Apolemia lanosa, Siebert, Stefan, Pugh, Phil R., Haddock, Steven H. D. & Dunn, Casey W., 2013
|
publication ID |
https://doi.org/10.11646/zootaxa.3702.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:E921A12B-1177-4D84-A422-E0EF4C915FF9 |
|
DOI |
https://doi.org/10.5281/zenodo.6152217 |
|
persistent identifier |
https://treatment.plazi.org/id/64678795-FFE1-121B-FF52-0682FD50E22F |
|
treatment provided by |
Plazi |
|
scientific name |
Apolemia lanosa |
| status |
sp. nov. |
Apolemia lanosa View in CoL sp. nov.
Material examined: Doc Ricketts Dive D327-D4, 4 Dec 2011, 35°56'N, 122°55'W, depth 636 m.
Doc Ricketts Dive D329-SS6, 6 Dec 2011, 36°6.99'N, 122°54.01'W, depth 1030 m.
Doc Ricketts Dive D423-D12, 1 Oct 2012, 36°15.02'N, 123°9.98'W, depth 1325 m.
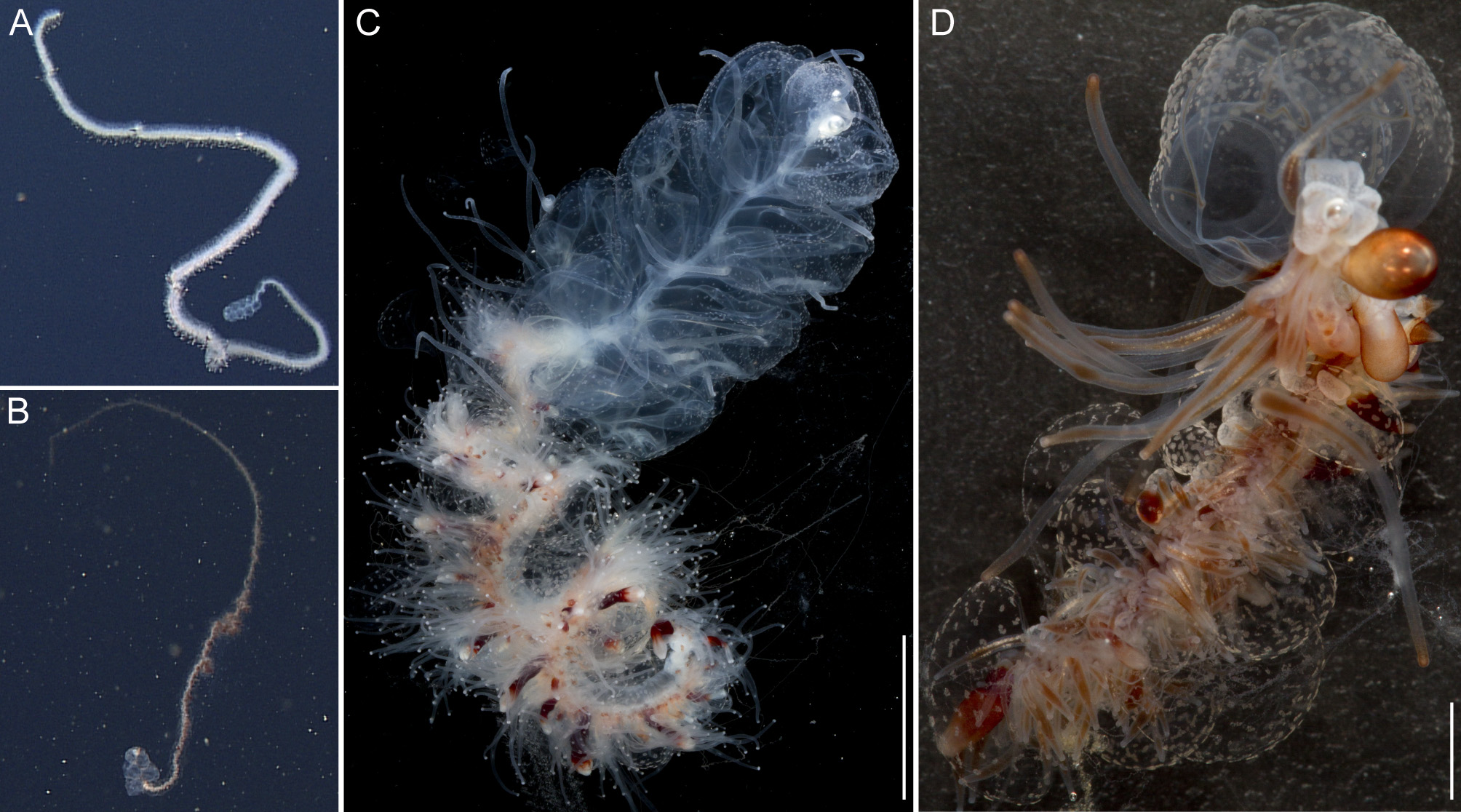
Due to their lengths, only the anterior parts of the specimens D327-D4 and D329-SS6, including the pneumatophore, nectosome, and anterior siphosome, could be collected. A posterior part of the siphosomal stem bearing gonophores was collected for D423-D12. In situ high definition (HD) videos were recorded for specimen D327-D4 and D329-SS6, and are provided as supplementary video (Supplementary video).
Holotype: The specimen D327-D4 has been designated as the holotype and specimens D329-SS6 and D423- D12 as paratypes. All three specimens have been deposited at the National Museum of Natural History, Smithsonian Institution, Washington DC ( USNM 1207944, USNM 1207945, and USNM 1207946, respectively). All of the following observations were made on the holotype and on living tissue, unless explicitly stated otherwise.
Diagnosis. Large nectophores with patches of nematocysts covering their upper and upper-lateral sides. Refractile cells scattered in and around these patches. No distinct diverticula on lateral radial canals. Number of nectosomal palpons per nectophore increasing in posterior direction, with an observed maximum of four. Bracts with similar patches and refractile cells on distal two-thirds of upper surface. Siphosomal growth zone with inconspicuous horn. Cormidia dispersed, each with one type of palpon. Cormidia with one primary gastrozooid and an increasing number of secondary gastrozooids in more posterior parts of the colony.
General appearance: The orange-white tinge of the siphosome, with deep red gastrozooids, contrasted with the complete lack of color in the nectosome ( Fig. 2 View FIGURE 2 A,C). The zooids of the siphosome were densely packed, without any obvious bare stem between them. The bracts were conspicuous. Bracts, gastrozooids and numerous palpons gave the siphosome a relatively thick and well-defined fleece-like outline (Supplementary Video).
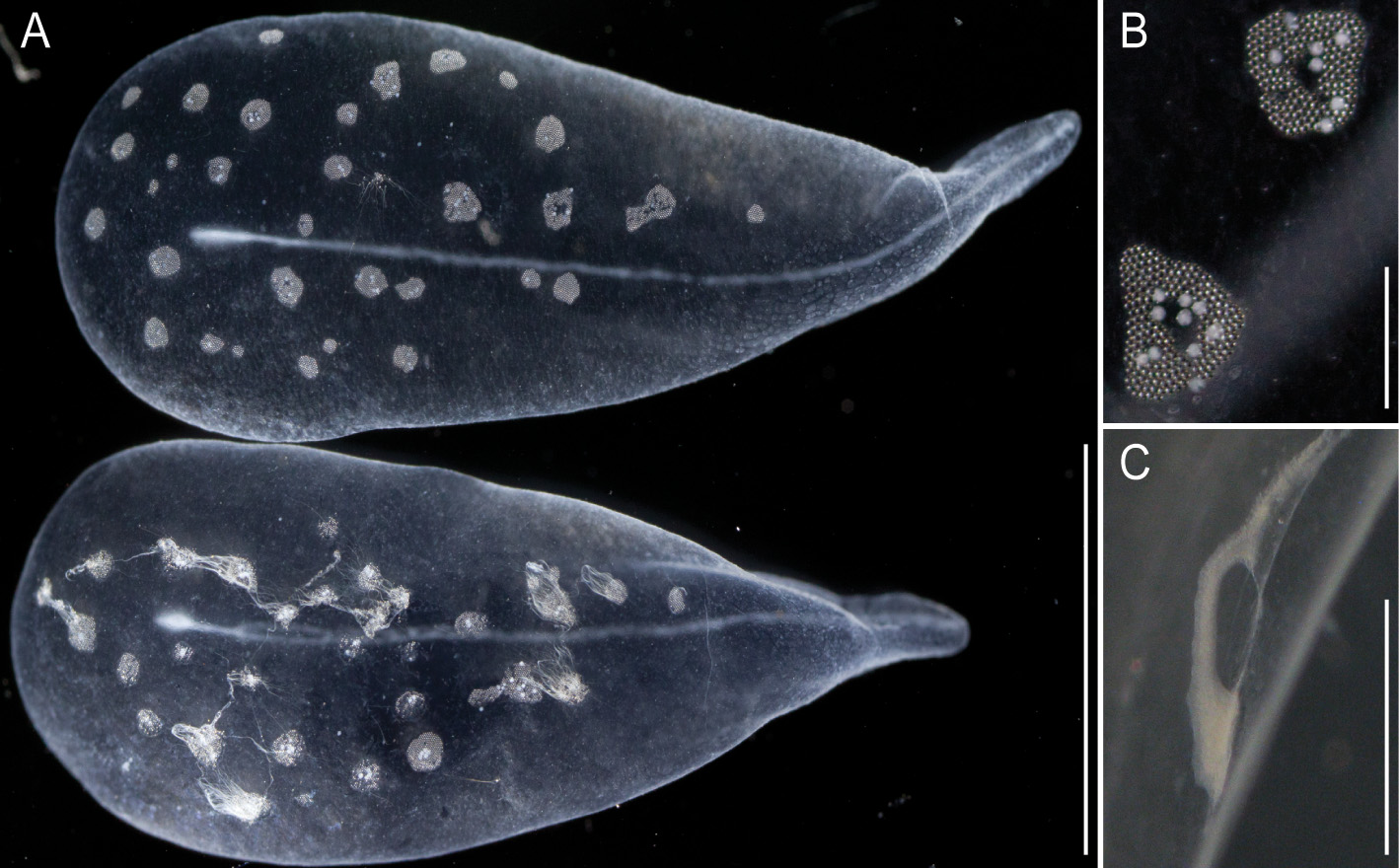
Pneumatophore: The pneumatophore of the holotype specimen was ovoid, 2.2 mm in height and 1.4 mm in maximum diameter, with a silvery appearance and no obvious pigmentation ( Fig. 3 View FIGURE 3 A).
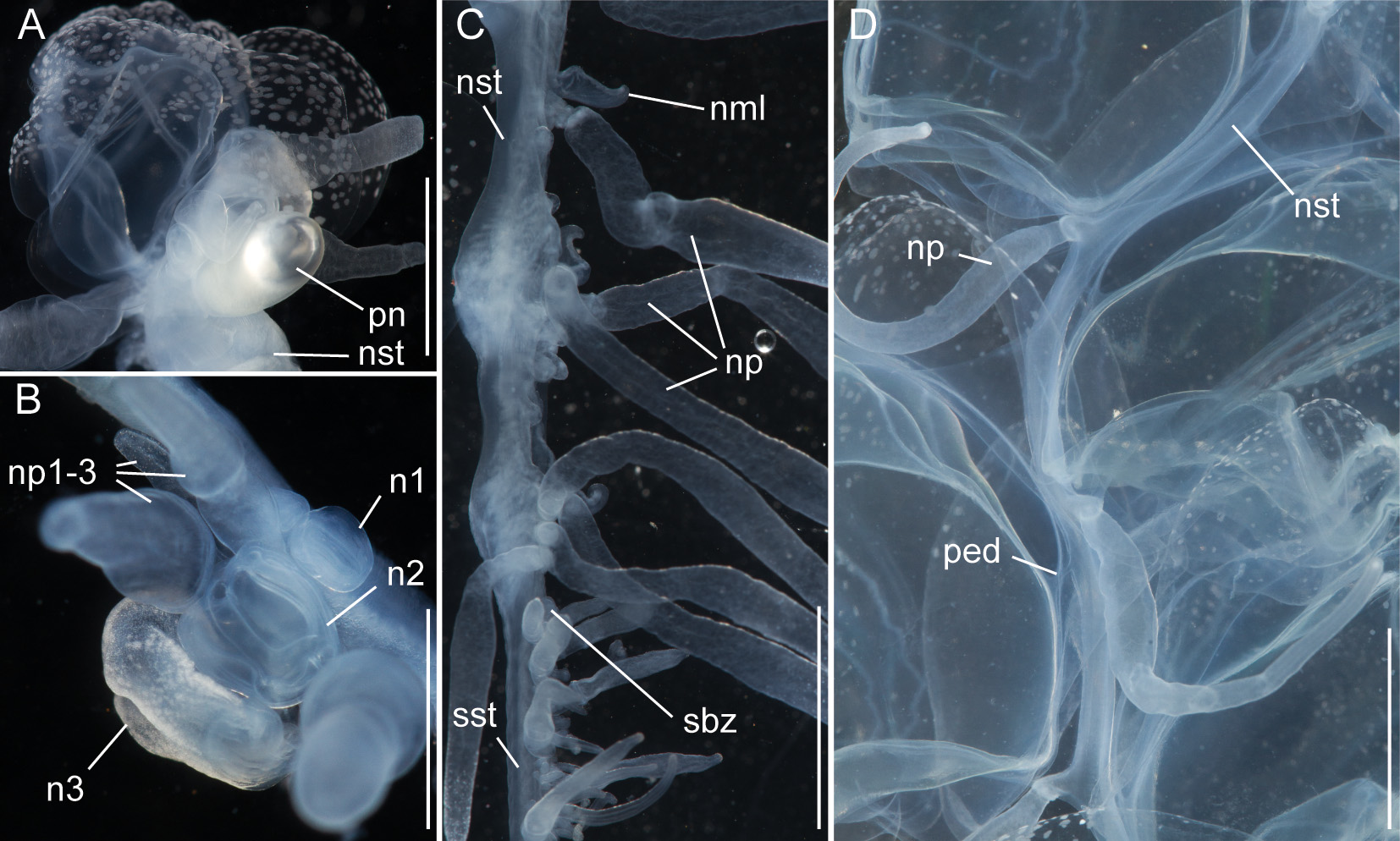
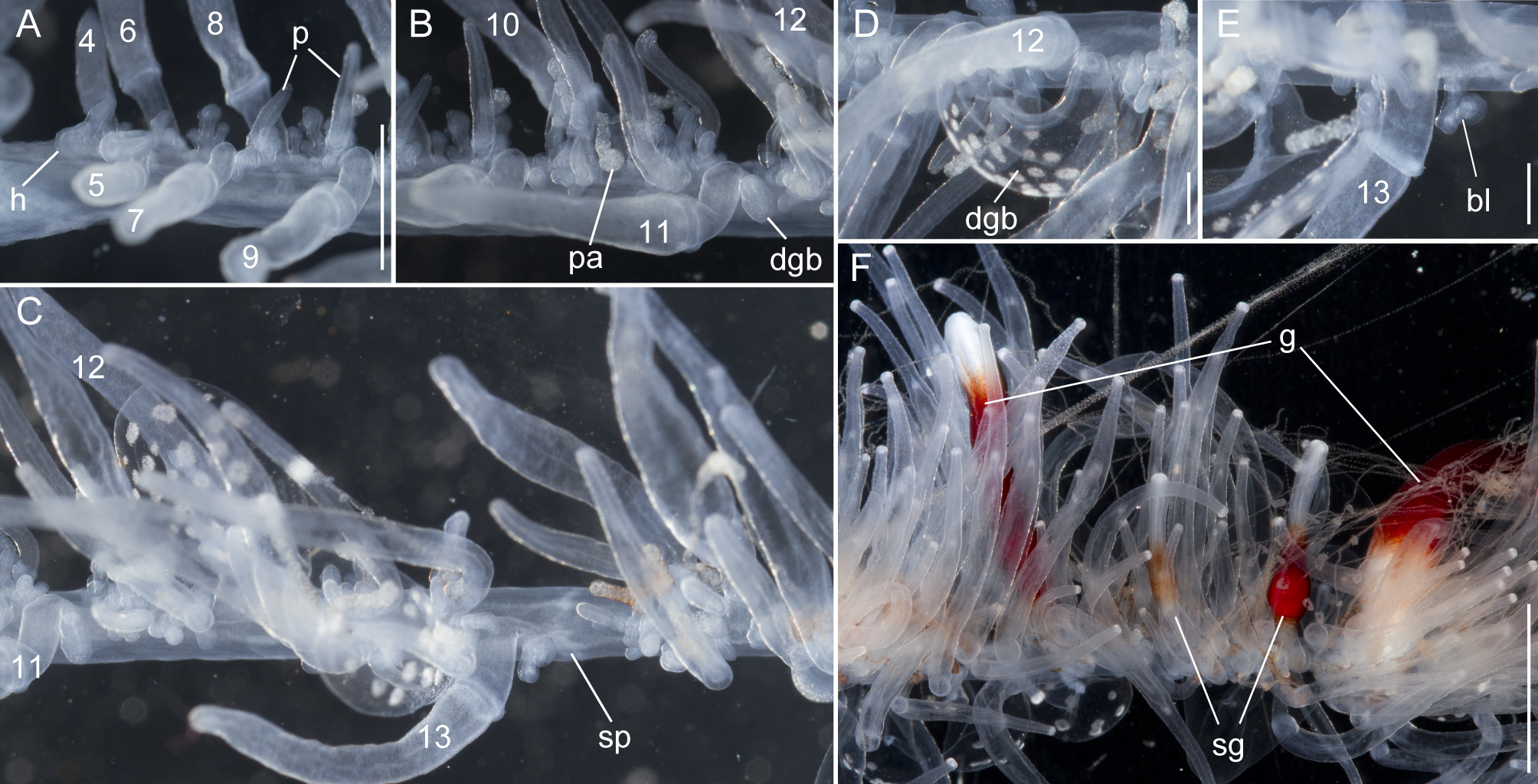
Nectosome: The colorless nectosome of the holotype measured 6 cm in length after relaxation ( Figs. 2 View FIGURE 2 C, 3). The nectophores were attached, in a single line, on the ventral side of the stem. In vivo the nectosomal stem was twisted. A 180º turn of the stem between two adjacent nectophores resulted in a biserial arrangement of nectophores ( Fig. 3 View FIGURE 3 D). Fourteen attachment lamellae were found on the nectosome of the holotype, including the ones with nectophores still attached. The youngest nectophores, toward the anterior end of the nectosome, were each accompanied by a single nectosomal palpon ( Fig. 3 View FIGURE 3 B). Further to the posterior, with the increasing age of the nectophores, the number of palpons per nectophore increased, such that there were four associated with the oldest nectophore of the holotype ( Fig. 3 View FIGURE 3 C). The nectosomal palpons lacked a palpacle and were attached directly to the stem to the posterior end and, in ventral view, left of the nectophore lamella. In a relaxed state these palpons were about three times as long as the associated nectophore. Nematocysts, occurring as small opaque spots, were scattered over the surface of the palpon, but were mainly concentrated in its distal region.
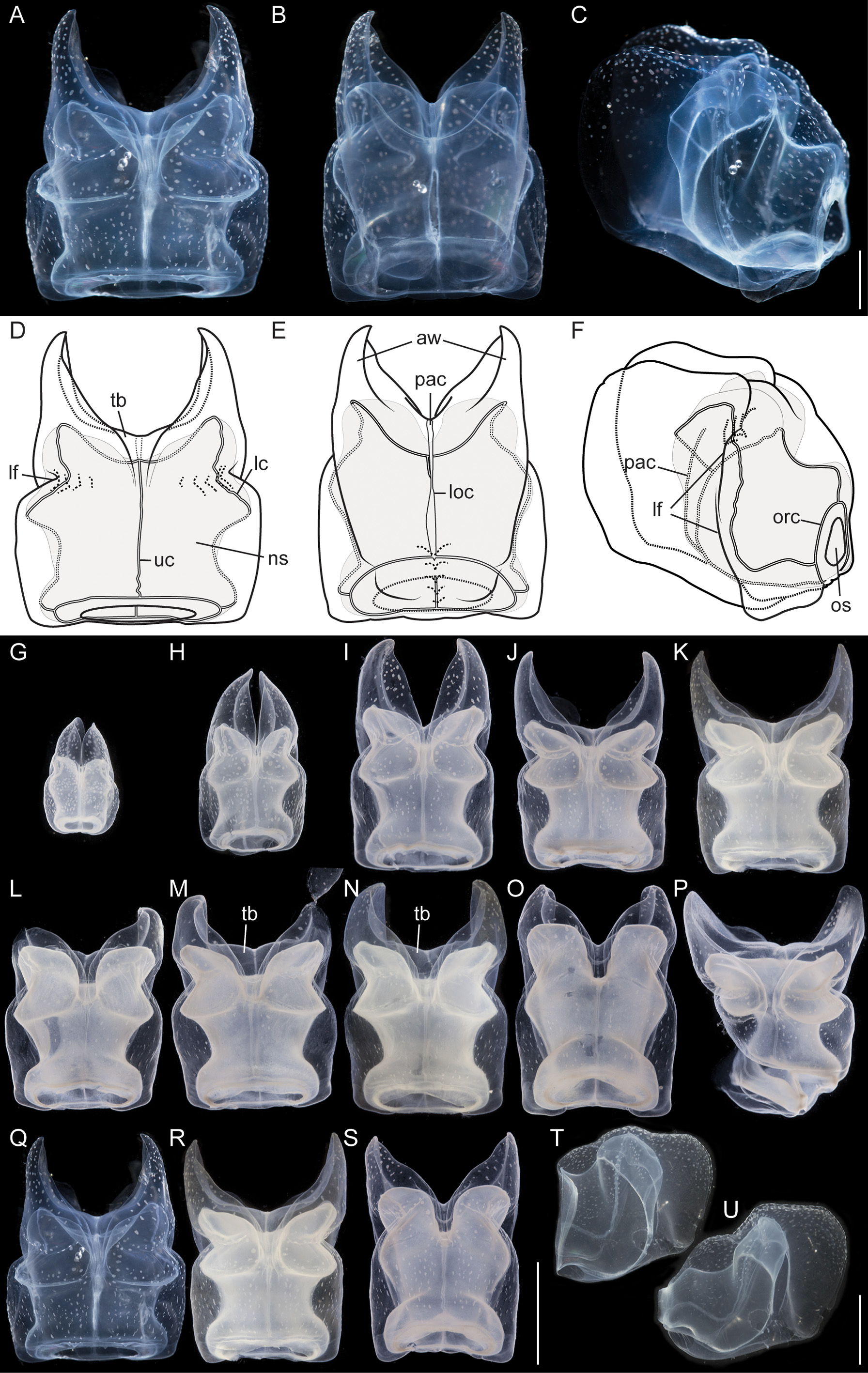
Nectophores: No sign of any pigmentation was seen on the nectophores ( Figs. 2 View FIGURE 2 C, 3A, 4A–C, 4G–U). The largest nectophore of the holotype specimen had a length of 21.4 mm and a width of 17.6 mm ( Fig. 4 View FIGURE 4 P). Living and fixed nectophores differed in appearance. In general, furrows tended to be more pronounced in fixed tissue. The appearance of a fixed nectophore varied with the presentation in the dish (compare Figs. 4 View FIGURE 4 R, 4S). In vivo, the state of contraction influenced nectophore appearance, which in consequence might influence the resulting nectophore morphology at the time point of fixation (compare Figs. 4 View FIGURE 4 T, 4U). The youngest nectophores (n1–2, Fig. 3 View FIGURE 3 B) within the growth zone had very prominent canals and a translucent epidermis, while older ones (n3) were slightly opaque. The upper and upper lateral surfaces of the latter were densely covered with nematocysts, which were organized into defined patches in older nectophores ( Figs. 3 View FIGURE 3 A, 4A–C, 4G–U). The distance between these patches was correlated with the size of the nectophore ( Figs. 4 View FIGURE 4 G–P). This suggests that the patches have a similar total area through the course of maturation, and that the patches become further separated as the nectophore grows. On the axial wings of more mature nectophores these patches tended to be more rounded, while those on the main body of the nectophore were more elongate ( Figs. 4 View FIGURE 4 A–C). In addition, refractile cells were sparsely scattered between and within these patches of nematocysts.
The large, flared axial wings occupied about one third the total length of the younger nectophores and about one fourth of the mature ones ( Figs. 4 View FIGURE 4 G–P). The central thrust block was very narrow in early nectophore development and well developed in mature nectophores ( Fig. 4 View FIGURE 4 G–P). A broad, relatively shallow lateral furrow extended almost vertically up each side of the nectophore, from the lateral margins of the lower surface to the upper surface. In most of the nectophores it reached the inner, upper margins of the axial wings ( Figs. 4 View FIGURE 4 A,D, 4G–P). On the lower surface of the nectophore, i.e. that which is closest to the stem, the lower margins of the axial wings began to approach each other from above the level of the proximal side of the nectosac, but each petered out well short of the mid-line ( Fig. 4 View FIGURE 4 B,E). There was a pair of small, slightly thickened cushions of mesoglea on the lower surface of the nectophore that delineated a shallow lower furrow, overlying the lower radial canal. The nectosac mostly filled the main body of the nectophore ( Fig. 4 View FIGURE 4 ). It was broadest just above the ostium and at about half of its length.
No descending pallial canal was present. From its origin from the pedicular canal, the ascending pallial canal ascended towards the top of the thrust block and ended at about two thirds of the total height of the nectosac ( Fig. 4 View FIGURE 4 C,F). The lower radial canal was frequently torn in the region where it ran in close contact with the lower part of the muscular attachment lamella, after which it passed along the base of the lower groove to reach the ostial ring canal ( Fig. 4 View FIGURE 4 B,E).
The upper radial canal gave rise symmetrically to the lateral radial canals close to the mid-height of the proximal side of the nectosac ( Fig. 4 View FIGURE 4 E,F). It then continued over onto the upper side of the nectosac and to the ostial ring canal. Although straight for the most part, just above the ostium it had a series of small bends ( Fig. 4 View FIGURE 4 D). The lateral radial canals, from their point of origin, curved away from the midline and upwards to the apico-lateral margins of the nectosac and passed, slightly undulating, over onto the upper surface before curving outwards to run obliquely downwards toward the lower surface of the nectosac, but curving upwards again before reaching that surface and then bending to run directly to the ostium, reaching it at about half its height ( Fig. 4 View FIGURE 4 D, F). No distinct diverticula could be observed on the lateral canals but occasionally small protrusions were observed.
Siphosome: The full length of the siphosome prior to collection was about 2m in the state of relaxation as pictured in Fig. 2 View FIGURE 2 A. After relaxation, the siphosome of the partial holotype fragment that was collected measured 12 cm in length ( Fig. 2 View FIGURE 2 C). The stem and the attached palpons were milky-white, while the basal region of the palpacles had a faint red tinge. The body columns of the gastrozooids were of deep red color.
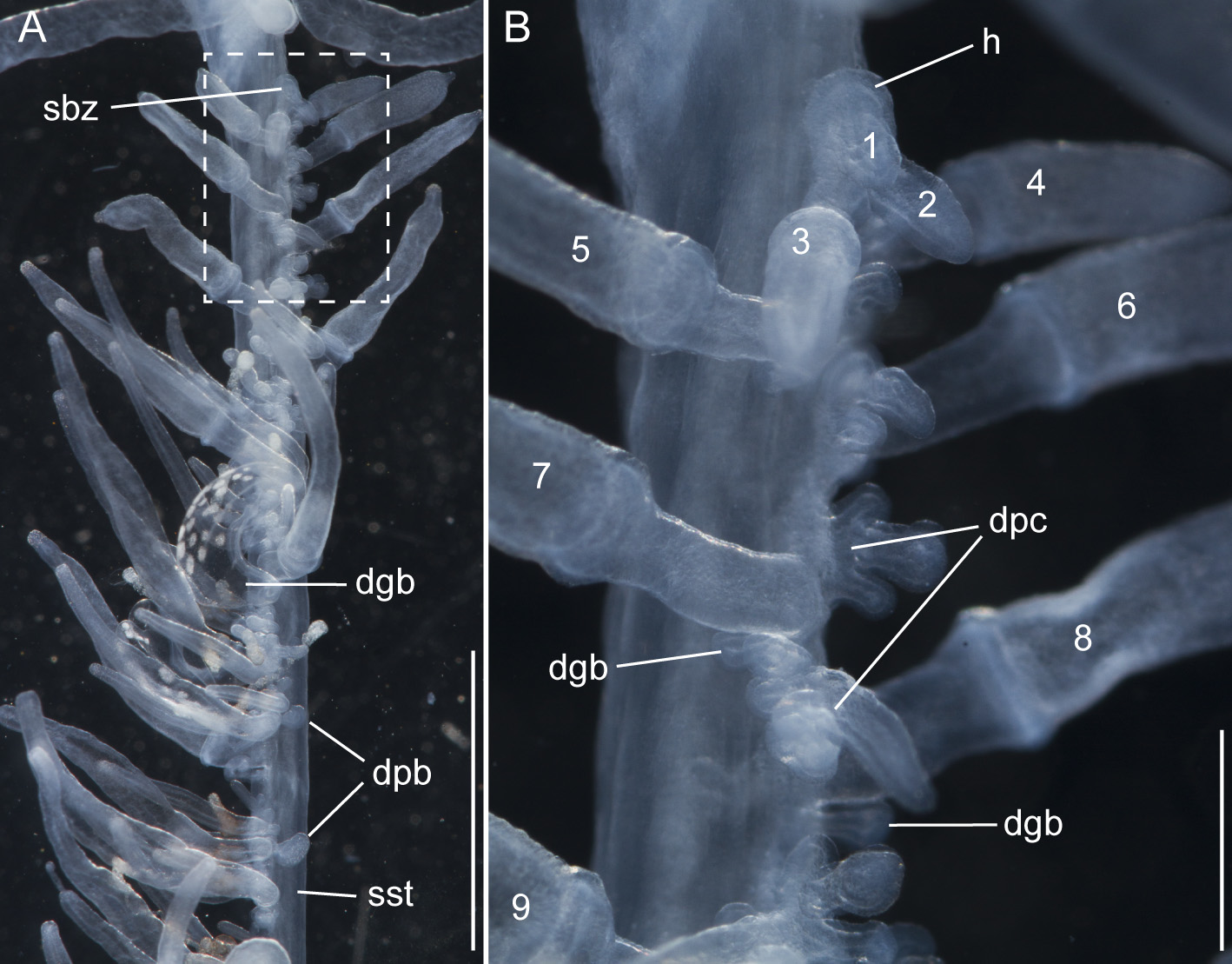
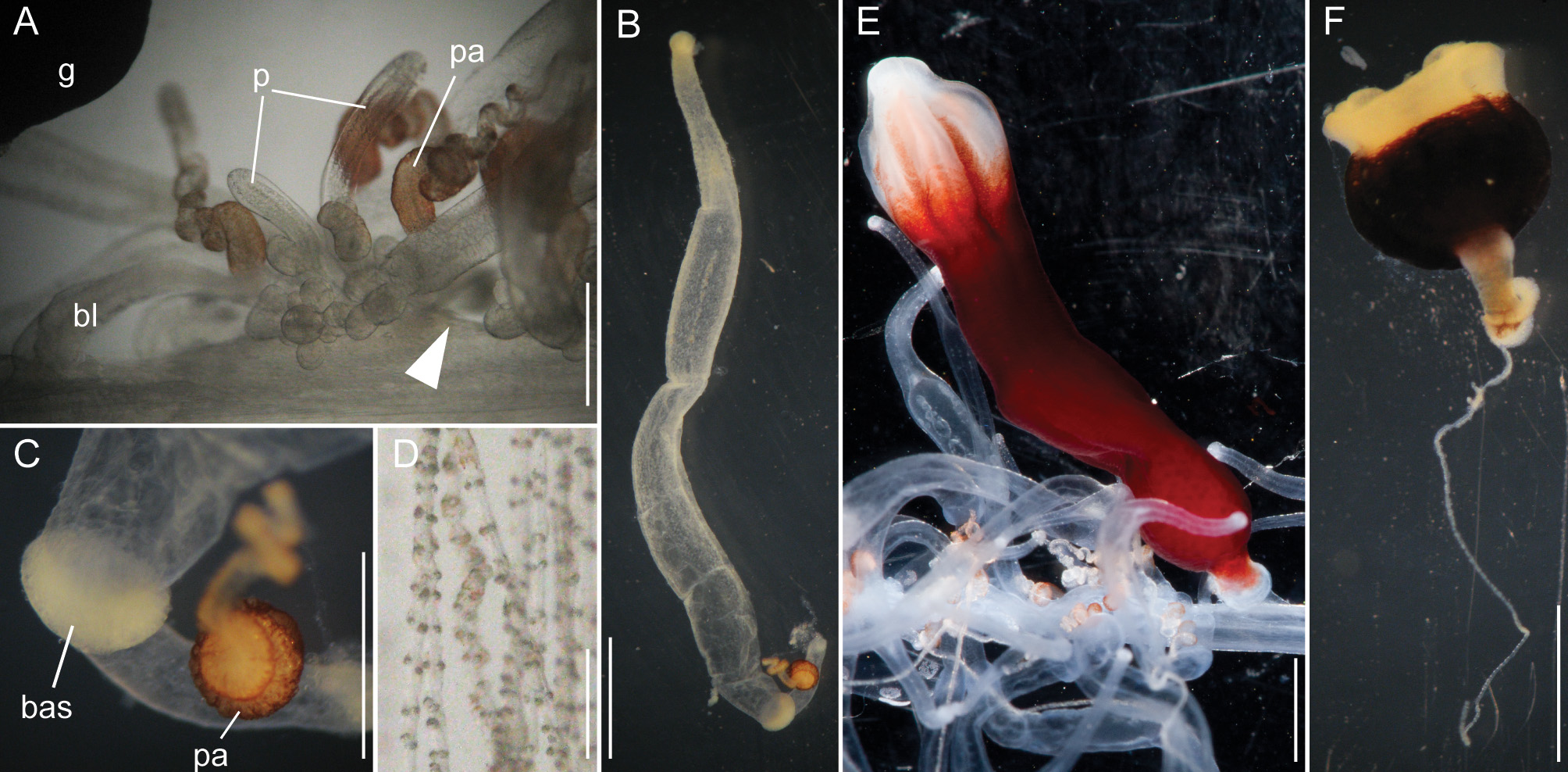
Siphosomal growth zone and early zooid development: The siphosomal growth zone had an inconspicuous horn that pointed in the anterior direction ( Figs. 5 View FIGURE 5 , 6 View FIGURE 6 A). Gastrozooid buds originated on the horn. Gastrozooid morphology, with a peduncle, ring-like basigaster, and proboscis was apparent at very early stages ( Fig. 5 View FIGURE 5 B). These primary gastrozooids were alternately displaced to the left and right slightly off the ventral midline resulting in a biserial organization that was most conspicuous in the youngest cormidia ( Fig. 5 View FIGURE 5 B). To the anterior of the gastrozooid peduncle, attached directly to the siphosomal stem, was a compound palpon bud. Each compound palpon bud gave rise to a palpon cluster. Key aspects of palpon morphology were in place by cormidium eight ( Fig. 5 View FIGURE 5 B). Developing palpacles had an opaque appearance ( Fig. 6 View FIGURE 6 B), first of a yellowish coloration, while older palpacles showed an intense orange at their proximal ends ( Fig. 6 View FIGURE 6 C, Fig. 8 View FIGURE 8 A,E). In the case of the gastrozooids, no tentacle formation could be observed during early zooid development. Palpacle formation, therefore, preceded gastrozooidal tentacle formation. Bract formation was first observed in association with gastrozooids ( Fig. 6 View FIGURE 6 B). Each gastrozooid was accompanied by a single bract attached to the ventral midline just to the posterior of the gastrozooid ( Fig. 6 View FIGURE 6 B). This gastrozooidal bract marked the posterior end of each cormidium. A stem sphincter is located just posterior to the gastrozooidal bracts ( Fig. 6 View FIGURE 6 C). Removal of the gastrozooidal bract left a curled attachment lamella ( Fig. 6 View FIGURE 6 D,E). A second type of bract was associated with the palpons. These were located laterally. The first of these lateral bracts to form arose toward the posterior end of each palpon clusters. As the palpon clusters grew, more bracts were added in the anterior direction. In older parts of the colony new secondary gastrozooids formed in between primary gastrozooids ( Fig. 6 View FIGURE 6 F) indicating that the distance between the latter increased with the age of the cormidium. The addition of new zooids, therefore, occurred along the siphosomal stem and was not restricted to the siphosomal growth zone.
Bracts: As the ROV approached, the colony rapidly began to autotomize its mature bracts, and so it was difficult to document their in vivo arrangement. In addition, a large fraction of the bracts were lost during the sampling procedure. Thus, by the time the colony was examined, few mature bracts remained attached directly to the siphosomal stem. Developing gastrozooid and palpon lateral bracts were very similar, though they became more distinct as they matured. Mature gastrozooid bracts were very elongate and straight without an obvious keel ( Fig. 7 View FIGURE 7 A). Mature palpon bracts tended to be more ovoid in shape and more curved, and they tapered to form a more rounded proximal end on the upper surface. In this case the mesoglea was generally thicker and, on the lower surface, it bulged out slightly to form a small, but distinct, keel. The bracteal canal arose from the proximal end of the bract. Nematocyst patches were scattered over the distal two-thirds of the upper side of the bracts ( Fig. 7 View FIGURE 7 A). As observed in the case of nectophores individual large refractile cells could be found scattered between and within these nematocyst patches ( Fig. 7 View FIGURE 7 B). In all bracts the bracteal canals extended distally along the lower surface in the mid-line. At their distal ends they bent into the mesoglea and then back towards the lower surface ( Fig. 7 View FIGURE 7 A,C). However, the distinctiveness of this arch varied considerably, and it was in many cases difficult to detect.
Palpons: Only a single type of siphosomal palpon was observed. The maximum length of the palpon was 22 mm when fixed. In vivo, however, palpons could reach twice the length of gastrozooids ( Fig. 2 View FIGURE 2 C). Palpons were slightly opaque and of a milky-white color, with a more opaque, white region at their distal ends ( Figs. 2 View FIGURE 2 C, 6F). In older cormidia, new palpon cluster formation was inferred at the anterior end of each cormidium ( Fig. 8 View FIGURE 8 A) based on differences in the sizes of palpons. The individual palpon clusters were clearly separated from each other on the siphosomal stem ( Fig. 8 View FIGURE 8 A) even though this separation was not obvious when the specimen was observed macroscopically. The very proximal part of the palpacle was tinged orange, while distally it was a translucent white ( Fig. 8 View FIGURE 8 A–D). Particularly in fixed material a ring-like basigaster region of the palpon became obvious just distal to the palpacle attachment point ( Fig. 8 View FIGURE 8 C). Developing nematocysts were discerned in this region. Proximally, nematocysts were densely packed on one side of the palpacle, but more distally they were separated into pairs and continued as such along the remainder of its length ( Fig. 8 View FIGURE 8 D).
Gastrozooids: The maximum length of fixed gastrozooids was 18 mm. The peduncles of the mature gastrozooids were short, translucent and did not bear other zooids ( Fig. 8 View FIGURE 8 E). The proximal portion of the gastrozooid was deep red in live animals ( Fig. 8 View FIGURE 8 E). Gastrozooids had a ring-like basigaster just distal to the peduncle, where developing nematocysts were found. The distal end of the gastrozooid was milky-white. Stripes were visible in many cases, which were reflected by ectodermal grooves in the distal parts of the gastrozooids ( Fig. 8 View FIGURE 8 E). The diminutive gastrozooid tentacles were not conspicuous in macroscopic images, and were only apparent in fixed gastrozooids that were separated from the stem ( Fig. 8 View FIGURE 8 F). As in other apolemiids, they bore no tentilla.
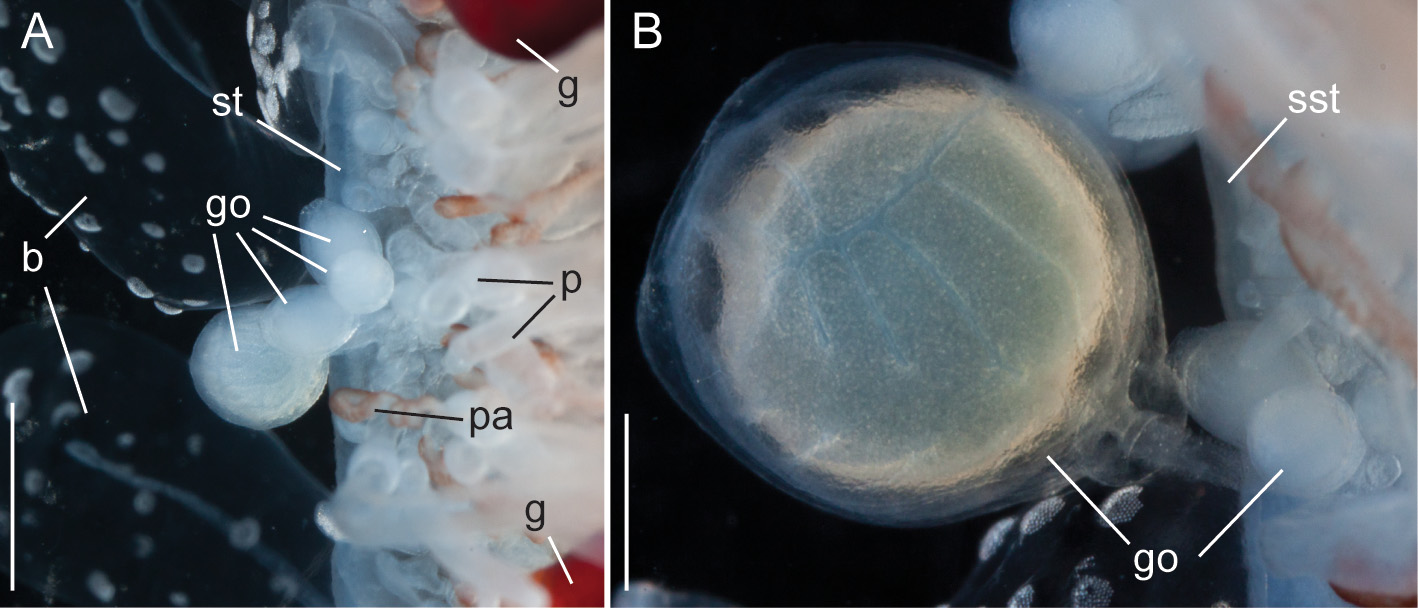
Gonodendra: Female gonophores were found along the siphosomal stem of specimen D423-D12 ( Fig. 9 View FIGURE 9 ). Gonodendra inserted directly on the siphosomal stem laterally to palpon clusters. There were several gonophores in different stages of maturation per gonodendron. Each gonophore was attached to the stalk of the gonodendron via a peduncle ( Fig. 9 View FIGURE 9 B). The stalks of the gonodendra were small and inconspicuous. Each gonophore contained a single egg.
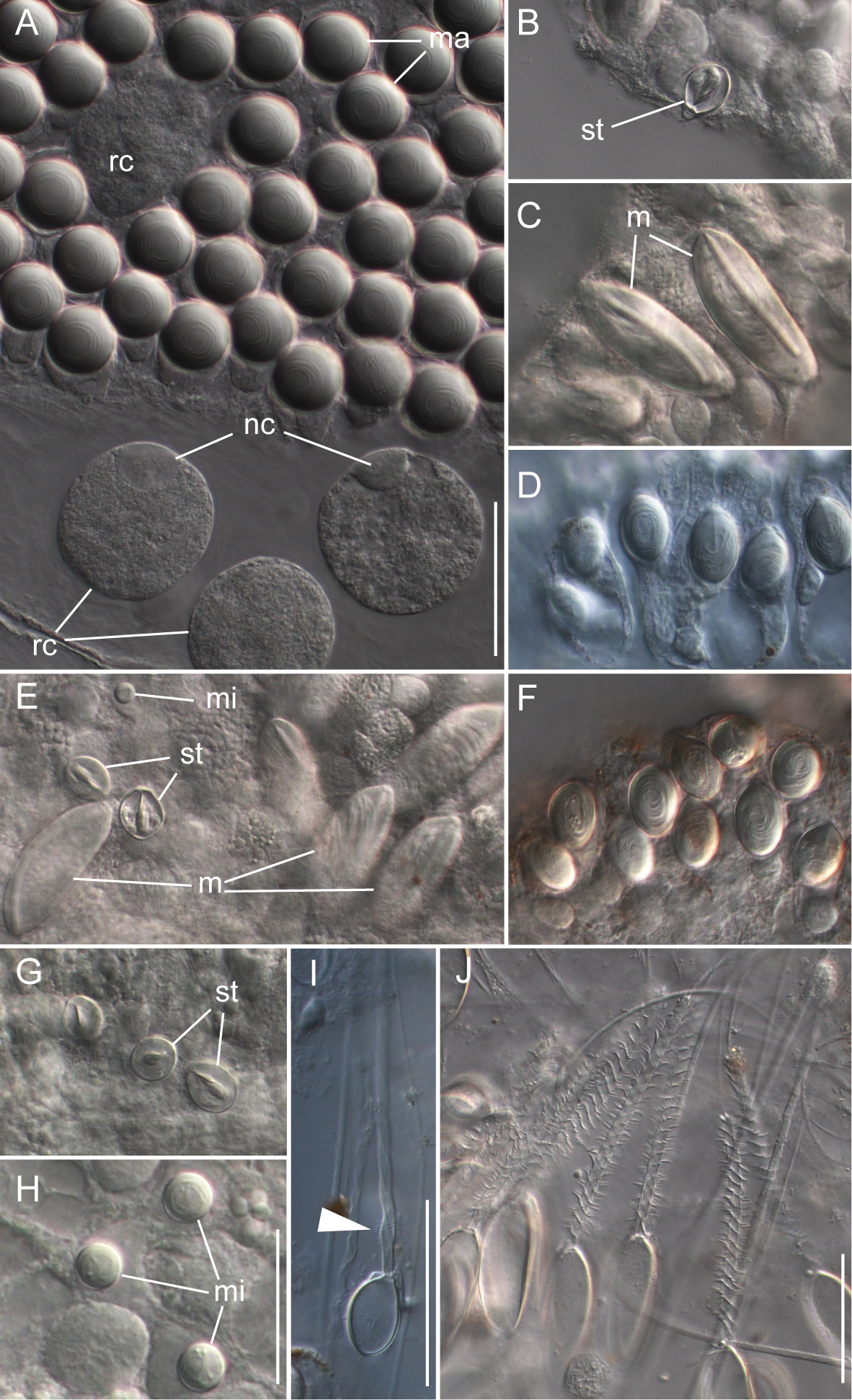
Nematocyst complement: Four types of nematocysts were documented across the different zooids of the colony ( Fig. 10 View FIGURE 10 ). These were:
a) Spherical isorhizas of two distinct sizes.
Macroisorhizas, with a mean diameter of 22.2 µm ( Fig. 10 View FIGURE 10 A). The evaginated tubule was isodiametric and holotrichous. These nematocysts formed the patches on nectophores and bracts. Spherical refractile spots (mean diameter 47.2 µm, n=12) present in between and within patches were cellular, as indicated by the presence of nuclei ( Fig. 9 View FIGURE 9 A).
Microisorhizas, 7.2 µm (mean) in diameter ( Fig. 10 View FIGURE 10 E,H) that were found at the distal ends of gastrozooids, palpons and nectophoral palpons, and were scattered along the body columns of these zooids in lower numbers.
b) Stenoteles, with a mean length of 17.9 µm and a mean width of 13.7 µm ( Fig. 10 View FIGURE 10 B,E,G). These were found in the same locations as the microisorhizas and were highly abundant at the distal ends of the three types of zooid.
c) Microbasic mastigophores ( Fig. 10 View FIGURE 10 C,E) could be found at the tips of gastrozooids, palpons and nectophoral palpons with densities being highest on gastrozooids and palpons. They were elongate (mean length 57.8 µm, mean width 21.9 µm) and slightly banana-shaped.
d) The capsules of tentacles and palpacles were ovoid in shape with a mean length of 22 µm and a mean width of 14.6 µm ( Fig. 10 View FIGURE 10 I). They were found on the palpacles and gastrozooidal tentacles. The evaginated tubule was isodiametric and holotrichous with a single dilation at the end of the shaft close to the distal end of the capsule. This was in contrast to the two dilations in birhopaloids as described for Apolemia uvaria ( Claus 1863; Totton 1965).
Distribution. The holotype and paratype specimens, described above, both came from the vicinity of Monterey Bay, California. Several other specimens from the same region have either been collected or identified from in situ photographs, as listed in Table 1 View TABLE 1 . The mean depth for all these specimens is 1193 ± 285.7 m. The species has also been pictured in Burton and Lundsten (2008) at the Davidson Seamount over a depth range 439– 1159 m. Photographic material strongly suggests that A. lanosa has been collected off Japan ( Lindsay 2005, 2006). The probability that there are further records is discussed below.
Etymology. The specific name lanosa , derived from the Latin lana, meaning woolly, refers to the fleece-like appearance of the siphosome of the living colony.
| USNM |
Smithsonian Institution, National Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |