Centroptilum volodymyri Martynov, Godunko and Palatov, 2022
|
publication ID |
https://doi.org/10.1080/24750263.2022.2090625 |
|
publication LSID |
lsid:zoobank.org:pub:1D2B7557-0DEC-4ACD-8CA3-A6B50FCC4A51 |
|
DOI |
https://doi.org/10.5281/zenodo.7741544 |
|
persistent identifier |
https://treatment.plazi.org/id/1D2B7557-0DEC-4ACD-8CA3-A6B50FCC4A51 |
|
taxon LSID |
lsid:zoobank.org:act:1D2B7557-0DEC-4ACD-8CA3-A6B50FCC4A51 |
|
treatment provided by |
Juliana |
|
scientific name |
Centroptilum volodymyri Martynov, Godunko and Palatov |
| status |
sp. nov. |
Centroptilum volodymyri Martynov, Godunko and Palatov , sp. nov.
( Tables I View Table I –III; Figures 1 View Figure 1 , 2 View Figure 2 , 3 View Figure 3 (a,c,e), 4 (a–e), 5, 6 (a,c,e), 7 (a,c,e,g), 8, 9 (a,c,e,g), 10, 11 (a,c,e), 12(a,c,e,g))
http://www.zoobank.org/urn: lsid:zoobank.org: a c t: 1 D 2 B 7 5 5 7 - 0 D E C - 4 A C D - 8 C A 3 - A6B50FCC4A51 Centroptilum sp. : Martynov et al. 2016: 170 [distribution in Georgia (Kintrishi River valley), with remark on belonging to undescribed new species] Centroptilum sp. : Bojková et al. 2018: 91, 96, tables 1, 2 [distribution in Iran within Zayanderud (based on Mahboobi Soofiani et al. 2012) and Gilan (two localities listed also in present contribution) provinces]
? Centroptilum sp. : Mahboobi Soofiani et al. 2012: 137 [Zayandeh-Roud River in the Zayanderud Province; this record should be verified]
Diagnosis [ based on larvae]. Larvae of C. volodymyri sp. nov. can be distinguished from the two other Western Palearctic representatives of the genus Centroptilum , namely C. luteolum and C. pirinense , by a combination of the following diagnostic characters: (1) the cuticle with flattened, stretched shallow triangular and semilunar-shaped corrugations, and relatively numerous scales and their bases covering the body surface; (2) labrum expanded laterally, with the U-shaped incurvation on the anterior margin; (3) apically rounded shape of the segment III of maxillary palps; (4) apically rounded shape of superlinguae of hypopharynx without a projection at the tip; (5) pretarsal claw with more than 60 small teeth in basal half of the claw, these teeth arranged into two rows; (6) the posterior margin of terga I– IV(V) with robust equilateral triangle-shaped spines scattered along the segment (equilateral spines sparse on terga (V)VI–X); (7) tergum VII near gill base with 3–4 relatively prominent posterolateral spines and sometimes several small spines; (8) gills II–VII with distinctly concave posterior margin.
Description. Based on specimens from the type locality only.
Mature larva. Body length 9.5–10.2 mm (male larvae slightly smaller than female larvae), length of cerci 5.5–5.8 mm (approx. 0.5× body length); length of paracercus 3.0– 3.3 mm.
General body colouration intensively brown/dark brown and blackish; base of fore wing pads, lateral and ventral side of meso- and metathorax darker, yellow to brownish black and black. Abdominal segments paler than thorax; legs and cerci lightest ( Figure 1 View Figure 1 ).
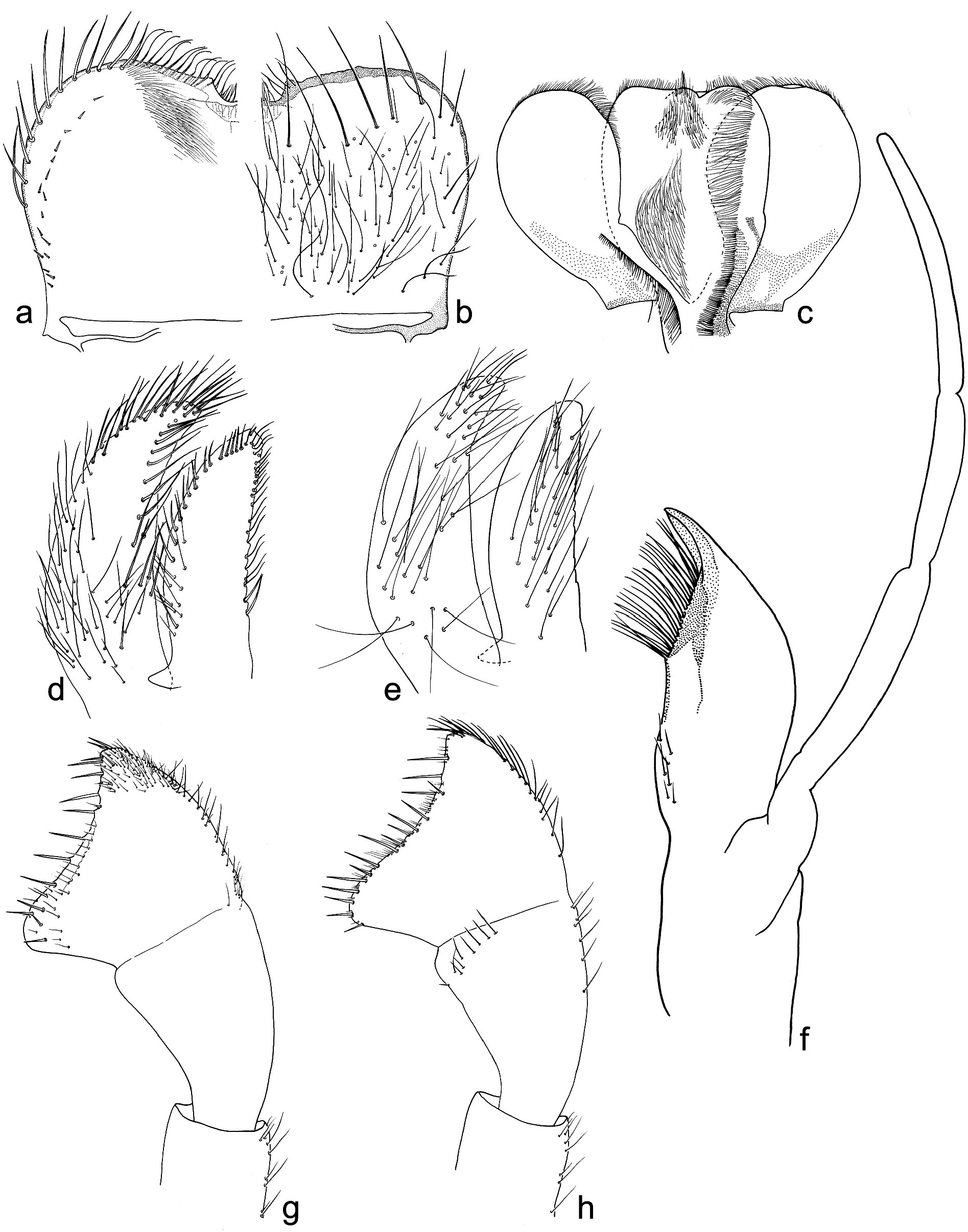
Head. Colour yellowish brown to light brown, with markedly darker area between ocelli, diffused light brown maculation on frons and vertex; clypeus and genae slightly darker, brown. Larval compound eyes brown. Antennae unicolor, light brown, distinctly longer than head and thorax. Frontal suture V-shaped. Head cuticle flattened, without pronounced corrugation. Surface of two first antennal segments and frons covered with sparse B and Hr setae ( Figure 3 View Figure 3 (a,c,e)); clypeus additionally with two groups of long setae grouped on both sides of body axis.
Labrum expanded laterally, approximately 1.8– 2.0× broader than long; median notch flattened; anterior margin concave, with U-shaped median incurvation relatively deep; anterior margin laterally from medial notch relatively symmetrically rounded. Dorsal surface of labrum covered with dense long and short Hr not grouped in rows and sparse B ( Figures 4 View Figure 4 (a) and 5(b)); ventral surface with row of submarginal short setae ( Figure 5 View Figure 5 (a)).
Superlinguae of hypopharynx rounded apically, without any projections ( Figure 5 View Figure 5 (c)).
Mandible incisors clearly divided into two groups, deeply separated throughout their length. Left mandibular incisor groups terminated by 4 + 2 stout denticles; left prostheca terminated by a group of slender setae (2–3 setae larger than other ones). Right mandibular incisor groups terminated by 3 + 2 stout denticles; right prostheca is stick-like ( Figure 4 View Figure 4 (c,d)).
Maxillae with long slender canines and dentisetae; 1st dentiseta is simple, 2nd and 3rd dentisetae are bifid; dentisetae not pressed, and well separated from canines. Maxillary palps 3-segmented: segment I distinctly wider than segment II; segment III distinctly longer than segment II, rounded apically, without stout setae apically of surface; surface of maxillary palps covered with sparse Hr and FT ( Figures 4 View Figure 4 (b,e,f) and 5(f)).
Labial palps 3-segmented. Segment III distinctly expanding apically, nearly trapezoidal with rounded outer angles; inner margin distinctly concave apically; posterolateral and posteromedian corners of different shape; stout setae along of apical margin and numerous Hr scattered on surface. Segment II distinctly wider than base of segment III; ventral side of segments I and II with scattered Hr; dorsal side of segment II with 6–10 elongated setae near to apicointernal margin ( Figure 5 View Figure 5 (g,f)).
Glossae nearly as broad as paraglossae ( Figure 5 View Figure 5 (d,e)). Paraglossae slightly longer; dorsal surface with single row of elongated stout setae along inner margin; ventral surface with fine long setae scattered over entire surface (apical setae shorter). Glossae with irregular row of submarginal short stout setae along outer and inner margins; denser setation shaped by fine long setae on dorsal side. Basal part of glossae and paraglossae with numerous fine long setae.
Thorax. Pronotum narrow, approximately 3.0– 3.5× longer than broad. Pronotum with two yellowish to light brown maculae centrally; two spreading maculae of same colour on darker background laterally [yellow to yellowish brown, with occasional dirty yellow diffuse spots laterally in specimens from Iran]. Mesonotum with elongated brown strips on light brown background; coxae intensively brown to black brown [slightly paler in specimens from Iran]. Metanotum with spreading yellowish spot centrally, yellowish brown to dark brown laterally. Thoracic pleura slightly darker than terga ( Figure 1 View Figure 1 (a,b)).
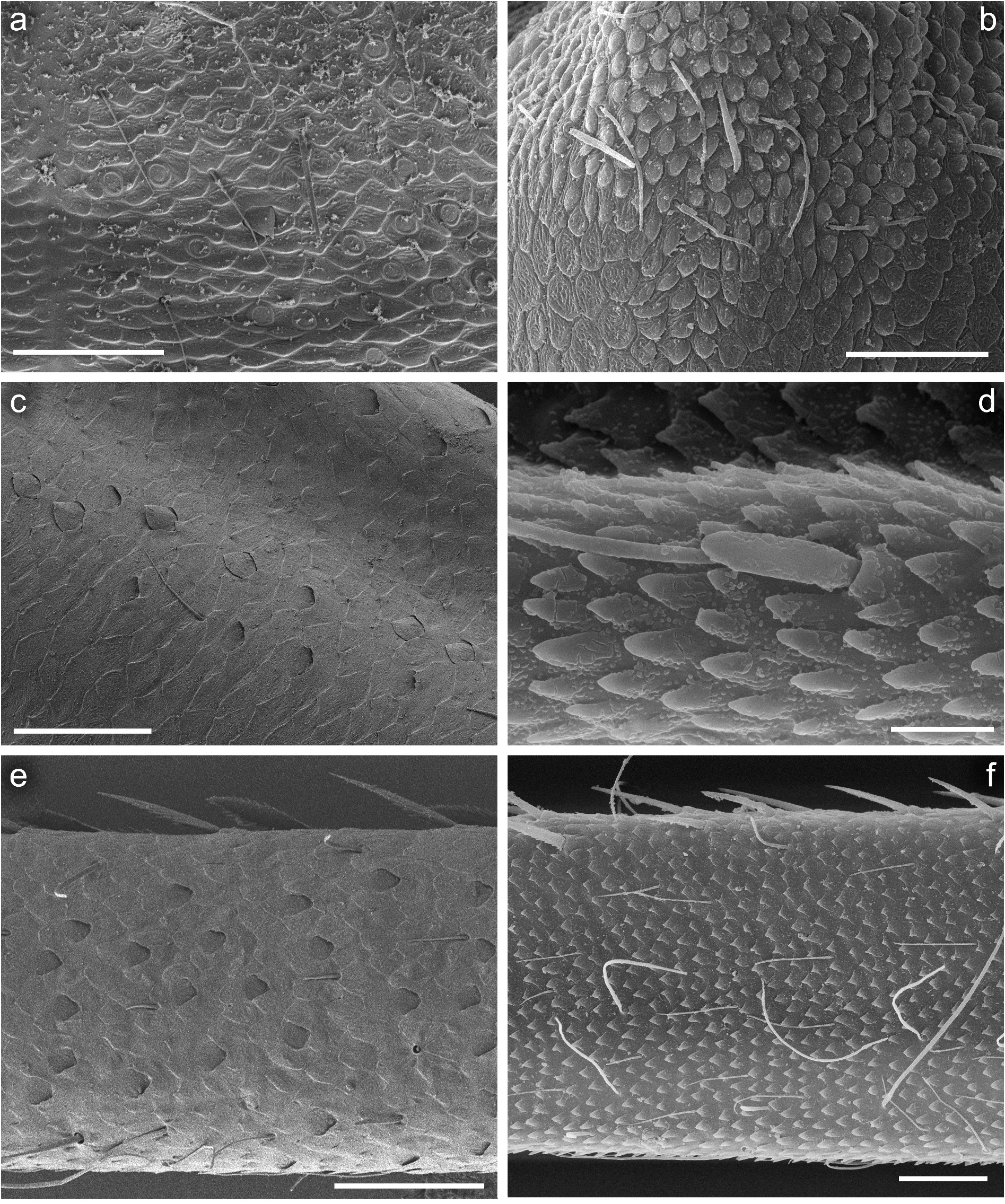
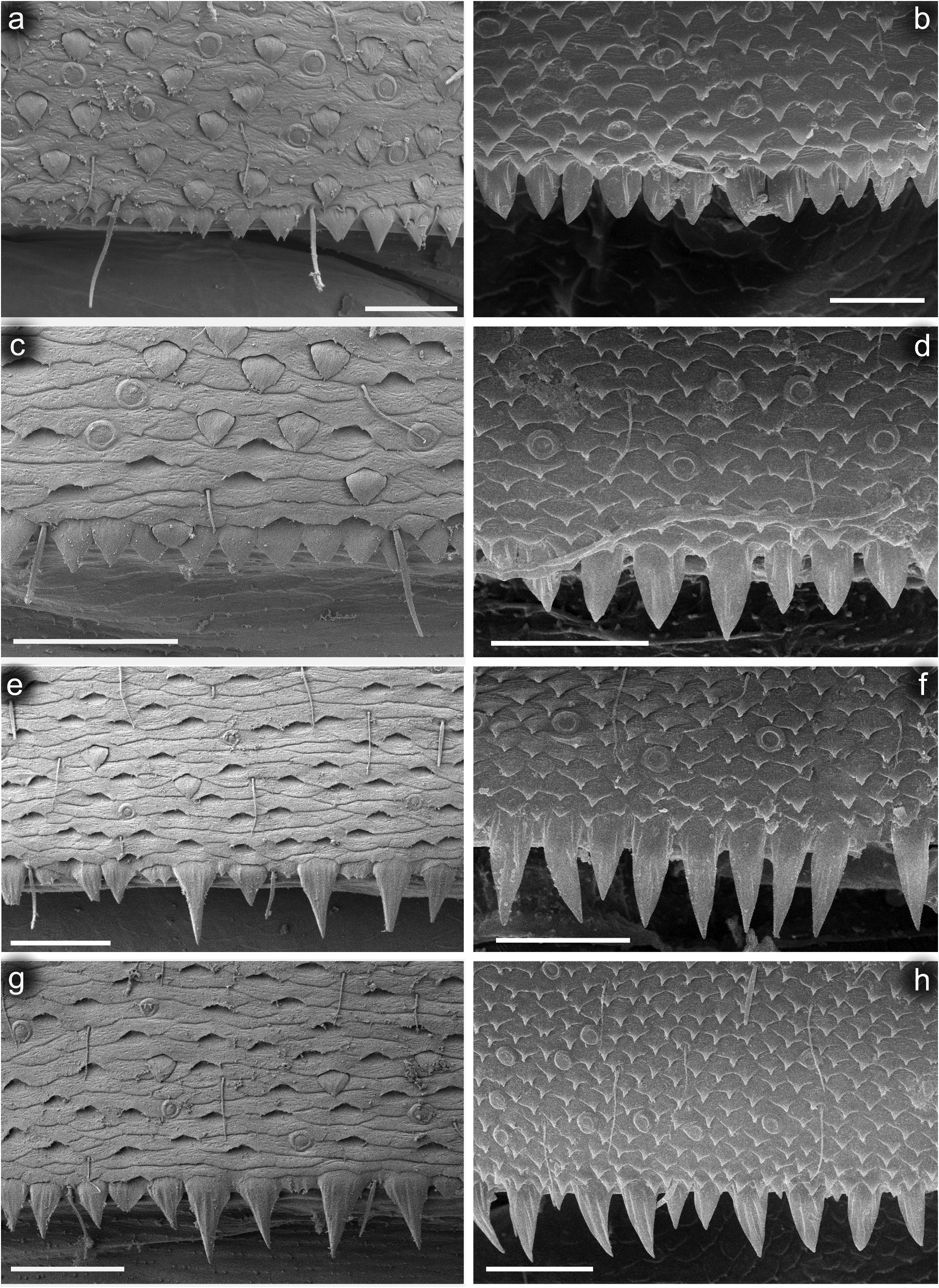
Hind wing pads well developed. Thoraxal cuticle formed by flattened, stretched shallow triangular and semilunar-shaped corrugations. Surface of pronotum covered with Hr, B, micropores [ chloride cells], sparse SC-et [6–8 μm in length] and SCS-et ( Figure 6 View Figure 6 (a)).
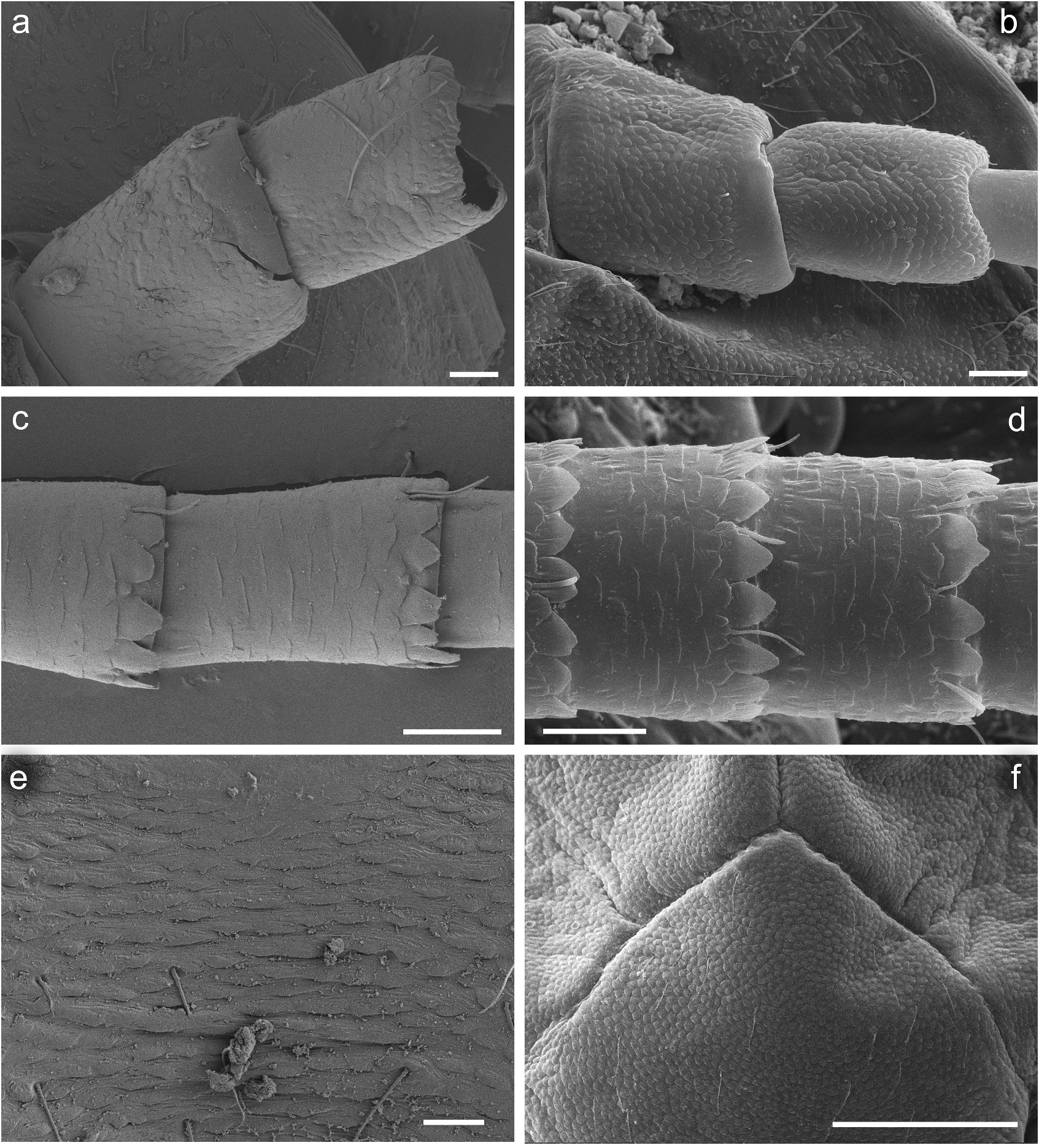
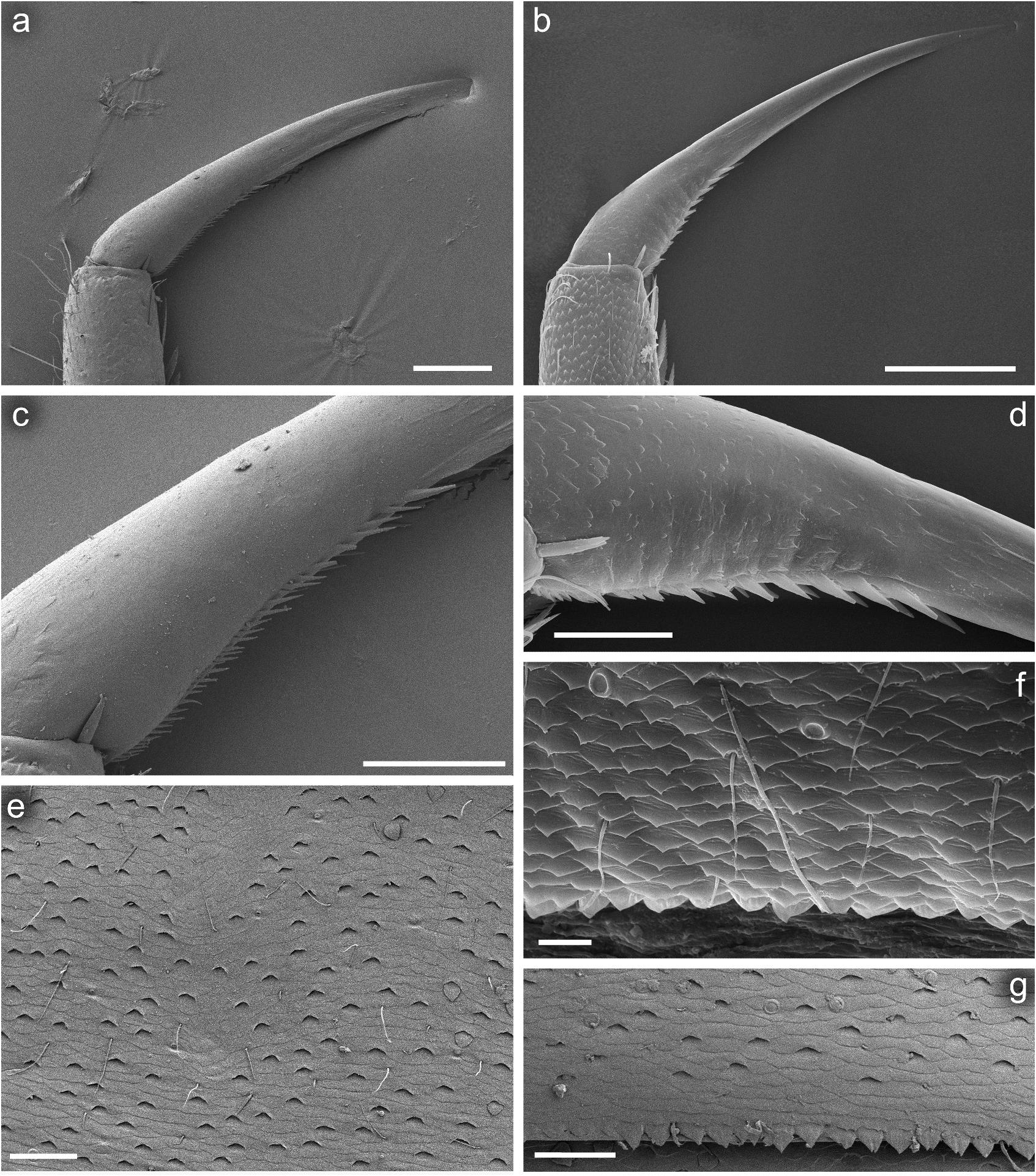
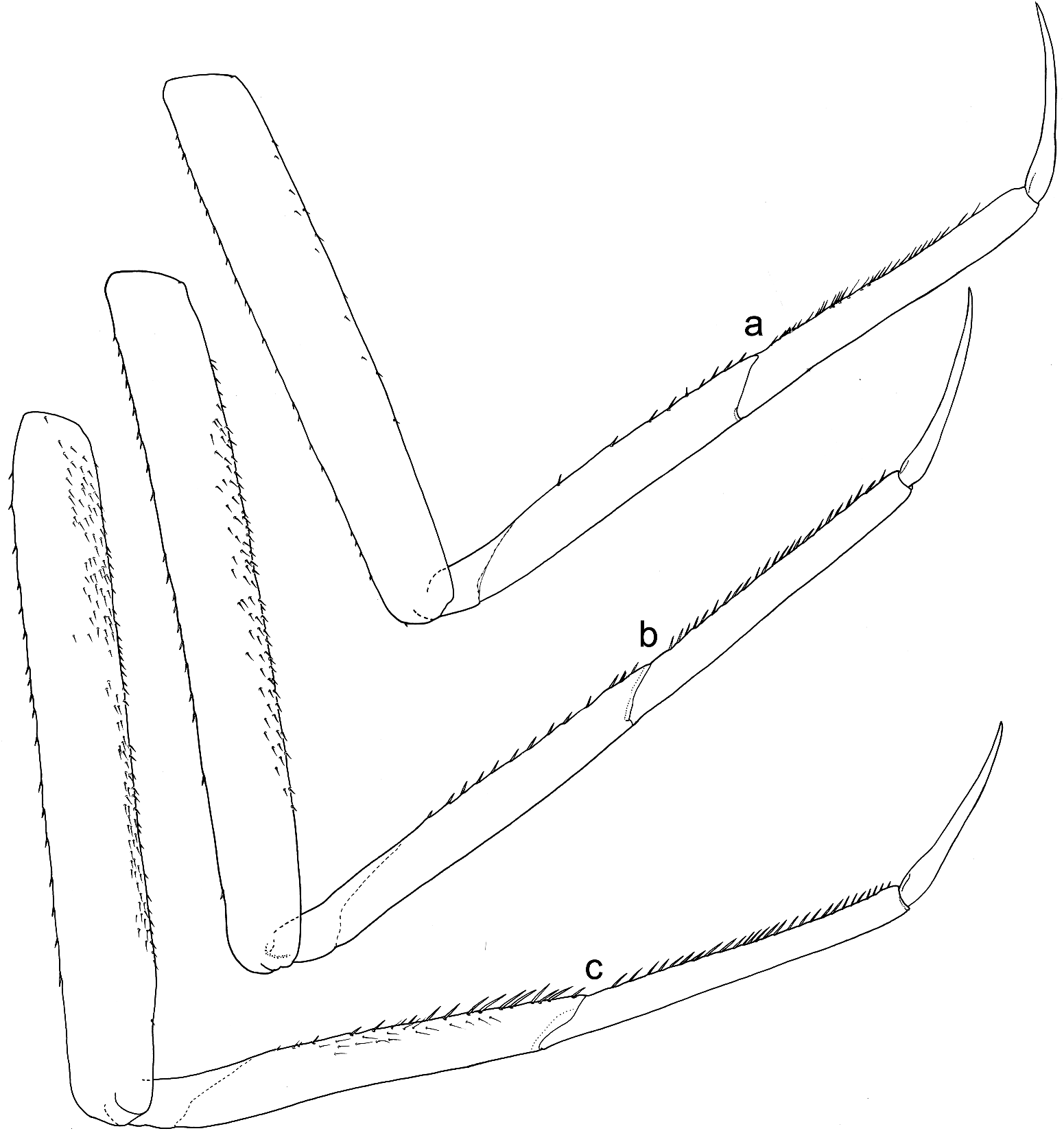
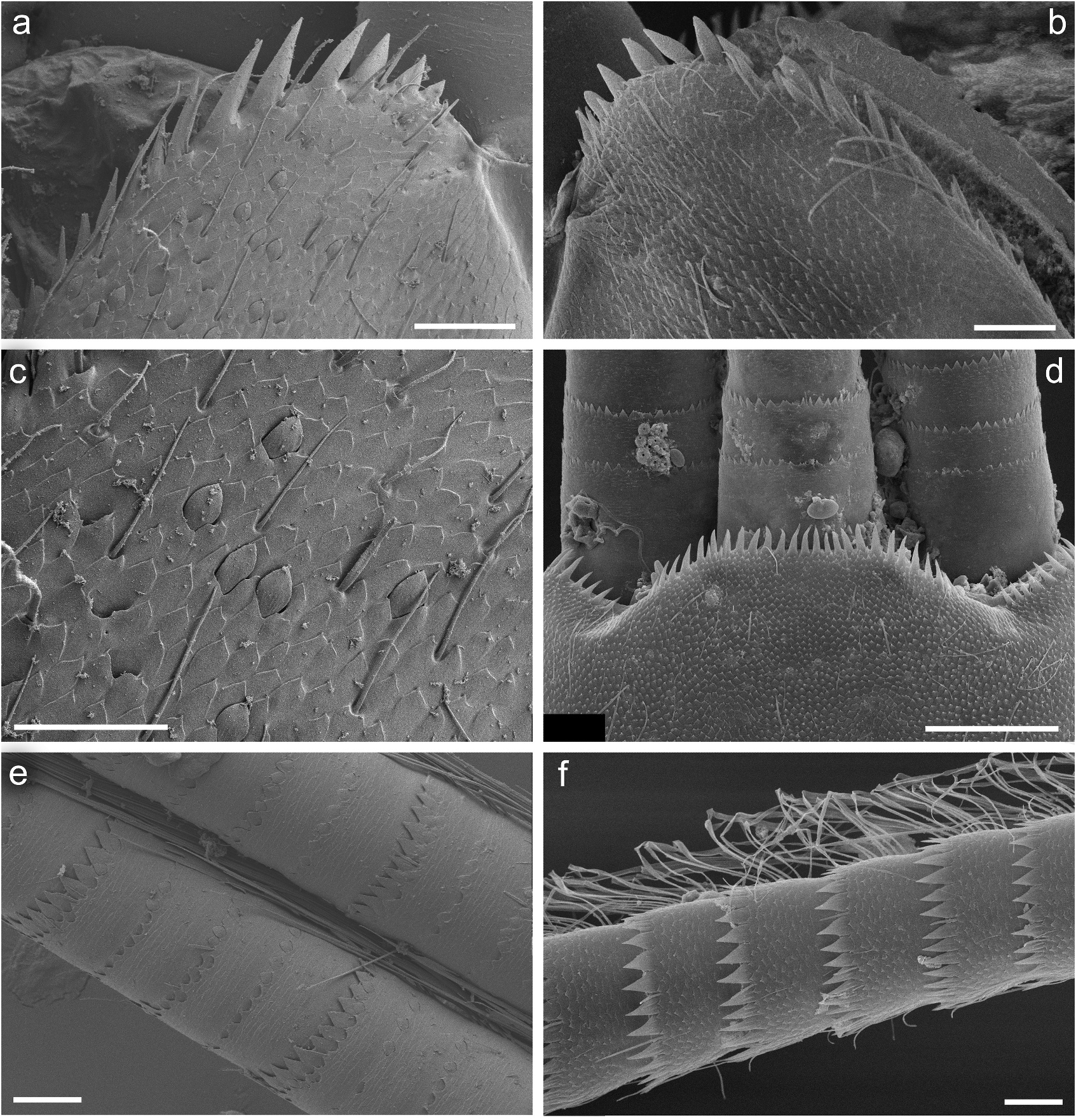
Legs relatively pale, whitish yellow to dirty brown [paler in specimens from Iran]; all leg segments slightly darker distally ( Figure 1 View Figure 1 (a,b)). Legs slender; femora parallel-sided; patella-tibial suture developed on all legs ( Figure 8 View Figure 8 (a–c)). Fore leg coxae pale, whitish yellow to intensively brown; coxae of middle and hind legs darker, intensively brown to dark brown. Trochanters with diffused brownish spot. Brownish elongated diffused macula along outer margin of all femora; tibiae yellowish to light brown; tarsi of the same colour as tibiae, occasionally slightly darker distally; claws yellowish brown. Cuticle of the legs formed by flattened equilateral triangle- and semilunar-shaped corrugations. Surface of femora with SC-et, SCS-et, Hr, B setae; the same types of scales and setae, with dominance of SCS-et distributed of surface of tibiae and tarsi ( Figure 6 View Figure 6 (c,e)). Numerous short, stout, pointed setae along outer margin of femora; numerous longer pointed setae along inner margin of tibiae and tarsi; outer margin of tibiae and tarsi covered with Hr and occasional B setae. Pretarsal claw length less than or equal to 1/2 of tarsus length ( Figure 8 View Figure 8 (a–c)); more than 60 small teeth arranged into two rows distributed in basal half to 2/3 of claw length ( Figure 7 View Figure 7 (a,c)).
Abdomen. Terga dark, yellowish brown to intensively brown ( Figure 1 View Figure 1 (a,b)); terga I with two broad spots centrally, and two smaller spots anterolaterally. Terga II–V with uniform colour pattern, viz. a small pale spot centrally near anterior margin of segment; large brownish diffused spot centrally, with paler maculae near posterior margin of segment; additionally, yellowish-brown to light brown elongated strips surrounding pale maculae. Tergum VI darkest, with colour pattern similar to those on previous segments, except of more diffused light spot anteriorly, and presence of two pale dots and two oblique strokes centrally. Terga VII–VIII with colour pattern similar to those on terga II–V (occasionally without small pale spot anteriorly), or uniformly light brown. Tergum IX uniformly light brown to brown without conspicuous pattern. Tergum X pale, yellow to light brown, with light transversal macula anteriorly, two unclear maculae centrally near anterior margin of segment and two brown small spots laterally. Anterolateral area of terga I–IX pale, yellowish brown.
Abdominal sterna predominantly uniformly coloured, whitish yellow to light brown, without distinct pattern. Occasionally sterna with contrasting pattern: sternum I whitish yellow, with dark brown spots anterolaterally; sterna II–V (VI) with diffused triangular brownish spot on yellowish background; sterna VII–VIII darker than previous segments, light brown to brown, with longitudinal pale band and two yellowish spots centrally; sterna VIII– IX darkest, intensively brown, with diffused paler maculation centrally and laterally; sternum X slightly paler than two previous segments, light brown.
Surface of terga with cuticle formed by flattened equilateral triangle- and semilunar-shaped corrugations; terga I–IV covered with micropores, Hr and B, dominant SC-et and more sparse SCS-et; terga V–X with the same type of micropores, Hr and B, sparse SC-et and dominant SCS-et ( Figure 9 View Figure 9 (a,c,e,g)). Posterior margin of terga I– IV (V) with robust equilateral triangle-shaped spines, alternating with sparse shorter ones and B setae; posterior margin of terga VI–X (occasionally tergum V) with thin and robust isosceles and equilateral triangle-shaped spines, alternating with shorter ones and sparse B setae. Tergum VII near gill bases with 3–4 relatively prominent posterolateral spines, and sometimes with several additional small spines; terga VIII–IX with 2–4 relatively prominent posterolateral spines, and sometimes with several additional small spines ( Figure 10 View Figure 10 (h)).
Cuticle of sterna shaped by flattened undulating corrugations covered with micropores, elongated Hr and B setae, sparse SC-et and dominant SCS-et ( Figure 7 View Figure 7 (e,g)). Posterior margin of sterna with robust equilateral triangle-shaped spines alternating with occasional short B setae.
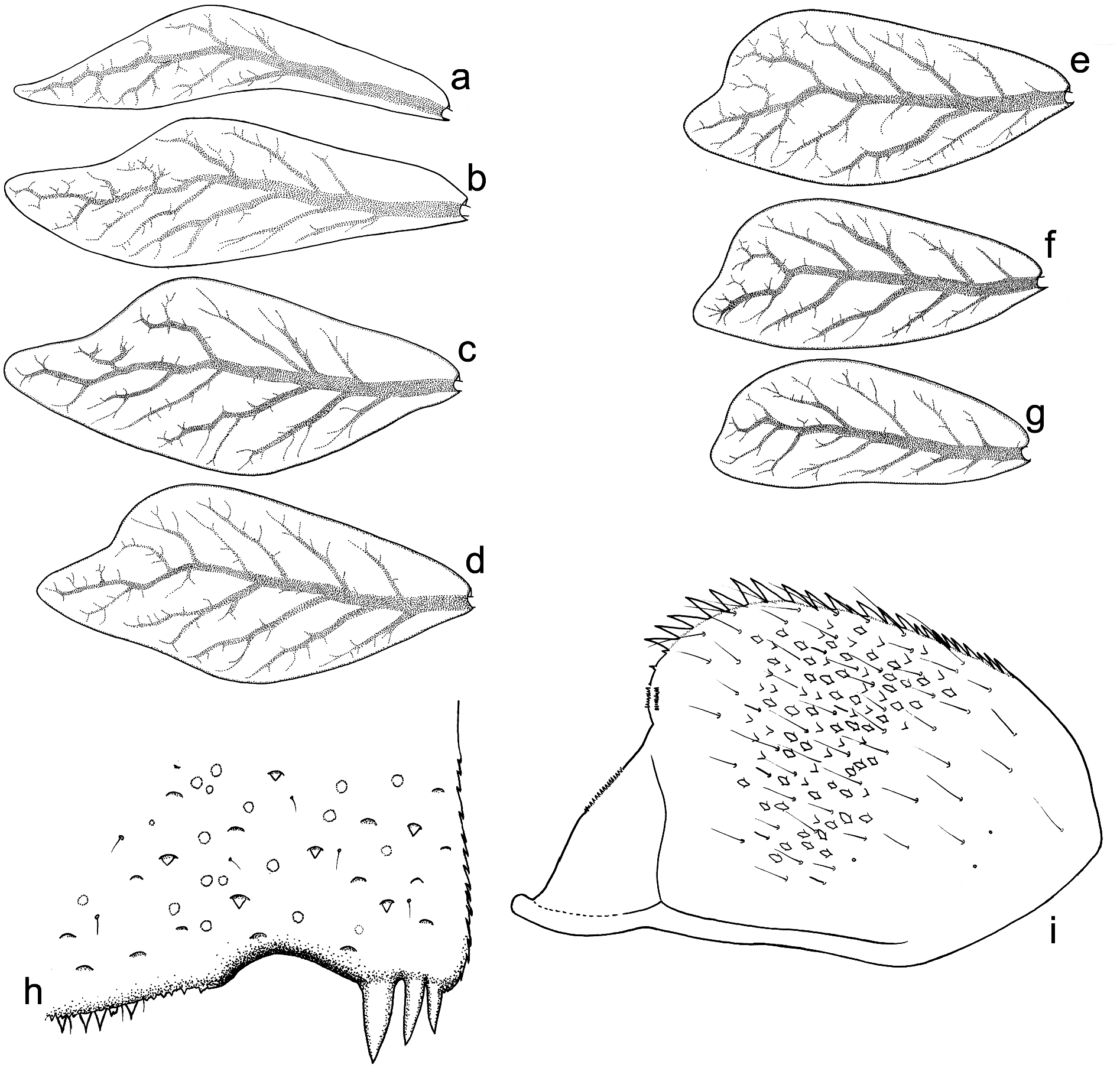
Gills whitish yellow, with well visible tracheisation, light brown to intensively brown coloured. All gills asymmetrical, rounded apically; gill I distinctly sinuous, narrow, lanceolate; gills II–VII with posterior margin distinctly concave ( Figure 10 View Figure 10 (a–g)).
Paraproct plate with 13–15 pointed strong teeth along inner margin; ventral surface of paraproct plate covered with leaf-shaped SC and their SCS grouped mainly centrally, elongated Hr and B setae ( Figures 10 View Figure 10 (i) and 11(a,c)).
Cerci and paracercus slightly paler than body, light brown, without coloured rings. Posterior margin of caudal filament segments with combination of robust marginal spines, occasional Hr and B setae; submarginal area with solitary SC-et ( Figure 11 View Figure 11 (e)). Cerci (inner margin) and paracercus (both margins) up to the apex bear well-developed primary swimming setae, except last one or two segments. Outer margin of cerci with relatively short Hr and B setae.
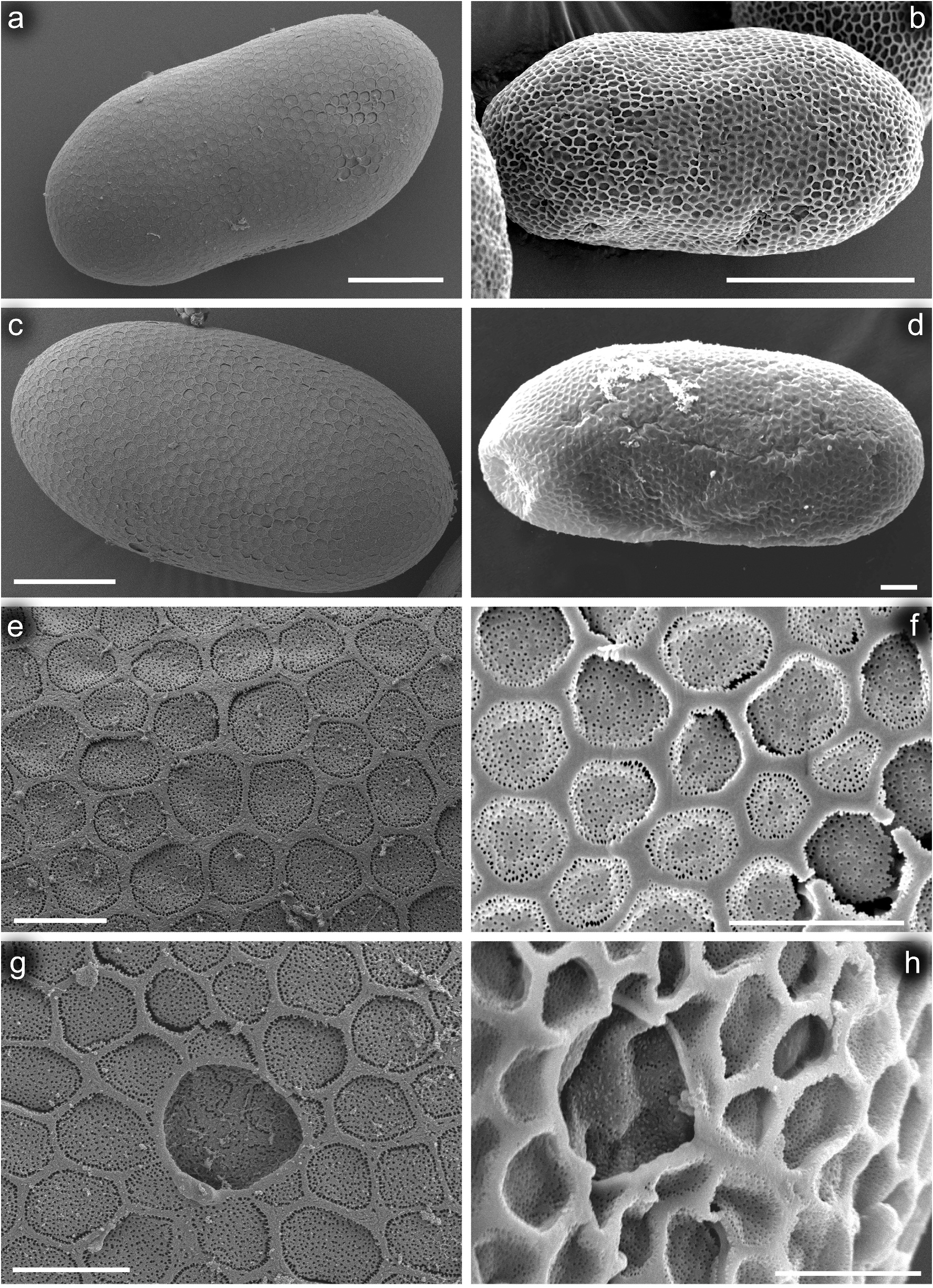
Egg (taken from larva). Oval; 130–140 µm long, 65–70 µm wide. Chorionic surface shaped by thin, flat reticulated ridges forming irregular polygonal mesh; cells (2.5–4.2 µm in diameter) of this mesh mostly with rounded margins; no knob-terminated coiled thread inside of chorionic cells ( Figure 12 View Figure 12 (a,c,e,g)). One, occasionally two oval micropyles in equatorial area, 5.5–5.8 µm long and 4.8–5.0 µm wide; no micropylar rim surrounding micropyle.
Imago and subimago. Unknown.
Type material
Holotype. Female mature larva, GEORGIA, Autonomous Republic of Adjara, Kobuleti District, vicinity of Chakhati village, valley of the Kintrishi River , small lentic water bodies, 41.762°N, 41.978° E, ′ 325 m a.s.l., 18 April 2016, Martynov A. V. leg. (inventory number in GoogleMaps NMNH NASU collection: Grg24Censp/1 ) .
Paratypes. Six larvae, GEORGIA, same locality and date as holotype (three larvae in slides 648 , 661 , 662 ; one larva in ethanol deposited in NMNH NASU with inventory number Grg24Censp/2 ) ;
49 larvae, TURKEY, Rize il [Province], Fındıklı Region , KaÇkar Mountains , Büyük [ Çağlayan ] stream, 41.236°N, 41.270°E, 337 m a.s.l., 18 August 2012, Palatov D. M. leg GoogleMaps .;
Non-type material. 10 larvae, IRAN Guilan Province, unnamed small brook (left tributary of Shalmanrud River ), SW of Amlash town, 37.046°N, 50.095°E, 185 m a.s.l., 21 May 2016, Bojková J., Soldán T., Imanpour Namin J. leg GoogleMaps .;
One larva, IRAN, ibid., SW of Amlash town, 37.037°N, 50.083°E, 287 m a.s.l., 21 May 2016, Bojková J., Soldán T., Imanpour Namin J., leg GoogleMaps .;
22 larvae, IRAN, Golestan Province, unnamed small brook (right tributary of Rude-Tengen River), S of Qarangi-ye Jangal town, 37.529°N, 55.792°E, 604 m a.s.l., 23 June 2019, Palatov D. M. leg GoogleMaps .
Etymology
The species is named in honour of Volodymyr Martynov, senior son of the first author, for his field assistance in mayfly sampling during a series of field trips.
Distribution and habitat
In Georgia, the larvae of C. volodymyri sp. nov. were collected in small pools, where small brooks flowed in, in the valley of the Kintrishi River at 325 m a.s.l. ( Figure 13 View Figure 13 (a,b)). They were shaded by the ancient colchic forests (chestnut, hornbeam and box trees). Pools were about 0.3 m deep, with bed substrate composed of silt and detritus, without macrophytes. No other mayflies were found there.
In Iran, the larvae were collected in two small brooks ( 1–3 m wide) flowing in steep terrain below 300 m a.s.l. on the northern slopes of the Alborz Mts., close to the Caspian Sea coastal area in Guilan Province ( Figures 13 View Figure 13 (c,d) and 14). They were shaded by humid deciduous forest and shallow, with an average depth of 0.05–0.15 m (max. 0.3 m in pools). They were characterised by alternating riffles and pools, where Centroptilum larvae were collected. Coarse bed substratum in riffles alternated with finer substrates formed by coarse and fine gravel in pools. Bedrock and boulders were sparsely covered with mosses. Water was relatively cold (16°C) and clear, although one brook was slightly turbid. The mayfly taxocene consisted of the following species: C. luteolum (dominant), Baetis ( Rhodobaetis) cf. gadeai Thomas, 1999 , Nigrobaetis ( Alainites) muticus (Linnaeus, 1758) (all Baetidae ), Electrogena pseudaffinis (Braasch, 1980) (Heptageniidae) , and Caenis macrura Stephens, 1835 (Caenidae) .
In Turkey, larvae were collected in a small stream ( 1.5–2.5 m width; 0.1–0.2 m depth) flowing in the forest at 337 m a.s.l. ( Figure 13 View Figure 13 (e,f)). Its bed substrate was composed of pebbles, detritus and silted stones and the flow velocity was 0.3–0.6 m.s −1. The mayfly taxocene consisted of the following taxa: Electrogena sp. (Heptageniidae) (dominant), Habroleptoides cf. confusa Sartori and Jacob, 1986 (Leptophlebiidae) , B. ( R.) cf. gadeai , and N. ( A.) muticus (Baetidae) .
Affinities
We attribute C. volodymyri sp. nov. to the genus Centroptilum based on larval and egg characters depicted by Jacob (1991), Kluge and Novikova (1992a, 1992b), and Bauernfeind and Soldán (2012). The attribution is confirmed by the presence of (1) epicranial suture met at an obtuse angle; (2) mandibular incisors deeply separated into two groups from their base; (3) segment III of maxillary palp not shortened, longer than segment II; (4) narrow pronotum, approximately 3.0–3.5 × longer than its width [4.0 times according to Kluge and Novikova (1992a, 1992b)]; (5) pretarsal claws with two rows of small teeth; (6) simple gills with pinnate tracheisation [according to Bauernfeind and Soldán (2012), in Centroptilum simple gills acutely pointed apically and almost symmetric, except for the very narrow gill I]; (7) abdominal segments almost without strong posterolateral setation, except for tergum VII covered by 1–3 relatively prominent spines [no strong posterolateral setation in Centroptilum according to Bauernfeind and Soldán (2012)]; (8) surface of abdominal terga with flattened, nonpointed corrugation and micropores [pointed chagrin structure in the form of minute spines, and circular sensillae according to Bauernfeind and Soldán (2012)]; (9) posterior margin of terga with robust spines alternating with shorter ones [the same for some terga of C. luteolum (see Figure 9 View Figure 9 (h)) and C. pirinense (according to Ikonomov 1962); the posterior margin of abdominal terga in Centroptilum with spines of subequal length according to Bauernfeind and Soldán (2012)]; (10) no long setation on last two caudal segments [basal and apical 1/ 5 part of caudal filaments are without setation according to Bauernfeind and Soldán (2012)]; and (11) chorionic surface of eggs with dense uniformly reticulated ridges, forming irregular polygonal cells [“meshes” according to Bauernfeind and Soldán (2012)].
Centroptilum volodymyri sp. nov. occupies a relatively independent position within the genus due to characteristics of the cuticle, setation of the posterolateral margin of tergum VII and the gill shape. At the larval stage, it clearly differs from the Western Palearctic representatives C. luteolum and C. pirinense by numerous scales and scale bases densely scattered on the body surface, some features of the mouthparts (particularly the shape of the labrum and superlinguae of the hypopharynx, and the proportions and setation of maxillary and labial palps; see also Table II), and dense denticulation of claws. Minor differences are found in the size of eggs and their cells within the chorionic mesh. The structure of the chorionic surface is thus very similar to that of C. luteolum (it was not described for C. pirinense ). A detailed comparative overview of the distinguishing characters for the delimitation of the three species discussed above is given in Table II.
Specimens of C. volodymyri sp. nov. collected in the Caucasus and Iran show some variability in colouration and morphology. Caucasian specimens are distinctly darker: their pleural sclerites and particularly anterior part of mesonotum and fore wing pads are markedly brown to dark brown. In contrast, Iranian specimens are distinctly paler, with body whitish yellow to brown. While Caucasian specimens had brown abdominal tergum and sternum of segment IX, Iranian specimens show a whitish-yellow central part of the tergum IX lined by two brownish oblique strips ( Figure 2 View Figure 2 (a)) and small light brown smudges on the sternum IX. Some minor differences between Caucasian and Iranian populations can be recognised in the structure of mouthparts and arrangement of scales and setae along the posterior margin of flagellar segments. Iranian specimens had a more elongated labrum width/length ratio (1.6–1.7, compared to 1.8– 2.0 in Caucasian specimens). Some Iranian specimens have fewer setae (fewer than six) on the dorsal side of segment II of the labial palp than the Caucasian specimens (6–8 setae). The setae under the maxillary crown are arranged in a nearly distinct row in specimens from the Caucasus, while they are mostly scattered in some specimens from Iran. The specimens from Georgia and Turkey have bluntly pointed or apically rounded scales on posterior margins of flagellar segments, while two or three pointed apices are found on each marginal scale in specimens examined from Iran. Nevertheless, these differences are not consistent in all specimens and characters, and we consider the specimens from Caucasus, Turkey and Iran to be conspecific, belonging to C. volodymyri sp. nov.
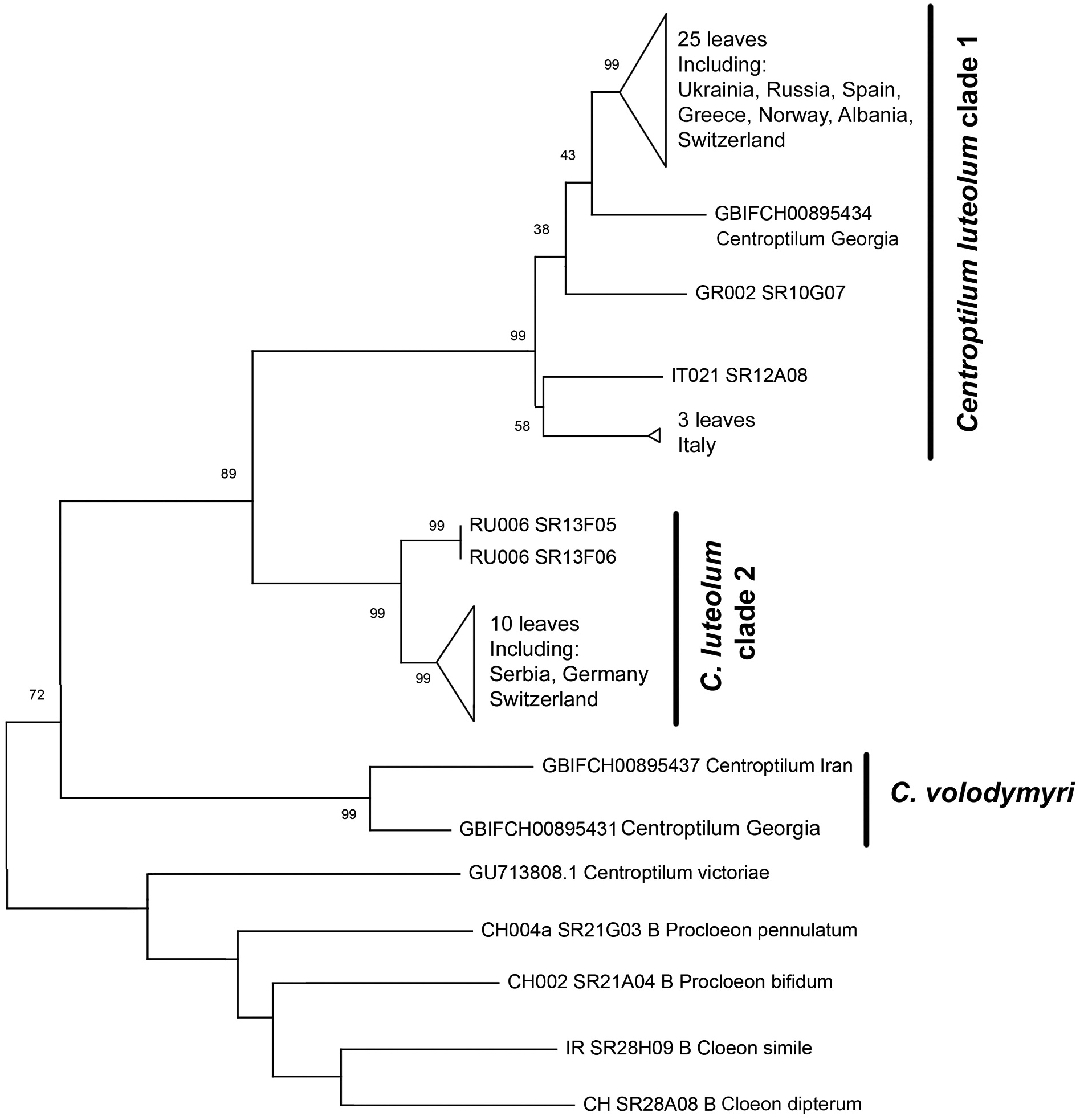
Molecular results
The molecular reconstruction highly supported C. volodymyri sp. nov. as a monophyletic clade, with a bootstrap support of 99% ( Figure 15 View Figure 15 ). Georgian and Iranian sequences are separated by genetic distances (13%, Table III) that are generally considered higher than intraspecific. Centroptilum volodymyri sp. nov. is recovered as the sister species of C. luteolum ; the genetic distances between C. volodymyri sp. nov. and the two clades of C. luteolum are higher than 25%. Centroptilum luteolum is composed of two monophyletic lineages (clade 1 and clade 2 in Table III and Figure 15 View Figure 15 ); both are highly supported as monophyletic and may represent distinct species (K2P distances higher than 20%).
Table I. Codes and origin of new sequences used in molecular study. For each specimen, the specimen catalogue number (GBIF code), the sample information (country, locality,
| Specimen | GenBank | GenSeq | ||||||
|---|---|---|---|---|---|---|---|---|
| Species | catalogue number | Country | Locality | Latitude | Longitude | Date | ID | nomenclature |
| Centroptilum | GBIFCH00895431 | Georgia | Adjara, Kobulety District, vicinity of Chakhati village, valley of | 41.762222 | 41.978111 | 18 April | OL960099 View Materials | genseq-2 COI |
| volodymyri sp. | the Kintrishi River, small lentic water bodies [Grg24Censp] | 2016 | ||||||
| nov. | ||||||||
| Centroptilum | GBIFCH00895432 | Iran | Guilan Province, SW from Amlash town, unnamed small brook | 37.036944 | 50.082500 | 21 May 2016 | OL960097 View Materials | genseq-3 COI |
| volodymyri sp. | ||||||||
| nov. | ||||||||
| Centroptilum | GBIFCH00895437 | Iran | Golestan Province, Kalaleh County, vicinity of Zav-e-Bala | 37.529433 | 55.791550 | 23 June 2019 | OL960098 View Materials | genseq-3 COI |
| volodymyri sp. | village, unnamed stream [Iran3 | |||||||
| nov. | Censp/1] | |||||||
| Centroptilum | GBIFCH00895434 | Georgia | Adzharia, territory of Kobulety town, Kintrishi River | 41.803889 | 41.775833 | 4 June 2013 | OL960101 View Materials | genseq-4 COI |
| luteolum | [Grg6Cenlut] | |||||||
| Centroptilum | GBIFCH00895435 | Ukraine | Luhansk Region, Antratsyt District, vicinity of Khrustal’nyi | 48.182321 | 38.877363 | 30 April | OL960102 View Materials | genseq-4 COI |
| luteolum | village, Khryshtaleva River [Lug 94] | 2012 | ||||||
| Centroptilum | GBIFCH00895441 | Switzerland | Burtignière, Vallée de Joux | 46.561667 | 6.170000 | 23 August | OL960100 View Materials | genseq-4 COI |
| luteolum | 2001 | |||||||
| NMNH |
USA, Washington D.C., National Museum of Natural History, [formerly, United States National Museum] |
| NASU |
NASU |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |