Rhithrogena sumatrana ( Ulmer, 1939 ), 2014
|
publication ID |
https://doi.org/10.11646/zootaxa.3802.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:8585D3AD-653C-4ED6-8523-FB6427A313C5 |
|
DOI |
https://doi.org/10.5281/zenodo.6141715 |
|
persistent identifier |
https://treatment.plazi.org/id/617C87EF-FF8C-7E37-97C1-FAB870D8FF49 |
|
treatment provided by |
Plazi |
|
scientific name |
Rhithrogena sumatrana ( Ulmer, 1939 ) |
| status |
comb. nov. |
Rhithrogena sumatrana ( Ulmer, 1939) comb. nov.
Ecdyonurus sumatranus Ulmer, 1939 , holotype female only, not nymph
Rhithrogena parva View in CoL (?) Ulmer, 1939, nymph, not imago
Ecdyonuroides sumatrensis [sic] Dang, 1967 , type species of the genus Ecdyonuroides
Ecdyonurus sumatranus Kluge, 1989
Thalerosphyrus sumatranus Wang & McCafferty, 2004 View in CoL
Material. One female imago holotype: Indonesia, South Sumatra [actual province of Bengkulu], Tjurup [ Curup ], at light trap, 7.V.1929, Prof. Feuerborn leg. [ ZMH]
Specimen kept in alcohol, except one hindleg mounted on slide in Canada balsam.
Seven nymphs: Indonesia, Java, Kali Kemantan in Kari Highlands , mountain stream at ca 1500 m, P2, 18.X.1928, Prof. Thienemann leg. [ ZMH, MZL]
Specimens in alcohol, one specimen partially mounted on two slides by Ulmer (Ulmer 1939, figs 467–469) in ZMH, one other specimen entirely mounted on microscopic slide [ MZL]
One nymph: Indonesia, Java, Buitenzorg in Tjiliwung River, FB3, 25.V.1929, Prof. Feuernborn leg. [ ZMH]
One nymph entirely mounted on microscopic slide: Indonesia, Java, Malang Batu Jalang , forested stream with waterfall, 9.V.2010, J.-M. Elouard leg. [ MZL]
Two nymphs, one entirely mounted on microscopic slide: Indonesia, Lombok, Nusa Tenggara Barat , Aik Jud River , 1 km north of Sesaot , 350 m, 23.X.1985, J.T & D.A. Polhemus leg. [ MZL].
Supplementary description of the female holotype. Ulmer (1939, p. 558, figs 129–131) gave a detailed description of this specimen, which is correct. As usual with Ulmer’s collection, the specimen is entirely faded, so coloration pattern is not more visible, except for the hindleg on slide.
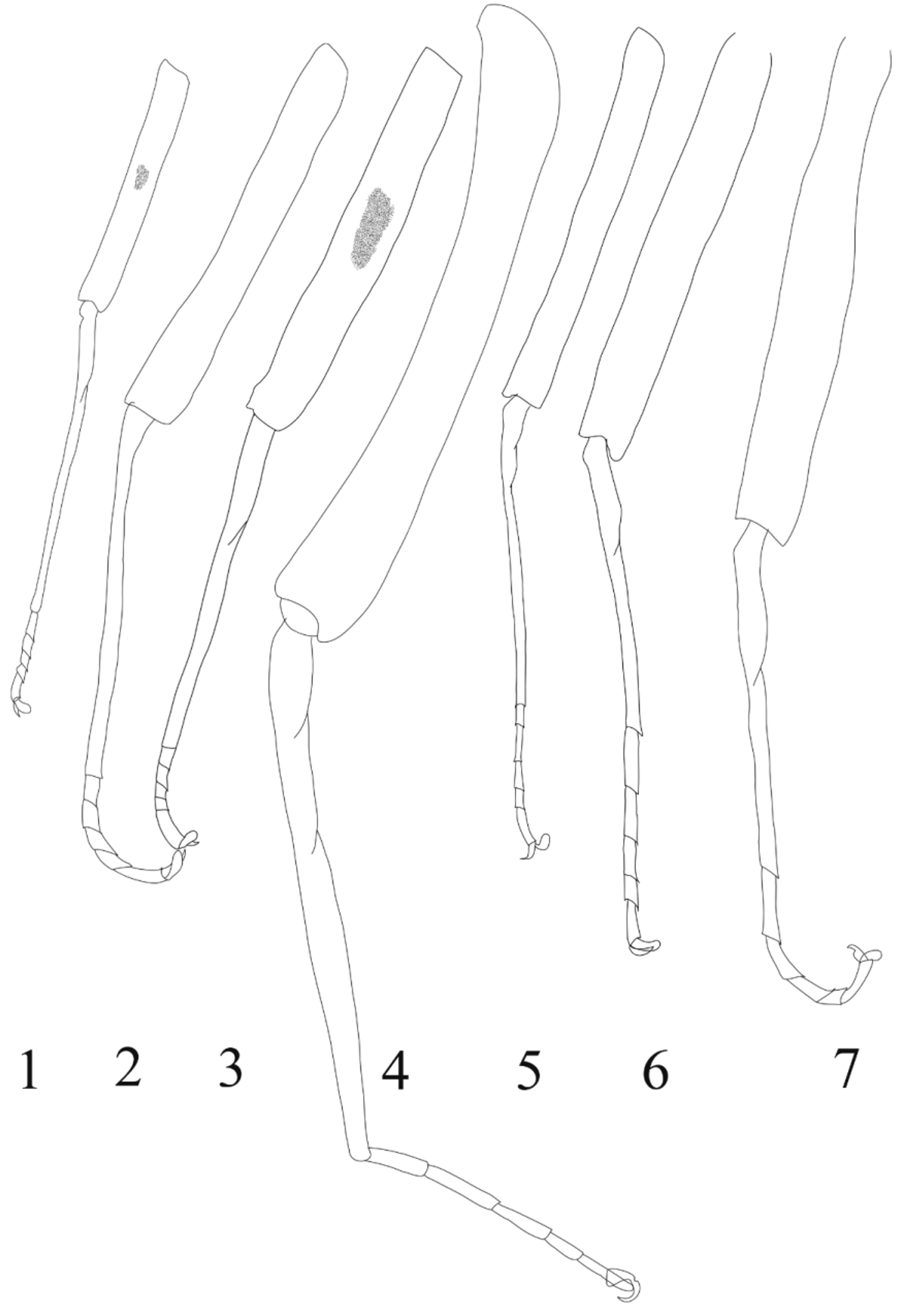
Of importance are the following characters: the dorsal face of femora bears a dark macula ( Ulmer 1939, p. 559: … mit hell honigfarbenen Schenkeln, die in der Mitte einen deutlichen schwarzen Punkt haben”); the hind tarsi are short, reported as one third the length of the tibia by Ulmer (1939, p. 559), they are in fact closer to one fourth ( Fig. 1 View FIGURES 1 – 7 ); the subanal plate is deeply cleft and bilobate.
Not mentioned by Ulmer are the following details of the thorax: mesonotum with a transverse suture; medial depression of mesothoracic furcasternum is narrowed anteriorly.
Description of the eggs. Size: ca 150 µm x 80 µm, regularly ovoid ( Fig. 8 View FIGURES 8 – 9 a). Chorionic surface covered by macrogranulae; macrogranulae asymmetrical and elongated (ca 3.5 µm x 2 µm), each directed toward one pole. Opposite pole covered with large KCTs (ca 5 µm in diameter), smaller KCT’s present between macrogranulae (2.5–3 µm in diameter) ( Fig. 8 View FIGURES 8 – 9 b). Micropyles oval (ca 8 µm x 5 µm) and located in equatorial area, with smooth margins ( Fig. 8 View FIGURES 8 – 9 c).
Supplementary description of the supposed nymph. Described under the name Rhithrogena parva (?) by Ulmer (1939), supplementary information is as follows.
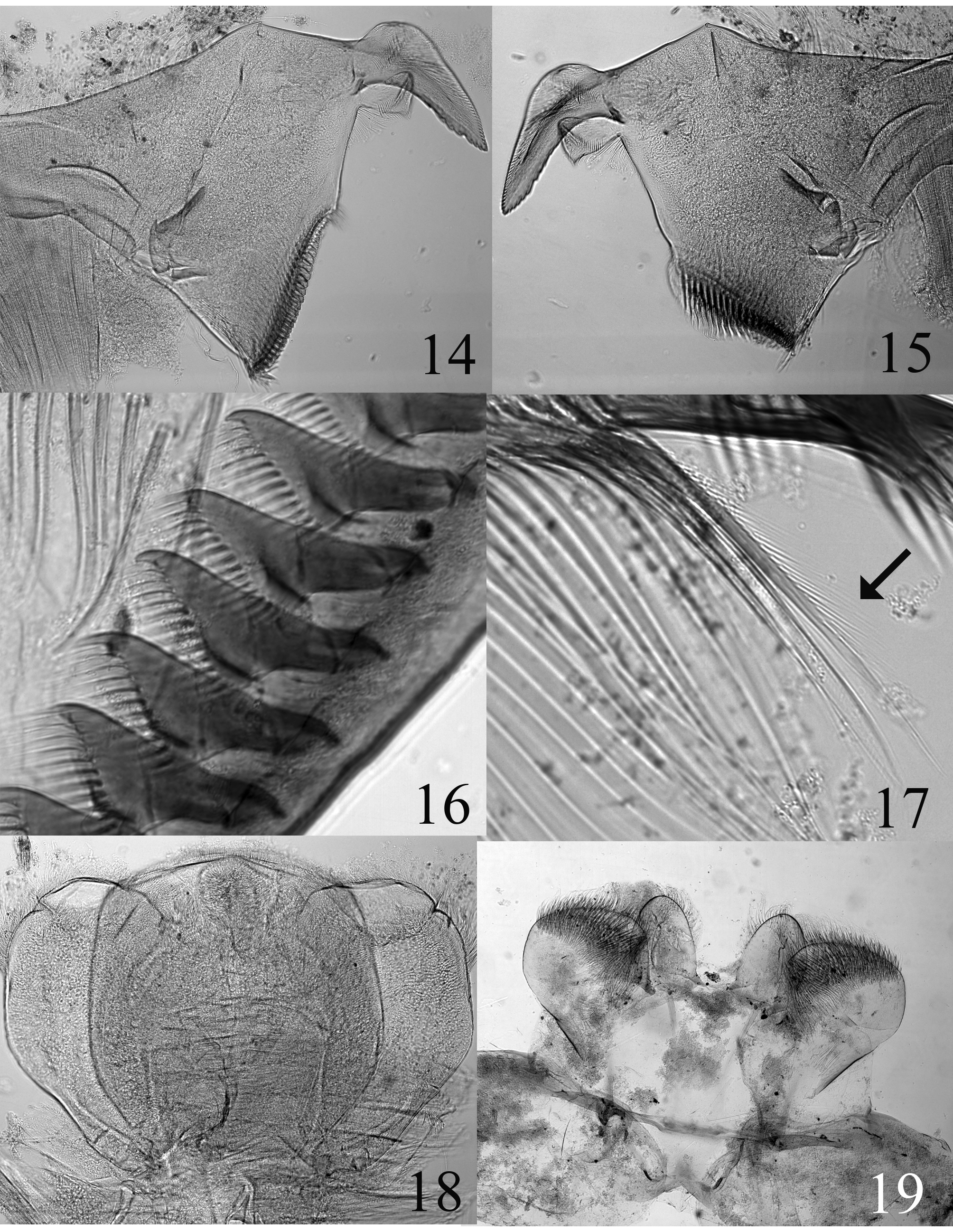
Labrum ca 2.5x wider than long ( Fig. 10 View FIGURES 10 – 13 ); lateral margins slightly angled, anteromedian emargination small and rounded, presence of irregular and pointed teeth in median area; anterior margin covered with long and thin setae up to two thirds of margin; tuft of short and thin setae in median position. Mandibles ( Figs 14–15 View FIGURES 14 – 19 ) with outer margin covered with long and thin setae, outer incisor ca 2–2.5 times longer than wide at base, below inner incisor one row of thin setae decreasing in length and ending ca at half distance to mola. Left mandible ( Fig. 14 View FIGURES 14 – 19 ) with tuft of dense and thin setae above mola and one row of numerous thin and long setae below mola. Right mandible ( Fig. 15 View FIGURES 14 – 19 ) without tuft of dense and thin setae above mola, and with row of numerous thin and long setae below mola. Galea-lacinia of maxillae with ca 12 comb-shape setae, median one composed of ca 13–15 teeth ( Fig. 16 View FIGURES 14 – 19 ). Outer dentiseta fringed on its outer margin ( Fig. 17 View FIGURES 14 – 19 ). Hypopharynx ( Fig. 18 View FIGURES 14 – 19 ) with stout and almost quadratic lingua, without distal emargination, with one row of thin and short setae anteriorly, superlinguae oval, bearing one row of thin and long setae down to ca ¼ margin. Labium ( Fig. 19 View FIGURES 14 – 19 ) with glossae rib-shaped, 1.5 times longer than wide at base; paraglossae quadrangular with well-marked inner angle and outer margin rounded.
Each leg with distinct blackish rounded macula in middle of dorsal face of femora. Bristles on dorsal face elongated, with slightly divergent margins, and with rounded apex ( Fig. 20 View FIGURES 20 – 23 ). Tibio-patellar suture on hind tibiae with row of ca 8–10 spatulate bristles. Tarsal claw with 2–4 teeth ( Fig. 21 View FIGURES 20 – 23 ).
Gill I ( Fig. 12 View FIGURES 10 – 13 ) with regularly crenulated margin, and with very long and thick plica; gills II–VII with entire, smooth margin. First abdominal sternite with lateral sclerites directed perpendicular to body axis ( Fig. 22 View FIGURES 20 – 23 ). Posterior margin of abdominal tergites ( Fig. 24 View FIGURES 24 – 26 ) with one row of irregular teeth, some long and thick, others smaller and thinner, and some submarginal microdenticles present.
Discussion. According to Kluge (1989) and Webb & McCafferty (2008), the structure of the female furcasternum reveals that this species cannot belong to the subfamily Ecdyonurinae, and thus cannot be associated with Thalerosphyrus . Other characters which are never found in Thalerosphyrus include the presence of a macula on the dorsal face of the femora, the subanal plate being bilobate and hind tarsi that are so short ( Figs 1–7 View FIGURES 1 – 7 ).
So if the specimen at hand is not a member of Ecdyonurinae, to which subfamily does this female belong? The subfamily Heptageniinae has very few species in Southeast Asia. The only genus known for certain is Trichogenia Braasch & Soldán, 1988 , represented by 3 species known only as larvae from Thailand, Vietnam, Sumatra, Borneo and Sulawesi ( Braasch & Soldán 1988; Webb et al. 2006). Webb et al. (2006) proposed to include also in this genus the species Heptagenia nasuta Ulmer, 1939 , known only from the imaginal stage, on the basis that no other Heptageniinae has ever been collected in the area, and the similarity in the tinted violet tinge found on the H. nasuta forewing and on a dissected wingpad of a Trichogenia nymph. Whether or not correct, our female does not match any Trichogenia species because the latter all lack dark maculae on the femora; the shape of the subanal plate resembles the one of the H. nasuta female ( Ulmer 1939, fig. 158), but the proportions of the hindlegs are completely different, with hind tarsi ca. 8.0x the size of the tibia, compared to 0.33x maximum in my specimen. The subfamily Rhithrogeninae, however, remains a candidate for placement of this taxon under study, of which three genera are recorded from Southeast Asia: Epeorus Eaton, 1881 ; Paegniodes Eaton, 1881 and Rhithrogena Eaton, 1881 . The presence of a transverse suture on the mesonotum excludes Epeorus as a possibility ( Webb & McCafferty 2008). The female of Paegniodes cupulatus ( Eaton, 1871) possesses hind tibiae ca 2x the length of the tarsi that have a segment composition, in decreasing order, of 2=5>3>1>4 ( Fig. 4 View FIGURES 1 – 7 ), whereas it is 1=5>2>3> 4 in the examined female ( Fig. 1 View FIGURES 1 – 7 ). Moreover, the subanal plate of P. cupulatus is entire and not cleft, and the femora do not possess dark maculae (Eaton 1885). Additionally, the eggs of Paegniodes do not present the same chorionic arrangement as those of Rhithrogena species, in particular those of Rh. sumatrana ( Fig. 9 View FIGURES 8 – 9 ).
Thus Rhithrogena is the best candidate to accommodate Ecdyonurus sumatranus . All the mentioned characters are, or can be, found in members of Rhithrogena ., which is a diverse genus encompassing more than 150 species, most of which have Holarctic distributions ( Barber-James et al. 2008).
Five species of Rhithrogena are known from Southeast Asia
• Rh. parva ( Ulmer, 1912) , male and female imagoes described from Formosa ( Taiwan) under the name Ecdyonurus parvus , recombined later without more comment ( Ulmer 1920). Nymphs mentioned for the first time from Java ( Ulmer 1939) under the name Rhithrogena parva ? Nymphs and eggs illustrated from Taiwan by Kang & Yang (1994), without mentioning on which basis they associate their nymph with Ulmer’s species;
• Rh. diehliana Braasch & Soldán, 1986 , a single male subimago poorly described from northern Sumatra;
• Rh. ampla Kang & Yang, 1994 , nymphs described from Taiwan, which are barely distinguishable from those of Rh. parva , but present egg chorionic structure differences, and may live in higher altitudes;
• Rh. unica Zhou & Peters, 2004 , all stages described from southern China, and type-species of Tumungula Zhou & Peters, 2004 (see below);
• Rh. siamensis Braasch & Boonsoong, 2009 , all stages described from Thailand and placed by the authors in the subgenus Tumungula
Rh. ornata ( Ulmer, 1939) is not listed here because I cannot accept the synonymy with Rhithrogeniella Ulmer, 1939 ( type species Rhithrogeniella ornata, Ulmer, 1939 from Java and Sumatra) proposed by Wang & McCafferty (2004). Examination of the type material show Rhithrogeniella is not a Rhithrogeninae because the depression of the furcasternum is not narrowed anteriorly and consequently cannot be a synonym of Rhithrogena . Its exact status will be treated elsewhere ( Sartori 2014a).
All these species present a dark macula on the femora. The female of Rh. sumatrana can be compared to those of Rh. unica and Rh. siamensis , with which it shares the short hind tarsi (between one third and one fourth the length of the tibia), and the subanal plate deeply cleft. It differs from Rh. siamensis by the egg chorionic structure ( Boonsoong & Braasch 2013), those of Rh. unica being unfortunately undescribed. The female of Rh. parva is incompletely described, but based on its redescription (see below), Rh sumatrana differs by the hind tibiae being much longer than the femora (subequal in Rh. parva , compare Figs 1 and 3 View FIGURES 1 – 7 ), by the tarsal composition and by the subanal plate being not so cleft. It remains a possibility that Rh. diehliana is a junior synonym of Rh. sumatrana , but due to the scarcity of data, more material is needed.
In the absence of mature female nymphs, the association between the female adult and the nymphs described here is based on the following interpretations. In Southeast Asia, the genus Rhithrogena is not only poorly diversified, but is also very rare and uncommon in stream and rivers. The genus appears to be absent from Vietnam, Malaysia and the Philippines. Rhithrogena of the Sunda Islands are known from Sumatra, Java and Lombok; the conspecificity of the populations of Lombok and Java, which are located on each side of the Wallace line, makes the conspecificity of the populations of Java and Sumatra credible, although no Rhithrogena nymphs have been collected or reported from Sumatra (contrary to what was stated by Zhou & Peters 2004). Both islands share several mayfly species in common, such as Compsoneuria spectabilis Eaton, 1881 ; Compsoneuriella thienemanni Ulmer, 1939 , both in the Heptageniidae (Sartori, unpublished); Rhoenanthus speciosus Eaton, 1881 in the Potamanthidae ( Bae & McCafferty 1991) ; Potamanthellus caenoides ( Ulmer, 1939) in Neoephemeridae , with this species’ range of distribution also including Lombok ( Bae & McCafferty 1998); and Dudgeodes ulmeri Sartori, 2008 in Teloganodidae , recently also reported from Bali ( Sartori et al. 2008; Sartori 2014b).
The nymph of Rh. sumatrana is easily separated from all other Southeast Asian species by the numerous crenulations of the gill I. The mandible illustrated by Ulmer (1939: Fig. 467) is much more elongated than the one showed here, because on his slide preparation the mandible has been folded, appearing much more slender than it is actually.
It is impossible to know if Rh. sumatrana belongs to the subgenus Tumungula Zhou & Peters, 2004 or to the subgenus Rhithrogena , s.s. Tumungula is characterized by several unique apomorphies which are present only on the male imago, such as a hypertrophied foreleg claw, the first segment of the fore tarsi longer than the second, and various details of the genitalia. Zhou & Peters (2004) mentioned anyway, in the diagnosis of the subgenus, the presence of an incised subanal plate, as in Rh. sumatrana . This is also the case not only in the species Rhithrogena (Tumugula) siamensis as illustrated by Braasch & Boonsoong (2009), but also in some Rhithrogena s.s. species such as Rh. paulinae ( Sartori & Sowa 1992) from Iran. This character is rather rare within the genus Rhithrogena , where most of the Holarctic species possess an oval to ellipsoidal subanal plate that is entire or slightly concave (e.g. Needham et al. 1935; Sartori 1992; Sowa et al. 1985; Sowa & Soldán 1986). As in Rh. (Tumungula) unica , the first sternite of the nymph possesses lateral sclerites perpendicular to the body axis, contrary to Rh. parva and Rh. ampla (see below); the same also occurs in Rh. (Tumungula) siamensis (B. Boonsoong, pers. comm.). The combination of gill VII with a smooth margin, and lateral sclerites perpendicular to the body axis (and to some extent the maculae on the femora) may support the monophyly of these three species (hence the validity of the subgenus Tumungula ), as it has been shown also to some extent for European species ( Sowa 1984; Vuataz et al. 2011).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Rhithrogena sumatrana ( Ulmer, 1939 )
| Sartori, Michel 2014 |
Thalerosphyrus sumatranus
| Wang & McCafferty 2004 |
Ecdyonurus sumatranus
| Kluge 1989 |
Ecdyonuroides sumatrensis [sic]
| Dang 1967 |
Ecdyonuroides
| Dang 1967 |
Ecdyonurus sumatranus
| Ulmer 1939 |