Nerocila serra Schioedte and Meinert, 1881
|
publication ID |
https://doi.org/10.1080/00222933.2022.2075290 |
|
DOI |
https://doi.org/10.5281/zenodo.7012440 |
|
persistent identifier |
https://treatment.plazi.org/id/61555368-FFB1-FF9F-FF1B-2615263CFAAC |
|
treatment provided by |
Plazi |
|
scientific name |
Nerocila serra Schioedte and Meinert, 1881 |
| status |
|
Nerocila serra Schioedte and Meinert, 1881 View in CoL
( Figures 1–7 View Figure 1 View Figure 2 View Figure 3 View Figure 4 View Figure 5 View Figure 6 View Figure 7 )
Nerocila Serra Schioedte and Meinert, 1881: 17 View in CoL , pl. 1, figs 12–14.
Nerocila serra View in CoL . – Gerstaecker, 1882: 260. – Stebbing, 1893: 352. – Nobili, 1903: 39. – Nierstrasz, 1915: 74; 1931: 124. – Barnard, 1925: 392; 1936: 163; 1940: 491. – Pillai, 1954: 12. – Morton, 1974: 46. – Kensley, 1978: 81, fig. 33E. – Bowman, 1978: 35. – Bowman and Tareen, 1983: 12, fig. 13. – Bruce 1987: 390, 406. – Bruce and Harrisson-Nelson, 1988: 597, fig. 6A–F. – Kazmi et al., 2002: 105, fig. 90. – Trilles et al., 2011: 453. – Saravanakumar et al., 2012: 2529–2531. – Trilles et al., 2013: 1273–1286, fig. 2h. – Rameshkumar et al., 2016: 940– 944, fig. 1H. – Ravichandran et al., 2019: 60 View Cited Treatment , fig. 8p–r.
Nerocila trivittata Bleeker, 1857: 23–24 View in CoL , pl. 1, fig. 2a–c [ nomen dubium].
Nerocila trivittata View in CoL . – Trilles, 1979: 254, pl. 1, fig. 4. – Jayadev Babu and Sanjeeva Raj, 1984: 821. – Ravichandran et al., 2019: 60.
Nerocila pulicatensis Jayadev Babu and Sanjeeva Raj, 1984: 818–823 View in CoL , pl. 1A–B, fig. 1a–j [new synonymy].
Material examined. Lectotype [ here designated]: ovig. ♀ (21.0 mm), Bangka Str. Salmin ( Bangka Island ), Indonesia, unknown host ( NRS TYPE-9047 (previously NRS-4974)).
Non-type: All specimens were collected from the host A. maculatus along the south-eastern coast of India: 1 ovig. ♀ ( 22.30 mm) Nagappatinam ( 10.7656°N, 79.8424°E), 8 January 2011, coll . G . Rameshkumar (ZSI/ MBRC D 1 -573 ); 2 ovig . ♀ (22.26 and 21.34 mm) ( CAS / MBRM C-661 , C-662 ); 1 ♂ (13.00 mm) ( CAS / MBRM C-665 ) 17 January 2018 Parangipettai ( 11.5084°N, 79.7568°E), coll GoogleMaps . P GoogleMaps . Vigneshwaran.
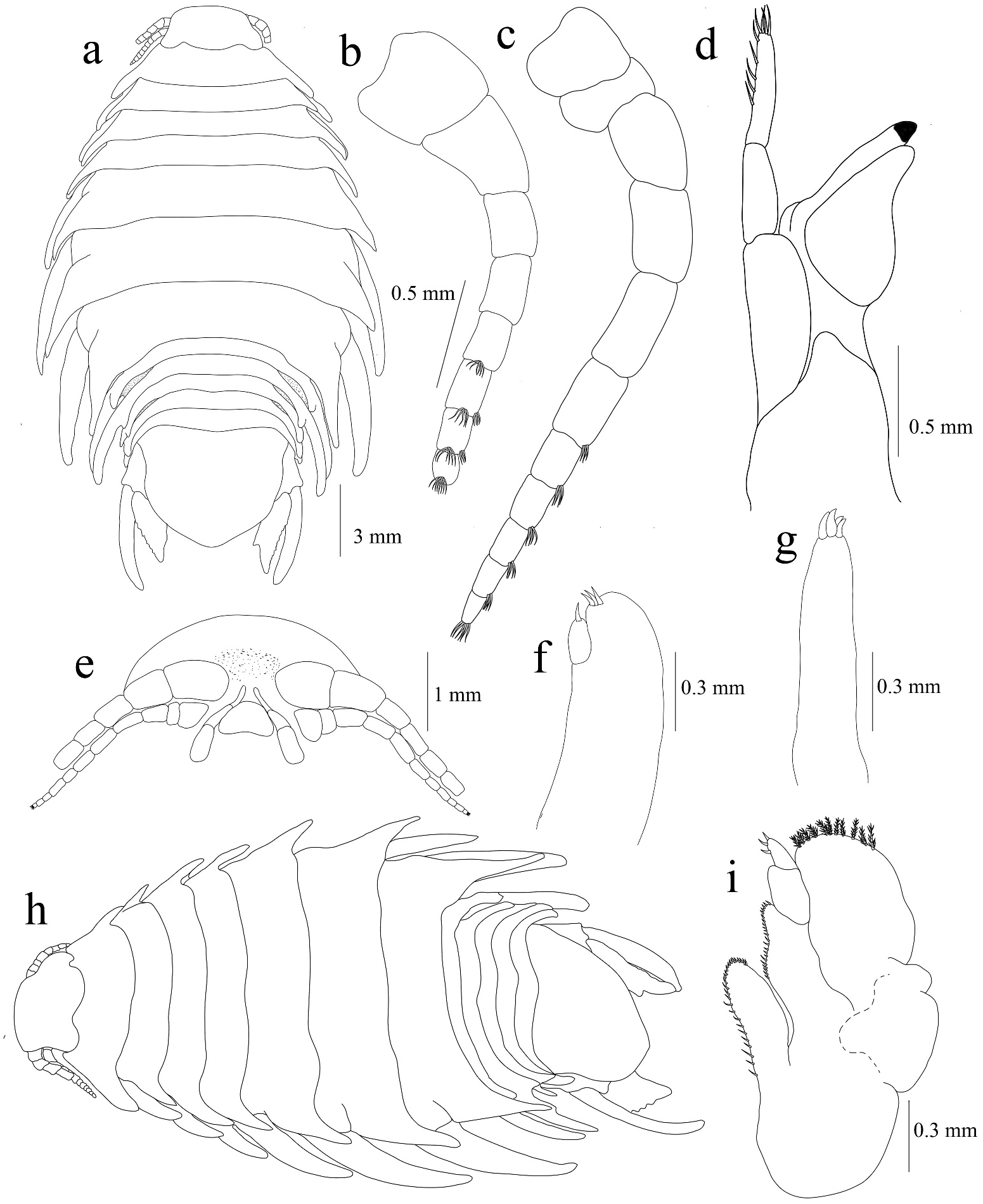
Description of ovigerous female lectotype ( Figures 1 View Figure 1 , 2a–c View Figure 2 , 3–5 View Figure 3 View Figure 4 View Figure 5 ). Body sub-oval, 2.3 times as long as wide, widest between pereonites 6 and 7. Cephalon 1.4 times as long as wide, anterior margin rounded; eyes with facets almost indistinct. Pereonites gradually increasing in width from 1 to 5; 6 and 7 longest and subequal,1 and 4 subequal in length, shorter than 5–7, 2 and 3 shortest and subequal. Posterolateral angle of all pereonites produced into narrow margin, increasing length progressively from pereonites 1 to 7; pereonites 5–7 with broad posteroventral regions. Coxae 2–7 acute, visible in dorsal view, gradually increasing in size; coxae 2–4 posteriorly and narrowly produced, not extending beyond posterior margin of pereonites; coxae 5–7 pointed with broad processes, longer than their respective segment. Pleon visible, pleonite 1 longest, 2–5 subequal in length. Pleonites 1 and 2 widest, ventrolateral margins of pleonites 1 and 2 enlarged, posteriorly directed, extending distinctly beyond pleonite 5, pleonites 3–5 not produced. Pleotelson 1.2 times as wide as long, smoothly rounded, with a distinct caudomedial lobe.
Antennula with eight articles, 1 and 2 larger than 3–8; articles 5–8 each with dense posterodistal cluster of setae. Antenna 11 articled, gradually decreasing in width, articles 1 and 2 larger than the others, articles 7–10 each with an anterodistal row of fine setae; article 11 with a cluster of apical setae. Mandible palp article 1 largest and widest and article 3 with setae on distolateral margin. Maxillula with four terminal spines slightly recurved. Maxilla bilobed, two spines on the median lobe and one spine on the lateral lobe. Maxilliped with oostegial lobe, distal palp segment with three terminal spines and smaller spine at about mid length of medial margin.
Pereopods gradually increasing in size from 1 to 5, dactylus 2 times longer than propodus. Nodules present on dactyli of pereopods 1, 2, 4 and 5; absent in pereopods 3, 6 and 7. Pereopods 1, 2, 4 and 5 without marginal spines, pereopod 3 with a single distal spine on the propodus. Pereopods 6 and 7 decreasing in length, with dactylus smaller than in other pereopods; pereopod 6 with three spines on carpus, four spines on merus and five spines on propodus; pereopod 7 with two spines on ischium, two rows of three and two spines respectively on carpus, two rows of three and four spines on merus and each rows with five spines on propodus. Brood pouch with five pairs of overlapping oostegites arising from the bases of pereopods 2–6. Pleopods without marginal spines; pleopods 1 and 2 endopods without lobes, pleopod 2 with appendix masculina about half length of endopod; pleopod 3 endopod with two folds; pleopod 4 endopod with two to three folds; pleopod 5 endopod with several large folds; pleopods 3–5 endopod with proximo-medial lobe well-developed and folded.
Uropod endopod 3.5 times as long as wide, extending beyond posterior margin of pleotelson, with a shallower notch on the medial margin and moderately coarse to finely serrate on the lateral margin. Exopod of uropod 0.95 times longer than endopod.
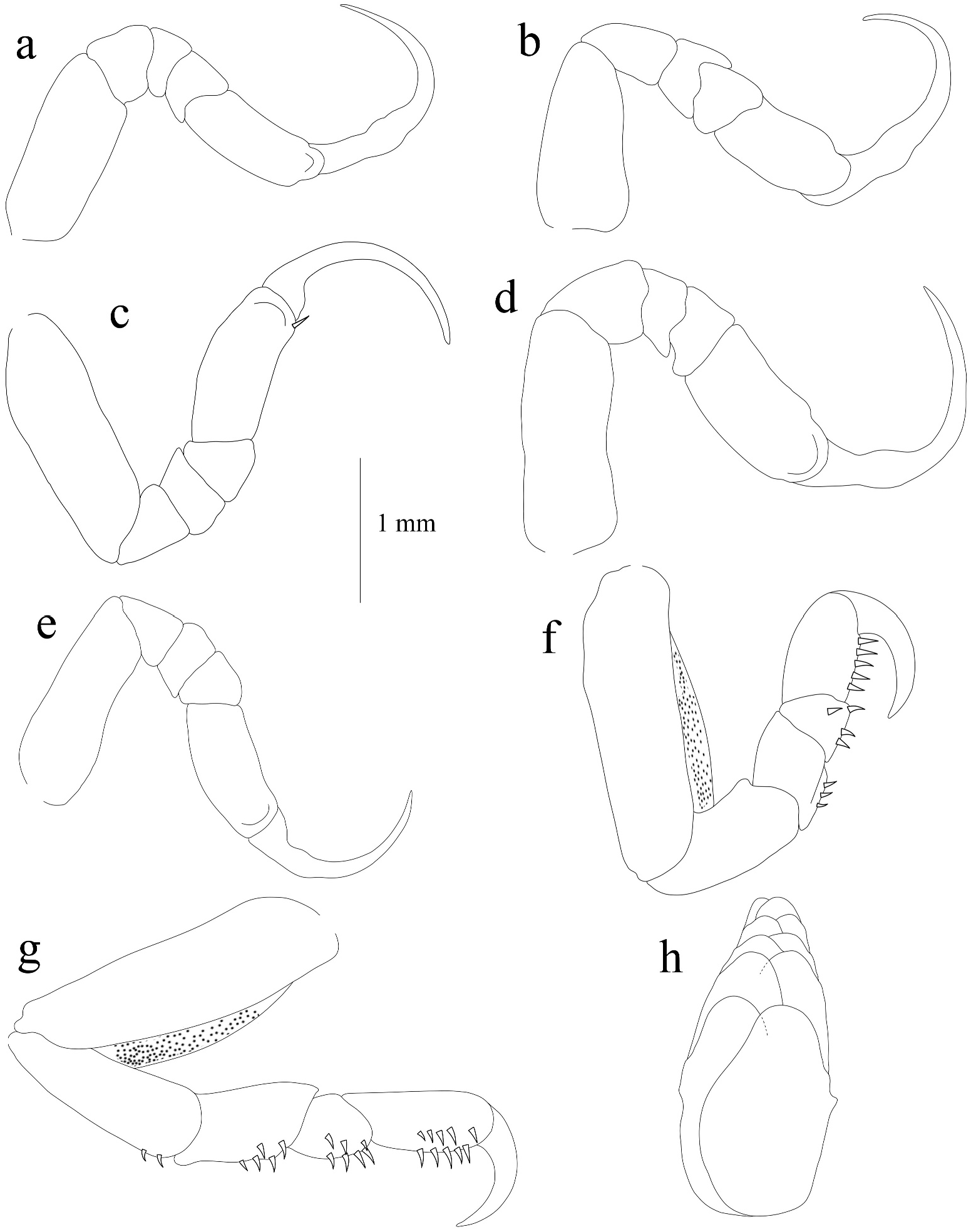
Description of male ( Figures 2d–e View Figure 2 , 6 View Figure 6 , & 7). Body symmetrical, about 2.4 times as long as wide, widest at pereonite 5–6. Cephalon 2.1–2.2 times as wide as long, anterior margin rounded. Eyes distinct. Pereonites 1 and 4–6 longest, 2 and 3 subequal;pereonite 7 slightly shorter than 6; posterior angles of pereonites 1–5 not produced; postero-lateral angles of pereonite 6 and 7 produced posteriorly as a pointed process; pereonites 5–7 widest and slightly wider than others. Coxae 2–7 partially visible in dorsal view, shorter than those of female, coxae 2 and 3 produced into rounded processes, not extending beyond posterior margin of pereonites; coxae 4–7 longer than anterior, not extending beyond posterior of pereonites. Pleonites all visible and subequal in length. Pleonites 1, 2 and 5 widest, ventrolateral margins of pleonites 1–2 enlarged, posteriorly directed, extending distinctly beyond pleonite 4, pleonite 3 and 4 not produced. Pleotelson about 0.74–0.88 times as wide as long, narrower than pleonite 1, distal margin narrowly rounded.
Antennula wider than antenna, with eight articles, distal margin of articles 6 and 7 with cluster of setae, article 8 with few terminal aesthetasc setae. Antenna with nine articles, decreasing gradually in width, articles 4, 5, 7 and 8 with cluster of setae, article 9 with few terminal esthetes. Mandible palp article 1 longest, 2 and 3 subequal in length; article 3 with about 4–5 small and two long setae on distolateral margin. Maxillula with four apical spines slightly recurved. Maxilla bilobed, with two spines on median and lateral lobes. Maxilliped with oostegial lobe and three recurved spines on article 3.
Pereopod dactyli without nodules, pereopods 1, 2, 4 and 5 without marginal spines, dactylus longer than propodus; dactylus of pereopods 2 and 7 smaller than others. Pereopod 3 with two spines on ischium and merus and propodus with one spine on ventrolateral margin. Pereopods 6 and 7 sub-equal in size, each with carina on mediolateral margin; pereopod 6 with two spines on ischium, five spines on carpus and merus, nine spines on propodal palm and one spine on anterolateral margin of propodus; pereopod 7 with three spines on ischium, three spines on carpus, two rows of three and two spines respectively on merus, two rows of five and six spines on propodus. Penes visible on sternite 7. Pleopods not distinctly visible in dorsal view; pleopods 1 and 2 endopods without lobes, protopod medial margin with 2 or 3 plumose setae. Pleopod 2 with appendix masculina about half the length of endopod; pleopod 4 endopod with a single lobe, pleopod 5 with 2 to 3 lobes several large folds. Proximo-medial lobe of pleopods 3–5 well developed and folded.
Uropod rami slender, tapering and sub-linear exopod and endopod; exopod and endopod subequal in length and extending scarcely beyond the posterior margin of pleotelson; endopod with serrate on the lateral margin.
Size. Ovigerous female: 21–23 mm; male: 13 mm.
Colour. Pale tan for live specimens and light brown to tan in ethanol-preserved specimens, often with three distinct longitudinal bands.
Distribution. Known from Indo– Malaysia ( Schioedte and Meinert 1881), Western Australian coast ( Bruce 1987), Northern Arabian Sea ( Kazmi et al. 2002) and south-east coast of India ( Barnard 1936; Saravanakumar et al. 2012; Trilles et al. 2013; Rameshkumar et al. 2016; Ravichandran et al. 2019).
Host. Nerocila serra have mainly been reported from catfishes of the order Siluriformes : Hexanematichthys sagor (Hamilton, 1822) and Arius maculatus (Ariidae) ( Barnard 1936; Trilles et al. 2013; Rameshkumar et al. 2016; present study); Plotosus canius (Plotosidae) and Mystus gulio (Bagridae) ( Jayadev Babu and Sanjeeva Raj 1984).
This species has also been recorded from sea snakes of the family Elapidae : Hydrophis obscurus Daudin, 1803 ( Barnard 1936) and Enhydrina schistosa (Daudin, 1803) ( Saravanakumar et al. 2012) . Though Barnard (1936) acknowledges their report to be an incidental occurrence, Saravanakumar et al. (2012) claim their findings of the cymothoid to be a significantly opportunistic association, reporting 374 of 886 E. schistosa infested with a whopping 664 female N. serra on various parts of the hosts’ bodies, of which most of the cymothoids were retrieved from female snakes ( 370 specimens). It is established that the cymothoids are highly host specific to teleosts, and therefore the association of N. serra with sea snakes ought to be revised and closely monitored for consistency of the report.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Cymothoida |
|
SuperFamily |
Cymothooidea |
|
Family |
|
|
Genus |
Nerocila serra Schioedte and Meinert, 1881
| Vigneshwaran, P., Ravichandran, S. & Ajith Kumar, T. T. 2022 |
Nerocila pulicatensis
| Jayadev Babu S & Sanjeeva Raj PJ 1984: 823 |
Nerocila trivittata
| Ravichandran S & Vigneshwaran P & Rameshkumar G 2019: 60 |
| Jayadev Babu S & Sanjeeva Raj PJ 1984: 821 |
| Trilles JP 1979: 254 |
Nerocila serra
| Ravichandran S & Vigneshwaran P & Rameshkumar G 2019: 60 |
| Rameshkumar G & Ramesh M & Ravichandran S & Trilles J-P 2016: 940 |
| Trilles J-P & Rameshkumar G & Ravichandran S 2013: 1273 |
| Saravanakumar A & Balasubramanian T & Raja K & Trilles J-P 2012: 2529 |
| Trilles J-P & Ravichandran S & Rameshkumar G 2011: 453 |
| Kazmi QB & Schotte M & Yousuf F 2002: 105 |
| Bruce NL 1987: 390 |
| Bowman TE & Tareen IU 1983: 12 |
| Kensley B 1978: 81 |
| Bowman TE 1978: 35 |
| Morton B 1974: 46 |
| Pillai NK 1954: 12 |
| Barnard KH 1940: 491 |
| Barnard KH 1936: 163 |
| Nierstrasz HF 1931: 124 |
| Barnard KH 1925: 392 |
| Nierstrasz HF 1915: 74 |
| Nobili G 1903: 39 |
| Stebbing TRR 1893: 352 |
| Gerstaecker A 1882: 260 |
Nerocila Serra
| Schioedte JC & Meinert FR 1881: 17 |
Nerocila trivittata
| Bleeker P 1857: 24 |