Proteocephalus misgurni, Tomáš Scholz & Alain de Chambrier & Takeshi Shimazu & Alexey Ermolenko & Andrea Waeschenbach, 2017
|
publication ID |
https://doi.org/ 10.1016/j.parint.2016.09.0161383-5769 |
|
DOI |
https://doi.org/10.5281/zenodo.6010900 |
|
persistent identifier |
https://treatment.plazi.org/id/632E7F4C-6F7A-FF91-FFF8-0584FD60FD70 |
|
treatment provided by |
Plazi |
|
scientific name |
Proteocephalus misgurni |
| status |
sp. nov. |
3.2.4. Proteocephalus misgurni sp. n.
Figs. 5 View Fig. 5 , 6 View Fig. 6
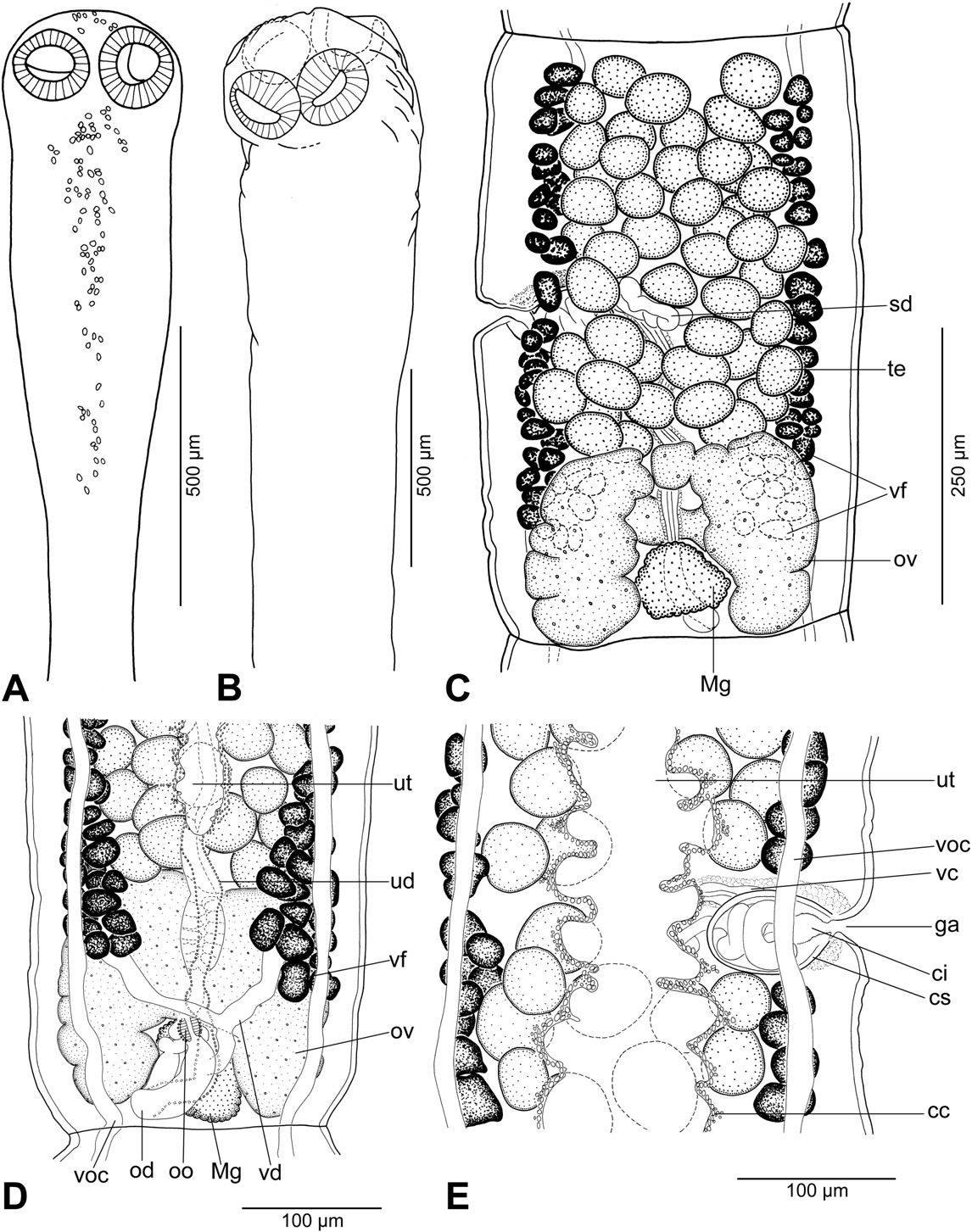
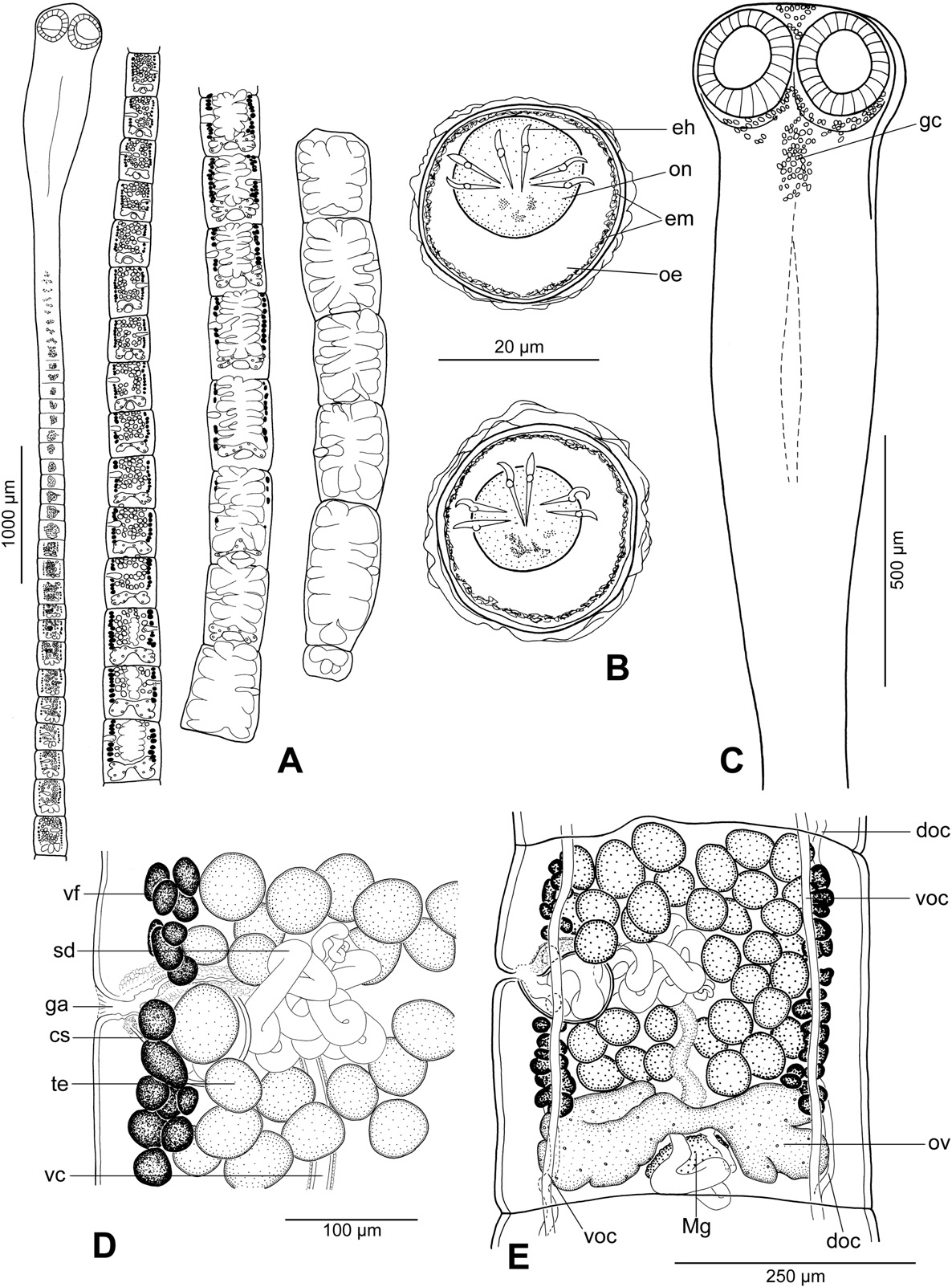
Description (based on 11 whole-mounted specimens and 2 scoleces studied using SEM; for morphometrical data – see Table 2): Proteocephalidae, Proteocephalinae. Testes , vitelline follicles, ovary and uterus medullary. Medium-sized tapeworm, body 9–21 mm long (n = 5), maximum width up to 530 (n = 10). Strobila acraspedote, anapolytic, consisting of 52–58 proglottids: 33–39 immature, 2 mature, 3–12 pregravid; 3–6 gravid proglottids ( Fig. 5 View Fig. 5 A). Immature proglottids and mature proglottids wider than long to longer than wide. Pregravid proglottids rectangular to longer than wide, gravid proglottids longer than wide.
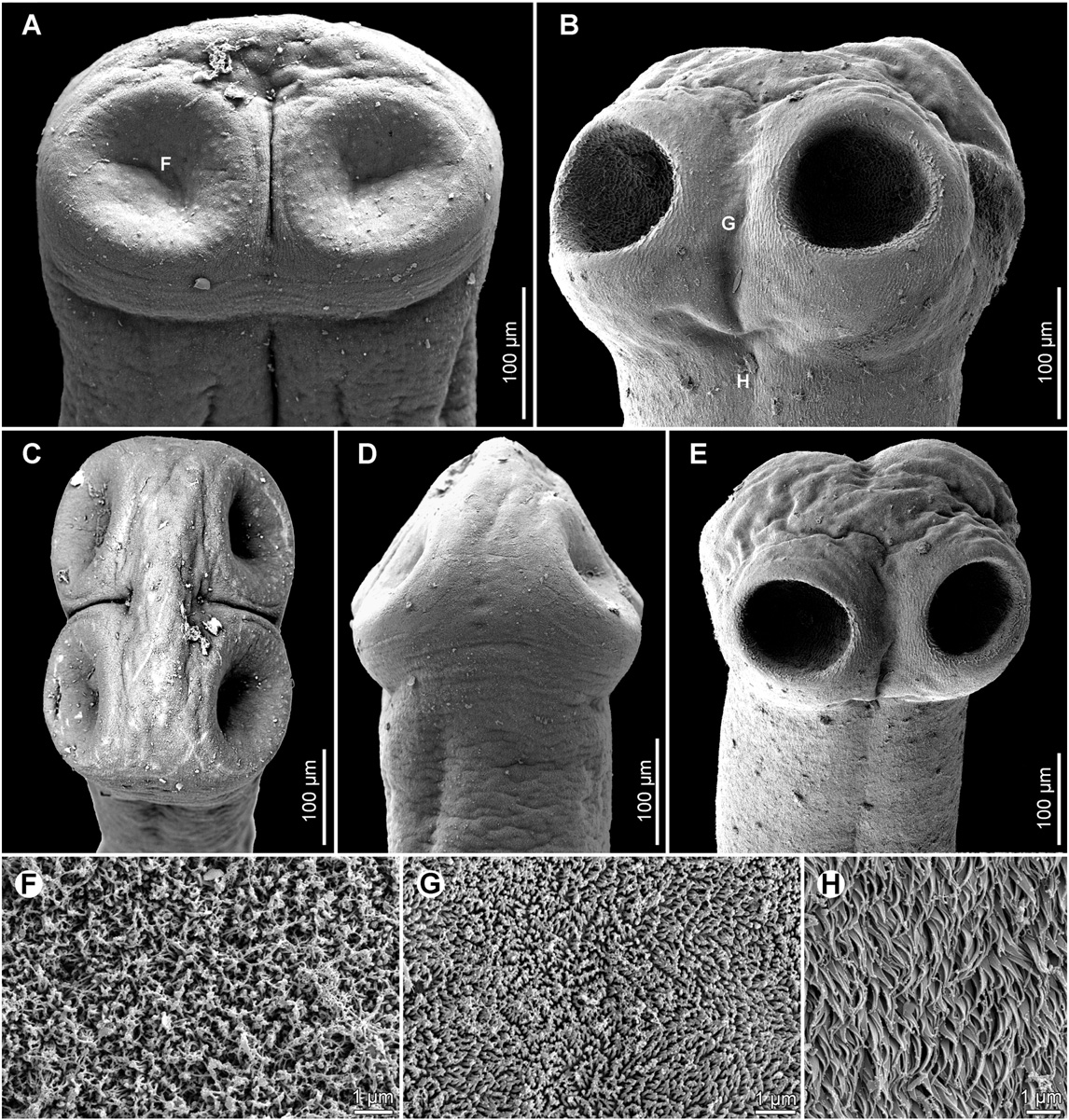
Scolex 250–350 long (n = 4), wider than neck ( Figs. 5 View Fig. 5 C, 6B, E). Suckers spherical, with deep cavity ( Fig. 6 View Fig. 6 B, E). Neck (proliferation zone) widened, 340–470 wide. Scolex covered with acicular filitriches and gladiate spinitriches (on suckers) and acicular filitriches (between suckers — Fig. 6 View Fig. 6 G, and on scolex apex); neck covered with gladiate spinitriches ( Fig. 6 View Fig. 6 H). Inner longitudinal internal musculature well-developed, anastomosed. Ventral osmoregulatory canals thin-walled, 10–12 wide, overlapping lateral bands of vitelline follicles, sometimes anastomosed, slightly sinuous ( Fig. 5 View Fig. 5 E). Dorsal osmoregulatory canals thick-walled, narrow, about 5 wide, difficult to observe in most mature or pregravid proglottids.
Testes 32–44 in number, spherical, small, in two irregular layers ( Fig. 5 View Fig. 5 E), forming one field separated aporally by well-developed sperm duct (vas deferens) ( Fig. 5 View Fig. 5 E). Testes present also in gravid proglottids. Vas deferens strongly coiled, with loops forming elongated field crossing median line of proglottids ( Fig. 5 View Fig. 5 D, E). Cirrus-sac ovoid to almost spherical, thick-walled; sperm duct (internal vas deferens) coiled. Cirrus muscular. Common genital atrium narrow, deep ( Fig. 5 View Fig. 5 D). Genital pores alternating irregularly, pre-equatorial to equatorial.
Ovary medullary, bilobed. Relative size of ovary (see [9]) about 15% of size of pregravid proglottids. Mehlis' gland 60–75 in diameter. Vaginal canal slightly sinuous in distal part; terminal part of vaginal canal (pars copulatrix vaginae) surrounded by chromophilic cells, without sphincter ( Fig. 5 View Fig. 5 D).
Vitelline follicles medullary, forming two long, narrow lateral bands slightly widened posteriorly at ovarian level ( Fig. 5 View Fig. 5 E). Follicles absent at level of cirrus-sac and vagina on ventral side ( Fig. 5 View Fig. 5 E). Posteriorly, follicles may overlap ovary ventrally ( Fig. 5 View Fig. 5 E). Uterus medullary, with development of type 2 according to [9]. Uterus occupies up to 38% of proglottid width in last immature proglottids, up to 87% in pregravid proglottids and up to 92% of gravid proglottids.
Eggs spherical, with hyaline outer envelope and bilayered embryophore; oncosphere containing six embryonic hooks 15–17 long ( Fig. 5 View Fig. 5 B).
Taxonomic summary
Type and only known host: Misgurnus anguillicaudatus (Cantor, 1842) ( Cypriniformes : Cobitidae ).
Site of infection: intestine.
Type locality: River Ilistaya near Chernigovka, Primorsky Region, Russia (44̊26′47″N, 132̊25′20″E).
Prevalence and intensity of infection (mean with range and number of fi sh examined in parentheses): 29% (n = 73); 1.3 (1–3).
Type material: Holotype (one whole-mounted specimen from host field No. RUS 77 collected on 21 June 2011; Coll. No. MHNG-PLAT 79136); two paratypes (whole-mounted specimens from hosts field Nos. RUS 75 and RUS 100 collected on 21 and 22 June 2011, respectively; MHNG-PLAT 79135 and 79142); two paratypes (whole-mounted specimens from host field Nos. RUS 91 and 121 collected on 22 and 23 June 2011, respectively; IPCAS C-728).
Etymology: the specific name is given according to the host's genus.
Differential diagnosis. Proteocephalus misgurni is most similar to P. midoriensis (see below), and differs from P. sagittus and P. demshini , in a similar shape of the body, with the club-shaped scolex conspicuously wider than immature and mature proglottids, the strobila composed of well-separated proglottids that are rectangular (most immature ones) or longer than wide (all remaining proglottids – Fig. 5 View Fig. 5 A), relatively few vitelline follicles that form narrow lateral bands, with posterior follicles overlapping the ovarian wings on the ventral side, a subspherical to almost spherical cirrus-sac, and low number of testes (<50) ( Figs. 2–5 View Fig. 2 View Fig. 3 View Fig. 4 View Fig. 5 ; Table 2).
This new species can be easily distinguished from P. midorensis by (i) its size (it is about twice longer and wider – see Table 2) including a much wider scolex (width 425–535 μm versus 285– 310 μm); (ii) less elongate proglottids (see Figs. 3 View Fig. 3 C and 5E); (iii) longer (horizontally) and narrower (in vertical direction, i.e. height) lateral wings of the ovary versus wings longer than wide in P. midoriensis ( Figs. 3 View Fig. 3 C and 5E); (iv) posterior extent of vitelline follicles, which overlap the ovary ventrally only up to its fifth in this new species (versus up to its half in P. midoriensis ), and width of their lateral bands (bands represent only 10–12% of the width of proglottids in P. misgurni versus 19–22% in P. midoriensis ); and (v) other metrical data such as the diameter of oncospheres, relative size of the suckers and relative position of the genital pore (see Table 2).
Proposal of P.misgurni as a new species is supported by molecular data (see Fig.1 View Fig. 1 , Suppl. Figs. 1 View Fig. 1 , 2 View Fig. 2 , 4 View Fig. 4 , Suppl. Table 1); uncorrected p-distances between this species and its sister species, P.midoriensis , are 1.5% for ssrDNA, 0.6% for lsrDNA and 2.5% for rrnL, respectively (Suppl. Table 1).
This new species seems to be a specific parasite of M. anguillicaudatus , which is native to Siberia (Tugur and Amur drainages), Sakhalin, Korea, Japan, China south to northern Vietnam, but has been introduced to several localities in the River Rhine ( Germany) and Ticino ( Italy, north of Milano) drainages, the Aral Sea basin, North America, Australia and Hawaii. This species proved successful in the aquarium fish trade and at least one country reports adverse ecological impact after introduction [1,2]. The parasite fauna of weather loaches is rather depauperate and cestodes were represented only by species of the caryophyllidean genus Paracaryophyllaeus (see [4]). The present authors and their co-workers (T. Shimazu in Japan and T. Scholz, M. Oros and his Slovak co-workers in China) examined as many as about 500 and 700 weather loaches in Japan and China, respectively, but no proteocephalidean cestodes were found. This indicates that P. misgurni is also endemic to the Primorsky Region of Russia as P. demshini .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.