silky setae on distal half. Body with clear clothing hairs.
Etymology. This species is named in honor of Dr. Boris Kondratieff, of Colorado State University, for his enthusiastic study of aquatic insect systematics, particularly that of Plecoptera and Ephemeroptera . His encouragement of the authors' larval-adult association studies resulted in the discovery of this species of Isoperla .
Diagnosis. The adult male I. borisi can be separated from most other eastern species by the highly sclerotized and uniquely toothed paraprocts ( Figs. 23 View Figs , 27 View Figs ). It can be further separated from the males of I. namata Frison, 1942 I. signata (Banks, 1902) , I. slossonae (Banks, 1911) ; and other species with toothed paraprocts, by the unique shape and armature of the male aedeagus, particularly the presence of paired basolateral lobes and a central posterior arrow-shaped spine plate, and by the shape of the vesicle. One reared I. borisi male had a second, smaller but similarly shaped vesicle-like lobe on the posterior margin of segment 7 ( Fig. 24 View Figs ). A similar lobe has been described as unique for I. maxana Harden & Mickel, 1952 known only from the holotype male from Minnesota ( Szczytko and Kondratieff, 2015a). However, the male aedeagus of I. maxana has not been described and additional material of this species has not been collected from the type locality since the original description leading to the supposition that the species may have been extirpated ( Szczytko and Kondratieff 2015a). Based on the occurrence of a second lobe on I. borisi it is possible that the original description of I. maxana ( Harden and Mickel 1952) may have been based on an aberrant specimen having an additional sternal lobe.
Female adults of I. borisi can be separated from most other eastern species based on the shape of the subgenital plate. Also, both male and females have dark vertical bands at the apices of the femora, a trait shared by I. slossonae and I. jamesae Grubbs and Szczytko, 2010 . Eggs of I. borisi have a unique chorionic sculpture of small circular depressions with a median raised bump.
Larvae can be separated from all other known eastern larvae by the unique head and abdominal pattern as well as the setation of the lacinia. The overall habitus of this species is similar to what was described as I. bilineata View in CoL by Frison (1935).
Type Locality. Isoperla borisi larvae have been extensively reared from Collins Creek, a small tributary of the Haw River, near Carrboro in Orange County, North Carolina. Initial collections of mature larvae were made from the headwaters of Collins Creek. At this location, Collins Creek is a second order stream approximately 2.0– 2.5 m wide with a drainage area of approximately 8.5 km 2 ( Fig. 44 View Fig ). Land use in the upper watershed of Collins Creek is primarily forest and agriculture, with few private residences.
As typical of small, permanent Carolina Slate Belt streams, Collins Creek has reduced flows during the summer months and may become a series of disconnected pools during dry years. However, water levels during the winter and spring are typically high and persist through the end of spring to early summer. In-stream habitat consists of riffle-pool sequences of bedrock and cobble with the pools typically silt-bottomed. The substantial amount of in-stream silt appears to originate from adjacent agricultural fields as there is little urban development in the watershed.
Additional Remarks. The larval habitus of I. borisi has historically been identified as an I. bilineata by taxonomists using Hitchcock (1974) and Unzicker & McCaskill (1982). Re-examination of archived NCDWR benthic macroinvertebrate samples has revealed this species to be restricted to the Carolina Slate Belt Level IV ecoregion where it is relatively common ( Fig. 45 View Fig ).
Mature Isoperla borisi nymphs were particularly abundant in riffles containing the moss Fontinalis sphagnifolia (Müller Hal.) Wijk & Margadant but were relatively scarce in other habitats (e.g. leaf packs). The associated larval EPT macroinvertebrate community collected concurrently with I. borisi was largely composed of facultatively intolerant taxa that included the mayflies Stenacron interpunctatum (Say, 1839) , and Baetis flavistriga McDunnough, 1921 , the stoneflies Amphinemura cf. nigritta (Provancher, 1876) and Perlesta spp. , and the caddisflies Ironoquia punctatissima (Walker, 1852) and Rhyacophila fenestra /ledra. An additional slate belt indicator species, the heptageniid mayfly Stenonema femoratum (Say, 1823) was also present along with Neophylax atlanta Ross, 1947 , a thremmatid caddisfly restricted to headwater piedmont streams.
Little is known about the ecology of the larvae or adults. Larvae were collected in abundance during late winter to early spring. A short adult emergence period occurred mid-April to mid-May with peak emergence in late April. Few adults were collected using traditional methods (beating sheet) and appeared to move immediately into the higher adjacent riparian vegetation after emergence. While I. borisi larvae appear to be tolerant of the high silt loads frequently present in Slate Belt streams, their response to other forms of disturbance is unknown as adequate verified records are not available to generate a rigorous tolerance value for use in the NCBI. However, this species does appear to be the only facultatively tolerant Isoperla in North Carolina based on the authors’ field observations.
Note on Phylogenetic placements. Considering the uniqueness of each life stage, both I. arcana and I. borisi are not presently assigned to any species group. Preliminary phylogenic analysis based on mitochondrial COI DNA indicates that each are distinct species. However, we use caution interpreting these barcoding results since both the number Isoperla species and the number of individuals assessed was small. Additional and expanded genetic analysis is required to firmly establish the relationships of I. arcana and I. borisi with their eastern congeners. Preliminary phylogenetic data is available for viewing at BOLD Systems (www.boldsystems.org) under the project name Nearctic Ephemeroptera and Plecoptera (NEAP) .
| NMNH |
Smithsonian Institution, National Museum of Natural History |
| R |
Departamento de Geologia, Universidad de Chile |
| V |
Royal British Columbia Museum - Herbarium |
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |