Apheloria virginiensis virginiensis ( Drury 1770 )
|
publication ID |
https://doi.org/ 10.5281/zenodo.5169106 |
|
publication LSID |
lsid:zoobank.org:pub:76EEFB2C-44E1-46F0-B985-1EADCC80D73D |
|
persistent identifier |
https://treatment.plazi.org/id/6312ED35-FFCC-FF8D-52B1-FC04FB55FB27 |
|
treatment provided by |
Felipe |
|
scientific name |
Apheloria virginiensis virginiensis ( Drury 1770 ) |
| status |
|
Apheloria virginiensis virginiensis ( Drury 1770) View in CoL
Fig. 1–5 View Figures 1–3 View Figures 4–5
Julus virginiensis Drury 1770: 99 , pl. 43, fig. 8. Say 1821: 107 (text).
Polydesmus virginiensis: Beauvois 1805: 56 . Brandt 1839: 311; 1841: 131. Newport 1844b: 264. Gervais 1859: 6. Saussure 1860: 62–63.
Polydesmus Virginiensis View in CoL (sic!): Gervais 1836. 378; 1847: 106.
Polydesmus virginensis (sic!): Gervais 1837: 43–44.
Fontaria virginiensis View in CoL : Newport 1844b: 264. Bollman 1893: 123 (in part), 152. Silvestri 1896: 195. Brimley 1938: 498. Chamberlin and Hoffman 1958: 34.
Polydesmus (Fontaria) virginiensis: Saussure 1860: 62–64 .
Fontaria coriacea: Brimley 1938: 498 View in CoL .
Apheloria aspila Chamberlin 1939: 10 View in CoL , pl. 4, fig. 31. Hoffman, 1949: 374. Chamberlin and Hoffman 1958: 18. New Synonymy.
Apheloria tigana Chamberlin 1939: 11 View in CoL . pl. 4, fig. 29. Loomis 1944: 173. Chamberlin and Hoffman 1958: 20. Wray 1967: 151. Shelley 1978: 63–66, fig. 62; 2000: 193. Hoffman 1999: 306. Marek et al. 2014: 8. New Synonymy.
Apheloria virginia Chamberlin 1939: 12 View in CoL , pl. 4, fig. 30. Wray 1967: 151.
Apheloria coriacea: Loomis 1944: 173 View in CoL . Hoffman 1949: 374.
Apheloria virginiensis: Shelley 1988: 1653–1654 View in CoL , fig. 34.
Apheloria virginiensis virginiensis: Hoffman 1999: 306 View in CoL . Marek et al. 2014: 8.
Type-specimens. Male neotype and 1 additional male and 1 female (FSCA), 1 male and 1 female (NMNH, VMNH), and 1 male (AMNH) collected by G. Phillips and R. M. Shelley, 8 July 2016, along VA Hwy. 40, 1.3 km (0.8 mi) W jct. VA Hwys. 40/ 644 in McKenney (36 o 59’37”N, 77 o 44’22”W), Dinwiddie Co., Virginia.
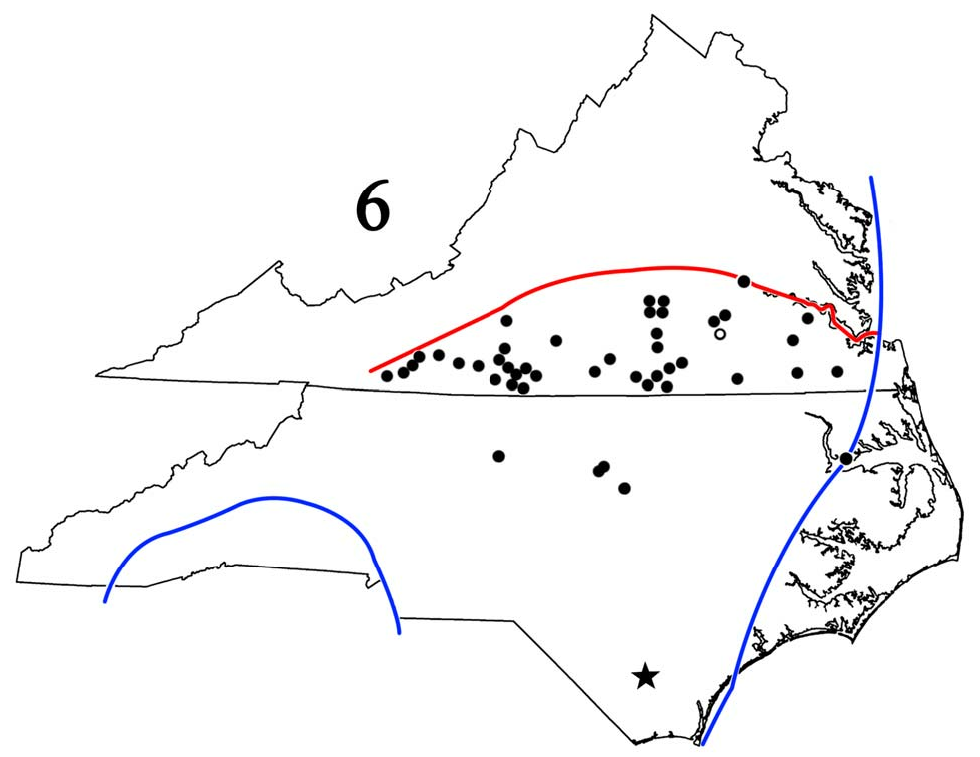
New synonymies. Hoffman (1999) placed A. aspila and A. waccamana , both authored by Chamberlin, in synonymy under A. tigana . He noted that A. aspila had page priority but chose A. tigana because the former’s type locality — Soco Falls, Jackson Co., NC – lay outside the species’ range and seemed erroneous. His reasoning was sound but unnecessary because the International Code of Zoological Nomenclature does not recognize page priority, and first reviser rights allowed him to choose either name. RMS recently examined both types; the vial label with A. aspila states “ Sigmoria aspila ,” and the above locality is preceded by the crossed-out word, “Durham,” a city in the “Triangle” (Raleigh/Durham/ Chapel Hill region of central NC), ~ 418 km (255 mi) to the east-northeast. As the A. aspila and A. tigana types are essentially identical, we believe the former did come from Durham but Chamberlin somehow became confused and crossed it out for what he erroneously thought was the correct site. Hoffman (1999) also stated that the types of all three names had been examined, but that of A. tigana could only have been viewed in situ because the gonopods had not been dissected when RMS examined it. Though never published, Hoffman considered A. tigana a full species because of a short vertical projection caudal to the prefemoral process (pers. comm. to RMS in 2011). However, viewing the dissected left gonopod from several angles, RMS saw no such structure; indeed, the gonopod is virtually identical to that of the J. virginiensis neotype! Consequently, A. tigana falls in synonymy under A. v. virginiensis as does A. aspila ; A. waccamana , whose type locality is Lake Waccamaw, Columbus Co., in southeastern NC ( Fig. 6 View Figure 6 , star), may apply to the form that Hoffman considered A. tigana , a matter that we are investigating.
Examining gonopods in situ for minute details is risky because, joined together by a sclerotized sternum or sternal remnant as well as membranous connective tissue and to the body by the latter alone, the appendages cannot be rotated or fully manipulated, which can lead to errors, misinterpretations, and misidentifications. For accurate and reliable determinations, at least one gonopod should be removed from the body, examined separately, and viewed from every perspective to fully grasp its structure. Hoffman’s (1999) error in considering A. tigana a separate species may reflect not doing so.
Color in life ( Fig. 1–3 View Figures 1–3 ). Epicranium, interantennal region, and frons black, fading into medium brown on clypeus and genae; antennae light brown. Prozonal and metazonal base colors dark ebony black; paranotal markings varying from pinkish-red to orange, subtriangular on collum and metaterga 2–3, extending mediad for varying lengths along caudal metatergal margins of metaterga 4–17. Collum with broad, bright yellow middorsal spot (1.3 x 0.7 mm) just caudal to anterior margin; metaterga 2–6 with small, paired, yellow middorsal spots with pinkish borders caudomediad, coalescing into one spot on 7 th metatergum, continuing and becoming fainter caudad, nearly absent on metaterga 18/ 19 in some individuals. Epiproct black basally, caudal half yellowish. Sides of metazonites reddish yellow, legs, sterna, paraprocts, and hypoproct subuniformly pale yellowish, claws dark brown.
Diagnosis. All but perhaps caudalmost metaterga with a small discreet single or two closely paired yellowish/pinkish middorsal spots slightly anterior to caudal margins. Gonopodal prefemoral process relatively long, broadly curved, and apically acuminate, extending into acropodital curvature and directed toward inner margin around 1/3 length, arising directly from prefemur, without nubbin-like pedicel but with basal medial flange and lateral lobe; acropodite smooth basally without one or more spurs or projections, curving broadly as described for the genus, noticeably swollen along outer margin at 2/3 length then narrowing smoothly and continuously to distal bend, distal zone moderately long, directed sublaterad, apically acuminate.
Neotype. Length 37.6 mm, maximum width 10.5 mm; W/L ratio 27.9%. Head smooth, glabrous; epicranial suture moderately distinct, terminating in interantennal region, not apically bifid. Interantennal isthmus 1.4 mm; genae medially impressed, ends extending beyond those of epicranium, width across genal apices 5.0 mm. Antennae extending backwards to midlength of 4 th metatergite; 1 st antennomere subglobose, 2–5 clavate, 6 longer and cylindrical, 7th truncate with four terminal sensory cones; 1 st –3 rd antennomeres sparsely hirsute, 4 th moderately so, 5 th –7 th densely pilose; relative lengths of antennomeres 6>3=5>4>2>1>7. Facial setae as follows: epicranial, interantennal, genal, and frontal series absent, clypeal about 9-9, labral around 12-12, merging with clypeal series and extending for short distances along genal margins, 3–4 setae per side.
Metaterga generally smooth, glabrous, and glossy, distinctly coriaceous anteriolaterad at bases of paranota beginning on 4 th tergite and becoming progressively more coriaceous caudad. Collum semilunar; metatergites 2–16 becoming slightly but progressively broader caudad. Paranota shorter than metaterga and continuing wrinkling from latter, anteriolateral margins curved, caudolateral corners slightly extended; peritermata distinct, strongly elevated above paranotal surfaces; ozopores located caudal to peritrematal midlengths on paranota 5, 7, 9–10, 12–13, opening subdorsad. Epiproct short, subtriangular, extending beyond caudal paraproctal margins, apically subacuminate.
Sides of metazona smooth, generally without grooves or impressions. Pregonopodal sterna with short, indistinct, medial lobes between 3 rd and 4 th legs, strongly depressed between 7 th legs to accommodate curvatures of gonopodal acropodites when segments compressed. Postgonopodal sterna of males and all sterna of females with faint bicruciform impressions but otherwise smooth, caudal margins sublinear or gently concave, with or without small setal tufts laterad but wholly without lobes or spines. First male legs short and crassate; 2 nd longer and subequal in length to remaining legs, gonapophyses long, prominent, and cylindrical, apices slightly flared. Postgonopodal legs becoming progressively less setose caudad; coxae with short, stubby, ventrally directed spurs, prefemoral spines longer, narrower, and directed laterad, arising on 6 th legs, longer and more spiniform from segment 9 caudad; tarsal claws gently curved. Paraproctal margins strongly elevated and thickened; hypoproct large and prominent, semilunar, slightly extended mediad with two long, subapical setae.
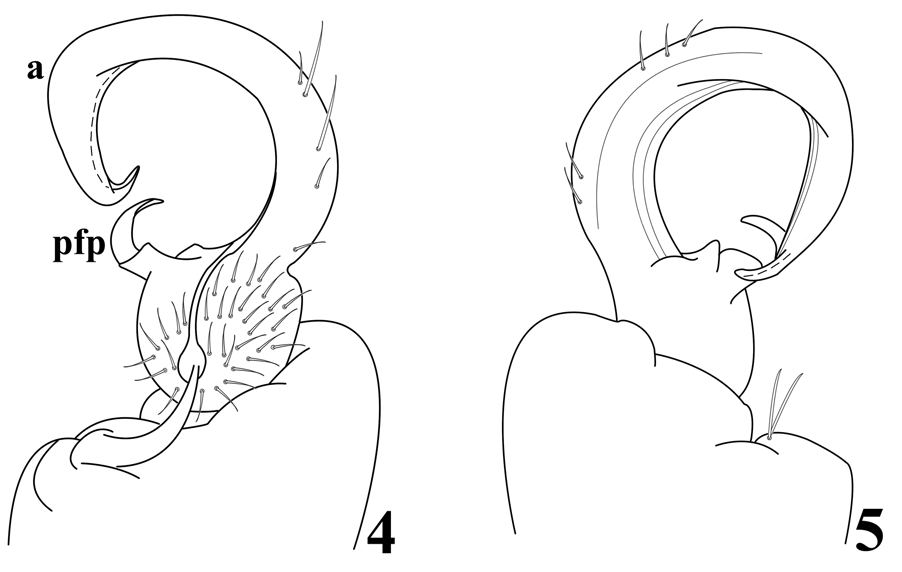
Gonopodal aperture ovoid, approximately 2.6 mm wide and 1.6 mm long at midpoint, lateral and caudal margins slightly elevated above metazonum, rims smooth, not thickened or flared. Gonopods in situ with one appendage lying transversely in aperture, its telopodite displacing that of opposite gonopod anteriad, overhanging anterior margin of aperture. Gonopod structure as follows ( Fig. 4–5 View Figures 4–5 ): coxa relatively large with two long macrosetae, without apophysis, connected to opposite member by membrane only, without sternal remnant; prefemur substantially smaller than coxa; prefemoral process relatively long, broadly curved, and apically acuminate, extending into acropodital curvature and directed toward inner margin around 1/3 length, arising directly from prefemur, without basal pedicel but with basomedial flange and lateral lobe; acropodite smooth basally without spurs or projections, curving broadly, swollen along outer margin at 2/3 length then narrowing smoothly and continuously to distal bend, distal zone moderately long, directed sublaterad, apically acuminate. Prostatic groove arising in pit in prefemur, running generally along inner surface of acropodite to terminal opening.
Additional specimens in neotype sample. They all agree closely with the neotype in somatic and gonopodal features except that in two males, both gonopods lie transversely, wholly inside the aperture,
with each telopodite lying over the opposing coxa and interlocking with its telopodite. Morphometrics for the five males and three females are as follows: Males: Length: range 31.7–35.9 mm, mean 35.1 mm, median 33.8 mm; width: range: 9.8–10.5 mm, mean 10.2 mm, median 10.2 mm. Females: Length: range 34.4–37.6 mm, mean 36.4 mm, median 36.0 mm; width: range 10.3–10.5 mm, mean. 10.4 mm, median 10.4 mm.
Ecology. The specimens were found under deciduous litter on black, organic substrate in a wet, mixed wooded ravine bisected by a slowly flowing creek. The area had received rain the previous day.
Distribution ( Fig. 6 View Figure 6 ). This study confirms Hoffman’s (1999) range statement of the Piedmont Plateau and inner Coastal Plain of Virginia south of the James River and extends it southwestward to the Blue Ridge foothills of Franklin, Patrick, Floyd, and Carroll Cos. and southward to the latitude of Raleigh, Wake Co., in the NC “Triangle.” Its widespread occurrence in the southernmost tier of Virginia counties strongly suggests comparable occurrence in the adjacent northernmost tier of NC counties where A. tigana was assumed to be the only generic representative ( Shelley 1978, 2000). The results of RMS’ examination of its holotype necessitates that all samples so identified be reexamined; indeed, the gonopodal illustration of a Wake Co. male of, ostensibly, A. tigana ( Shelley 1978, fig. 65–66) is really A. v. virginiensis . In southeastern Virginia, the nominate form, with small, discrete, metatergal spots ( Fig. 1–3 View Figures 1–3 ), seems tightly parapatric with banded A. v. corrugata ; the former occurs south of the James River in Surry, Sussex, Courtland, and Suffolk Cos. while the latter occurs to its north. Both forms inhabit inland Chesterfield and Carroll Cos., and west of the former, A. v. virginiensis curves southwestward as A. v. corrugata alone occupies Appomattox, Buckingham, and Cumberland Cos. Farther southwest in Bedford and northern Franklin Cos., the middorsal spots become larger and somewhat splotchy as their caudal margins spread laterad suggesting metatergal bands, and they become even more banded in northern Bedford Co. This “semi-banded” pattern arises in northern Franklin Co. and is so pronounced in Bedford that the millipeds cannot be labeled A. v. virginiensis ; we therefore place the boundary between these counties. Perhaps A. v. virginiensis intergrades with A. v. corrugata in the Blue Ridge foothills while they are parapatric in the Piedmont and Coastal Plain. While the gonopods remain relatively constant, the spots become smaller and more discrete in Floyd and Carroll Cos., and hence compatible with those in the neotype.
The position of the southern boundary is unknown, but it is at least as far south as Greensboro, Guilford Co., the “Triangle,” and, to the northeast, Albemarle Sound and Chowan Co. Though not yet found there or in southeasternmost Virginia, it seems safe to predict that only A. v. virginiensis occurs in the Dismal Swamp and between Albemarle Sound, NC, and lower Chesapeake Bay, Virginia. However, all samples from the northern tier of NC counties bordering Virginia and from counties immediately to the south must be reexamined; minimally, the roster includes those listed by Shelley (1978, 2000): Alamance, Caswell, Durham, Edgecombe, Forsyth, Franklin, Granville, Guilford, Halifax, Person, Rockingham, Stokes, Surry, Wake, Warren, and Yadkin. In characterizing middorsal spot variation on “Triangle” Aphelorias, Shelley (1978) stated, “Size and shape of the middorsal spots also vary, ranging from large, semilunar splotches to small, well-defined circles. On a few individuals, there is a progressively deeper indentation of the spot proceeding anteriorly, resulting in two small, paired middorsal spots on the anteriormost segments,” precisely the condition in the neotype of J. virginiensis ( Fig. 1–3 View Figures 1–3 ).
Deletions. Kentucky: Edmondson Co., Mammoth Cave, presumably in epigean Mammoth Cave National Park, not inside the cave itself (Chamberlin and Hoffman 1958). North Carolina: Moore Co., Southern Pines ( Brimley 1938), specimen lost but locality is south of the largest regional river, the Cape Fear, and too far [128.0 km (80.0 mi)] from the “Triangle” to be assumed to be A. v. virginiensis (= A. tigana ). Tennessee: Davidson Co., Ashburnham (Chamberlin and Hoffman 1958).
Published Records. North Carolina: Guilford Co., Greensboro ( Wray 1967). Wake Co., Raleigh ( Brimley 1938). Virginia: Dinwiddie Co., exact location unknown but likely the correspondent’s home, “The Grove” plantation near “Bolster’s Store” ( Drury 1770, Cockerell 1922, Shelley 1980, Hoffman 1999, Marek et al. 2014). Pittsylvania Co., Chatham ( Chamberlin 1939, Wray 1967).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Apheloria virginiensis virginiensis ( Drury 1770 )
| Shelley, Rowland M., Phillips, Gary & Smith, Jamie M. 2017 |
Apheloria virginiensis virginiensis: Hoffman 1999: 306
| Marek, P. & T. Tanabe & P. Sierwald 2014: 8 |
| Hoffman, R. L. 1999: 306 |
Apheloria virginiensis: Shelley 1988: 1653–1654
| Shelley, R. M. 1988: 1654 |
Apheloria coriacea:
| Hoffman, R. L. 1949: 374 |
| Loomis, H. F. 1944: 173 |
Apheloria aspila
| Hoffman, R. L. 1949: 374 |
| Chamberlin, R. V. 1939: 10 |
Apheloria tigana
| Marek, P. & T. Tanabe & P. Sierwald 2014: 8 |
| Hoffman, R. L. 1999: 306 |
| Shelley, R. M. 1978: 63 |
| Wray, D. L. 1967: 151 |
| Loomis, H. F. 1944: 173 |
| Chamberlin, R. V. 1939: 11 |
Apheloria virginia
| Wray, D. L. 1967: 151 |
| Chamberlin, R. V. 1939: 12 |
Fontaria coriacea: Brimley 1938: 498
| Brimley, C. S. 1938: 498 |
Polydesmus (Fontaria) virginiensis:
| Saussure, H. 1860: 64 |
Fontaria virginiensis
| Brimley, C. S. 1938: 498 |
| Silvestri, F. 1896: 195 |
| Bollman, C. H. 1893: 123 |
| Newport, G. 1844: 264 |
Polydesmus virginensis
| Gervais, P. 1837: 43 |
Polydesmus virginiensis: Beauvois 1805: 56
| Saussure, H. 1860: 62 |
| Gervais, P. 1859: 6 |
| Newport, G. 1844: 264 |
| Brandt, J. F. 1841: 131 |
| Brandt, J. F. 1839: 311 |
| Beauvois, A. M. F. J. P. 1805: 56 |
Julus virginiensis
| Say, T. 1821: 107 |
| Drury, D. 1770: 99 |