Apolemia rubriversa, Siebert, Stefan, Pugh, Phil R., Haddock, Steven H. D. & Dunn, Casey W., 2013
|
publication ID |
https://doi.org/10.11646/zootaxa.3702.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:E921A12B-1177-4D84-A422-E0EF4C915FF9 |
|
DOI |
https://doi.org/10.5281/zenodo.6152219 |
|
persistent identifier |
https://treatment.plazi.org/id/64678795-FFE9-1204-FF52-00E0FD49E1C7 |
|
treatment provided by |
Plazi |
|
scientific name |
Apolemia rubriversa |
| status |
sp. nov. |
Apolemia rubriversa View in CoL sp. nov.
Material examined: Doc Ricketts Dive D331-D4, 0 7 Dec 2011, 36°41.99’N, 122° 5.99’W, depth 650m.
Doc Ricketts Dive D195-D5, 0 7 Oct 2010, 36º 35.97’N, 122º 8.928’W, depth 780m.
Ventana Dive V 908, 20 June 1995, 36° 42.34’N, 122° 02.24’W, depth 609m.
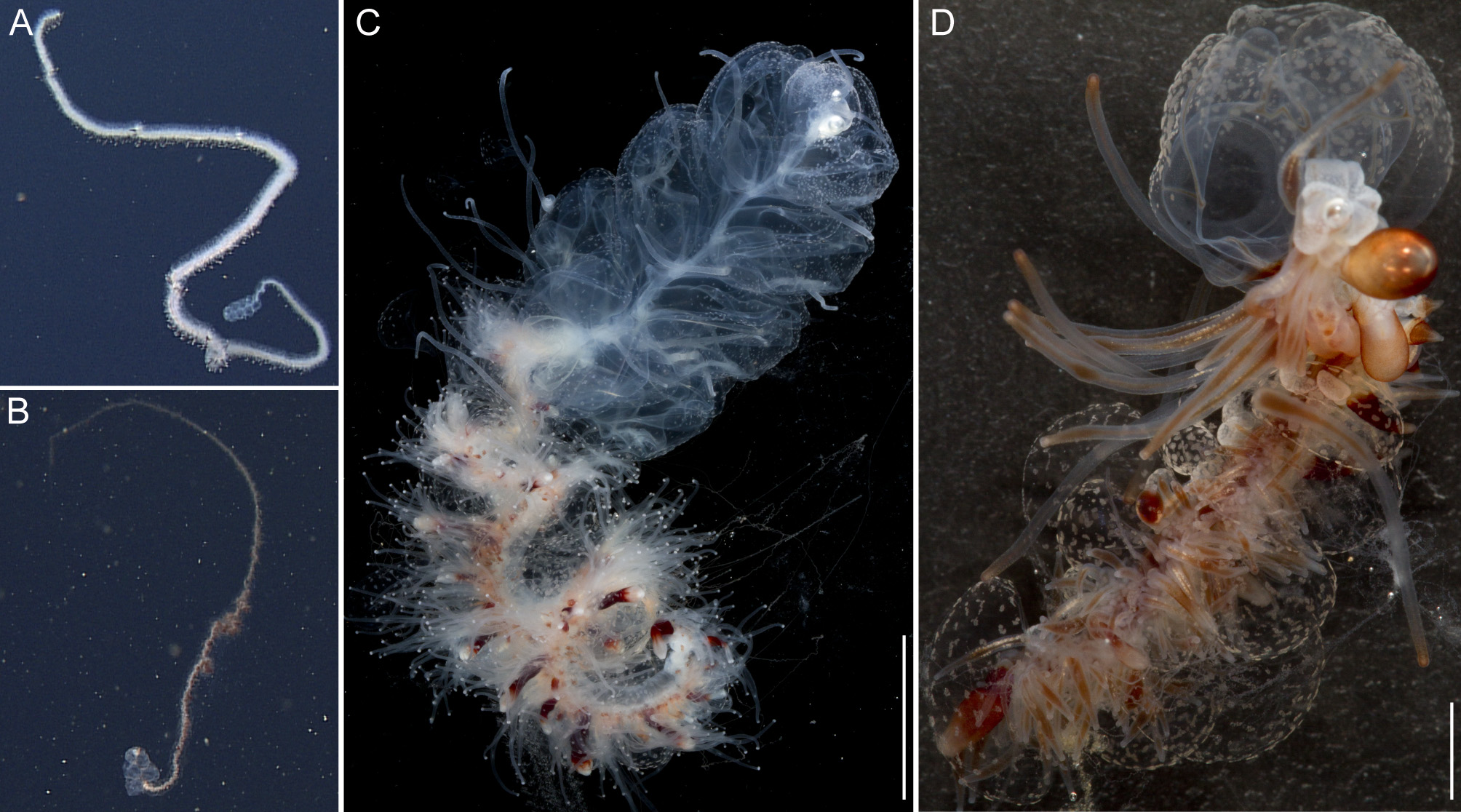
Only the anterior parts of the colonies, including the pneumatophore, nectosome, and anterior siphosome were collected. In situ high-definition videos were recorded for both Doc Ricketts specimens (Supplementary video, for Doc Ricketts D311-D 4 specimen). The following observations were made on the holotype and on live tissue if not stated otherwise.
Holotype: The specimen from Doc Ricketts dive D311-D4 has been designated as the holotype. The specimens from Doc Ricketts dive D195-D5 and Ventana Dive V 908 have been designated as paratypes. All three specimens have been deposited at the National Museum of Natural History, Smithsonian Institution, Washington DC ( USNM 1207947, USNM 1207948 and USNM 1207949, respectively).
Diagnosis. Nectophores, with upper and upper lateral surfaces densely covered in patches of nematocysts. Refractile cells sparsely scattered between, but not within, these patches. Lateral radial canals with up to three distinctive diverticula extending along the wall of the nectosac and not penetrating into the mesoglea. Two median patches of red pigmentation present on the lower surface; one in the vicinity of the thrust block and the other, more prominent, one in the ventral groove.
Siphosomal growth zone with pronounced horn. Cormidia pedunculate. All siphosomal zooids borne on the peduncle of the gastrozooid, with naked stem between them. The cormidia with distinct biserial arrangement and peduncles attach left and right from the midline. Upper surface of bract entirely covered with patches of nematocysts, with refractile cells present outside of nematocyst patches predominately at the margins of the upper bract surface. Bracteal canal usually with distinct diverticula penetrating into the mesoglea. One type of palpon.
General appearance: The overall appearance of the colony was brown-red in color. This was mainly caused by colored gastrovascular fluid within the gastric system ( Fig. 2 View FIGURE 2 B,D). The bracts were nearly transparent and gave the siphosome a ragged appearance rather than a well-defined outline ( Fig. 2 View FIGURE 2 B,D).
Pneumatophore: The pneumatophore of the holotype was oval, approximately 3.3 mm in height and 1.7 mm as maximum diameter ( Fig. 11 View FIGURE 11 A). The gas-filled pneumatosaccus was covered with orange pigment.
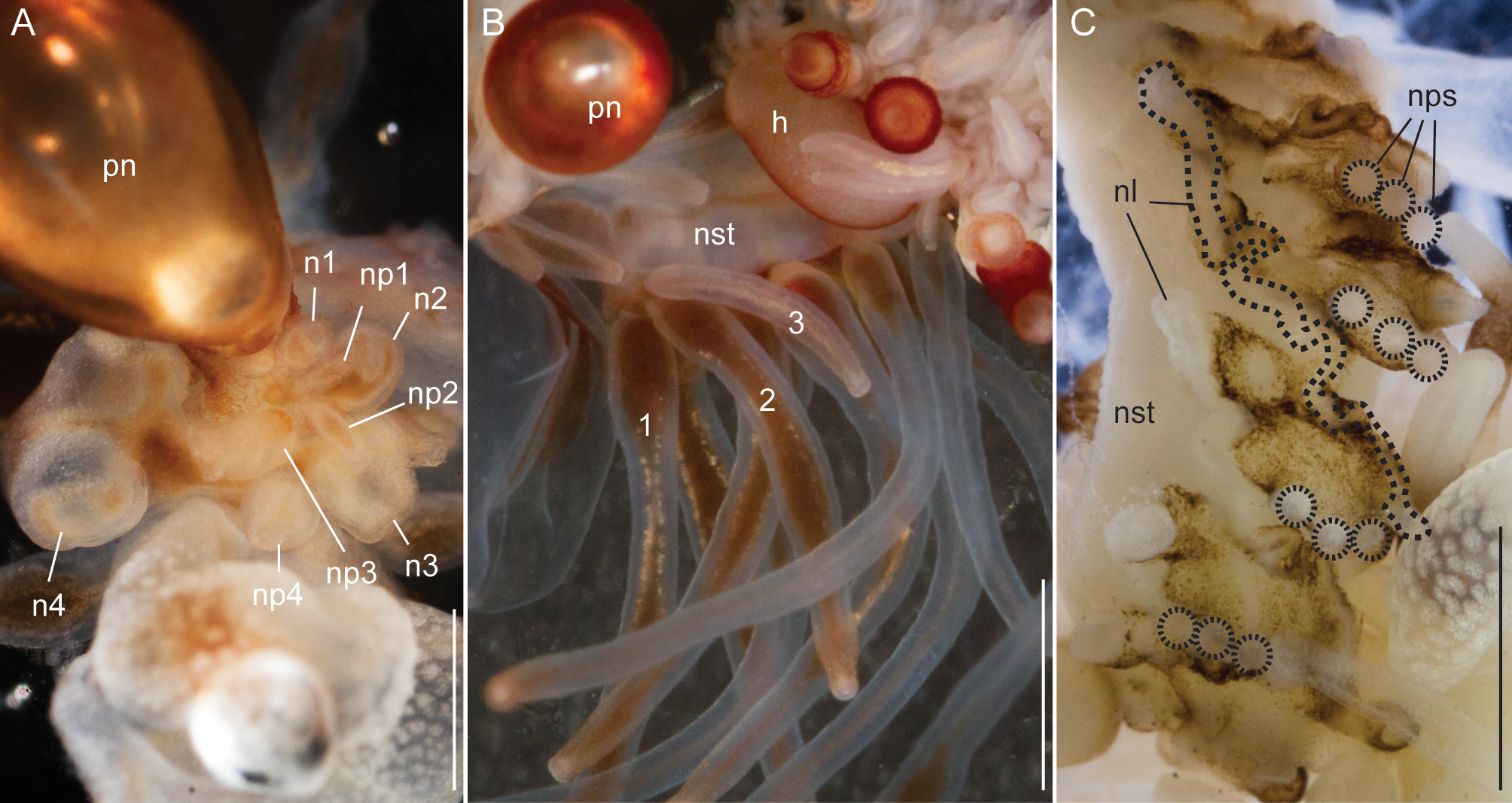
Nectosome: All but one of the mature nectophores of the holotype specimen became detached from the nectosome during the sampling procedure ( Fig. 11 View FIGURE 11 B) but were retained in the sampler. The youngest nectophores were each accompanied by a single palpon ( Fig. 11 View FIGURE 11 A). The nectophores were attached to the ventral side of the stem, i.e. on the same side that the siphosomal zooids were attached. Up to three palpons were associated with a single mature nectophore for the holotype and specimen D195 ( Fig. 11 View FIGURE 11 B,C). The palpons in the nectosome were translucent white, and were up to two times the length of associated nectophores in relaxed state. The nectophoral palpons were attached to a protruding tissue fold at the posterior end and, in ventral view, left of the nectophoral lamella ( Fig. 11 View FIGURE 11 C).
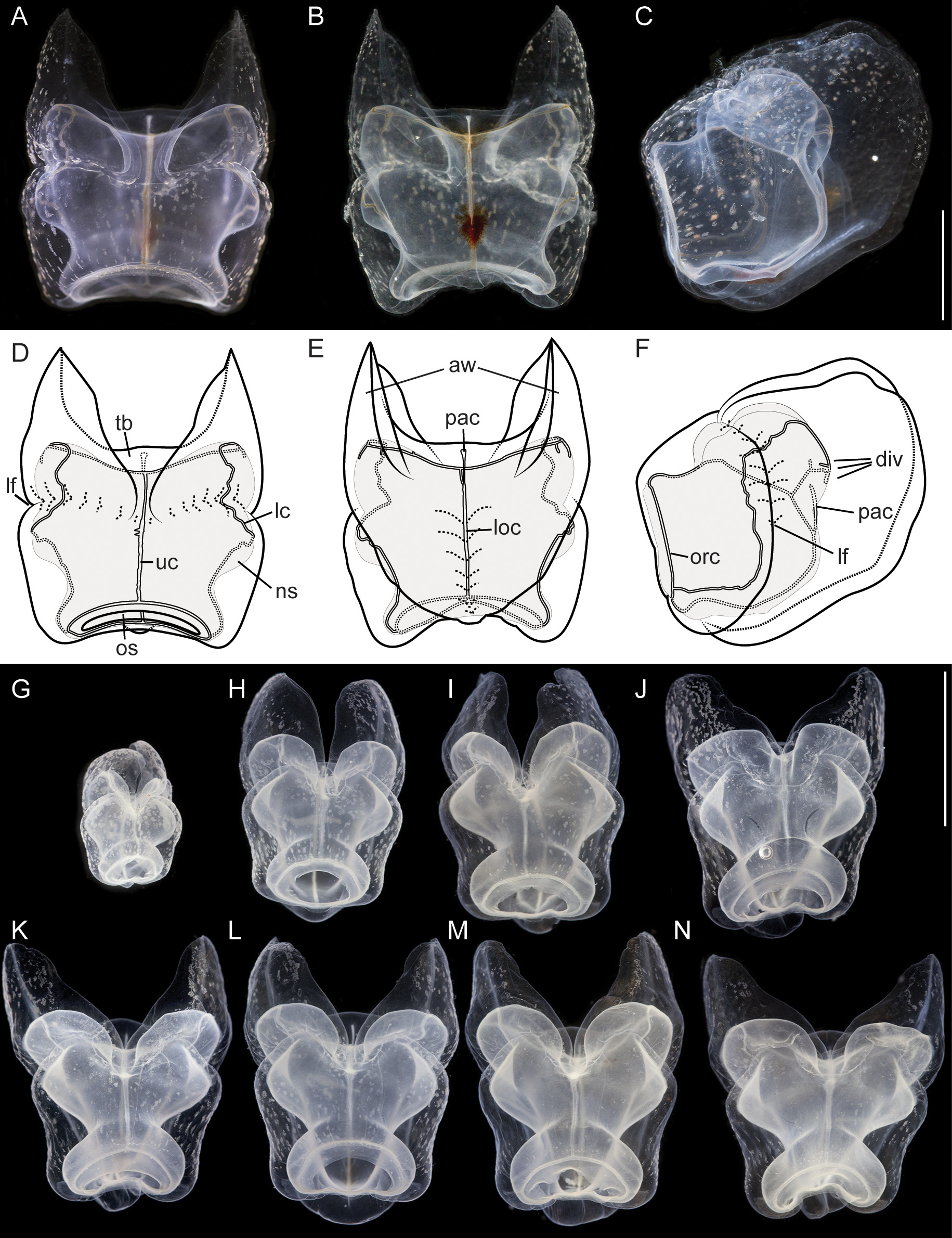
Nectophores: In the growth zone, the more developed nectophores were densely covered with opaque nematocysts ( Fig. 11 View FIGURE 11 A). In older nectophores these nematocysts were organized in distinct patches. The largest nectophore of the holotype had a length of 14 mm, and a width of 10 mm. Mature nectophores had large axial wings ( Fig. 12 View FIGURE 12 ). Whereas immature nectophores were widest at mid-height of the nectophore, mature ones were widest at the apical tips of the axial wings ( Fig. 12 View FIGURE 12 A,N). Patches of nematocysts covered the upper and upper-most lateral surfaces, including the axial wings ( Fig. 12 View FIGURE 12 ). There were no lateral nematocyst patches below the lateral furrows. Refractile cells were sparsely scattered between but not within these patches of nematocysts.
Deep lateral grooves approached the upper side at approximately half the length of the nectophore, which resulted in indentations in the sides of the nectosac. On the upper side of live nectophores these grooves ran slightly in the ostial direction before they met in the mid-line ( Fig. 12 View FIGURE 12 A,D). In fixed nectophores, however, the grooves were even more pronounced ( Fig. 12 View FIGURE 12 G–N). On the upper side close to the midline they bent through almost 90° to turn in the ostial direction and ran around the axial wing margins before turning toward the mid-line and uniting with the opposite groove. Laterally the grooves continued along the lower lateral sides of the nectophore and ended below the ostium ( Fig. 12 View FIGURE 12 F).
Nectophores had a wide ostium, and the nectosac was broad at the distal end of the nectophore, occupying most of its width. Slightly proximal to this region, the nectosac narrowed considerably, before expanding in width just before the lateral furrow. The nectosac was indented by the lateral furrows and expanded again to reach its maximum or equivalent width proximal to the lateral grooves ( Fig. 12 View FIGURE 12 ).
In lower view, the inner margin of the axial wings of the nectophore gradually curved toward the mid line and tapered out well apart from each other before reaching the mid-length of the nectophore ( Fig. 12 View FIGURE 12 B,E). Some red pigmentation was present in the region of the thrust block ( Fig. 12 View FIGURE 12 B). The outer wing margins curved inwards at about the mid-length of the nectophore and met below the ostium. On the lower side of the nectophores the mesoglea formed pillows on either side of the median lower furrow. Where the furrow was deepest and narrowest, red pigmentation was present on its walls ( Fig. 12 View FIGURE 12 B). In living nectophores the distal part of the lower surface only slightly protruded below the ostium ( Fig. 12 View FIGURE 12 A,D). In fixed nectophores this protrusion was very pronounced ( Fig. 12 View FIGURE 12 J–N). This suggests a stabilizing or antagonistic function of the lower mesoglea pillows flanking the lower furrow. This structural element seems to be less affected by the fixation procedure, whereas other parts of the nectophore might have a greater tendency to shrink, causing the differences in appearance between fixed and living nectophores.
The ascending pallial canal arose from the pedicular canal at about 1/4 the height of the nectosac and then ran toward the upper surface for a short distance to end half way up the thrust block ( Fig. 12 View FIGURE 12 F). The pedicular canal also gave rise to the upper and lower radial canals. The upper radial canal was fairly straight on the upper surface. Just distal to where the lateral furrows joined, the canal widened slightly, kept a larger diameter and ran down to the ostial ring canal ( Fig. 12 View FIGURE 12 D). Within the region where the lateral furrows joined small protuberances could be observed occasionally on the irregular wall of the upper radial canal.
The lateral radial canals arose at the same level from the upper radial canal at mid-height of the nectosac ( Fig. 12 View FIGURE 12 F). They ran away from the midline and slightly up to reach the sides of the nectosac just above its widest point. In mature nectophores, in this region the lateral radial canals had up to three distinct diverticula. The outermost, on the proximal-lateral margin, was the longest and pointed towards the ostium ( Fig. 12 View FIGURE 12 E,F). The other two were shorter, if present at all, with the one closest to the junction of the lateral canals ascending and the next descending. Presence or absence of diverticula was not a solely function of ontogenetic stage. In the holotype specimen, medium-size nectophores could be found without a single diverticulum whereas diverticula were clearly present in smaller and larger nectophores. At the position where the outermost diverticulum branched off, the lateral radial canal turned upwards and curved over onto the upper surface of the nectosac and eventually, in a slightly undulating manner, down. The canal either descended along the base of the lateral furrow, or more usually crossed that furrow and then began to descend parallel with it ( Fig. 12 View FIGURE 12 F). Before reaching the lower margin of the nectosac the canal then curved upwards and ran obliquely to join the ostial ring canal ( Fig. 12 View FIGURE 12 F).
In specimen V908, only one mature nectophore was available. It had all three characteristic diverticula (not shown).
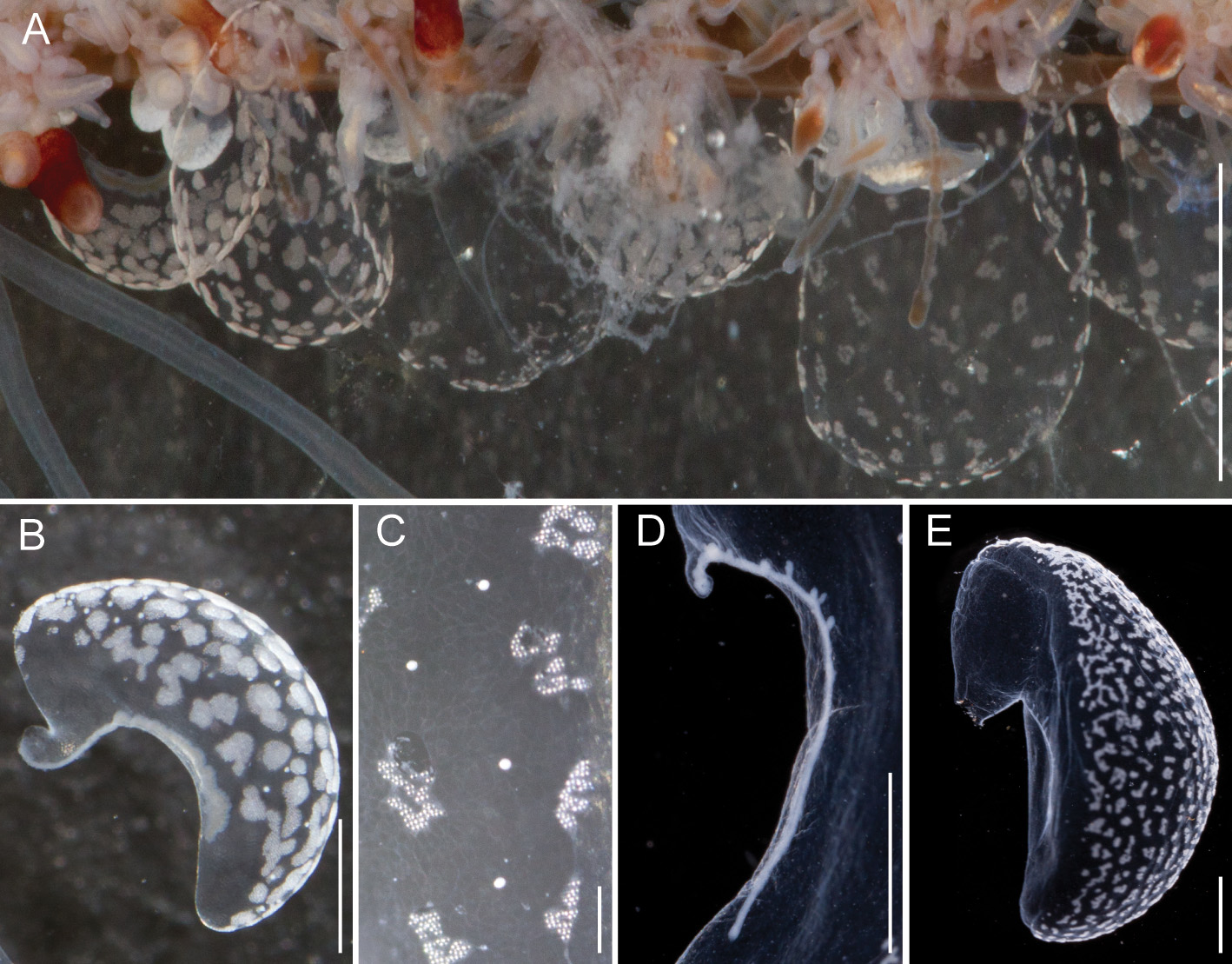
Siphosome: The full length of the siphosome of the holotype prior to collection was about 1.2m in the state of relaxation as pictured in Fig. 2 View FIGURE 2 B. After relaxation, the siphosome of the partial holotype fragment that was collected measured 1.8 cm in length ( Fig. 2 View FIGURE 2 D).
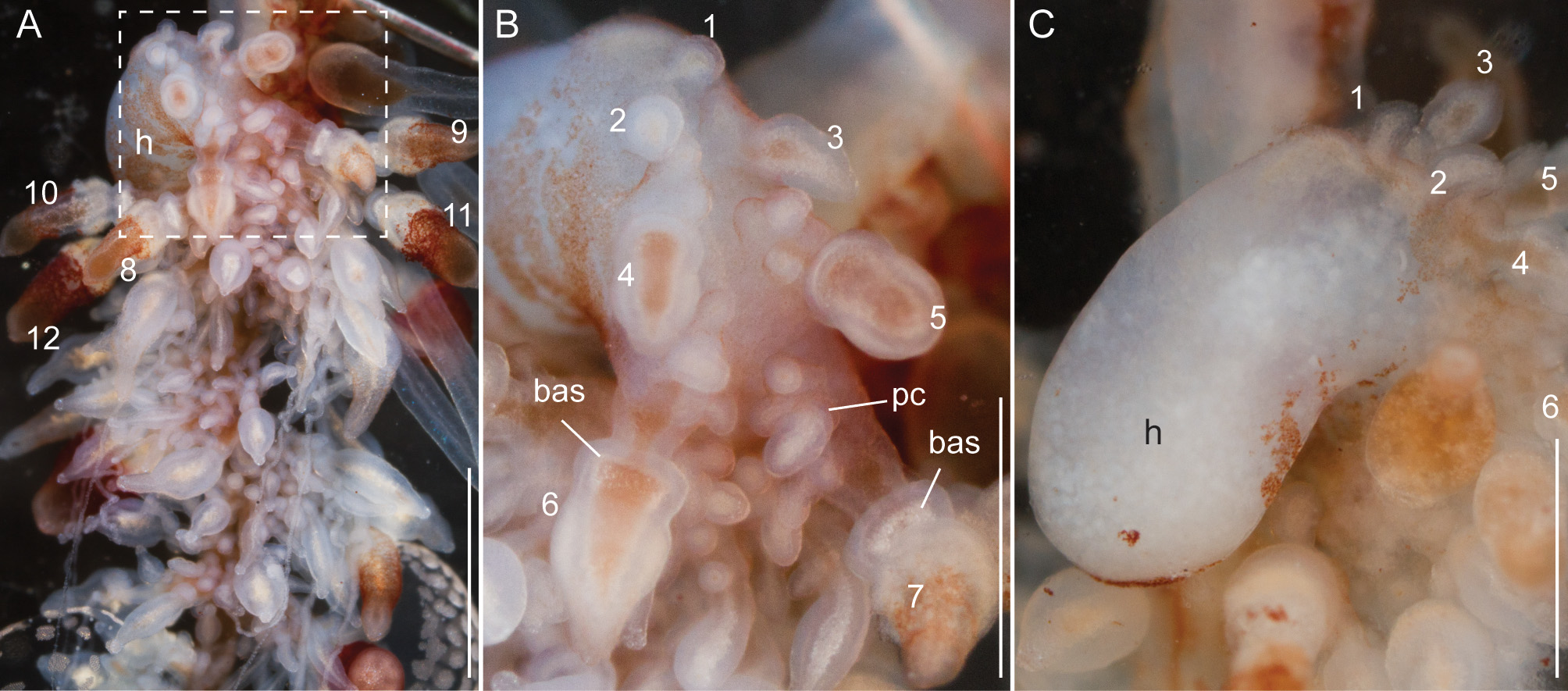
Siphosomal growth zone and early zooid organization. The siphosomal growth zone of the holotype had a massive horn that bent, in ventral view, to the left side of the colony ( Fig. 13 View FIGURE 13 ). The horn was covered with a thin layer of orange-red pigment, which was easily detached when the horn was manipulated ( Fig. 13 View FIGURE 13 B,C). The anterior end of the horn was free of zooids ( Fig. 13 View FIGURE 13 C). The first observable buds became gastrozooids, which were alternately displaced to the left and right off the ventral midline resulting in a pronounced biserial organization ( Fig. 13 View FIGURE 13 A, B). Additional buds became visible on the peduncle of the fourth-youngest gastrozooid ( Fig. 13 View FIGURE 13 B). At the distal end of the peduncle, the diameter of the developing gastrozooid widened and its body had a cone-shaped appearance (gastrozooids 3–7, Fig. 13 View FIGURE 13 B). Just distal to the base of the cone, a constriction became apparent in gastrozooid 6 which generated a ring-like structure (gastrozooid 7, Fig. 13 View FIGURE 13 B). The opaque appearance of this structure indicated a site of nematogenesis and thereby the formation of the basigaster region. By gastrozooid 9, it was obvious that the buds on the peduncle of the gastrozooid gave rise to palpons. No tentacle formation could be observed in early gastrozooids.
Bracts: Bracts were mottled with irregular shaped patches of nematocysts distributed across their upper surface ( Fig. 14 View FIGURE 14 ). These patches formed early in bract development and the distance between them increased as the bracts matured ( Fig. 14 View FIGURE 14 A). Refractile cells were scattered sparsely and predominantly on the periphery of the upper surface outside of the opaque nematocyst patches ( Fig. 14 View FIGURE 14 B,C). The bracteal canal ran close to lower surface throughout its length. At the distal end, however, it bent into the mesoglea ( Fig. 14 View FIGURE 14 D). The wall of the bracteal canal in young bracts was irregularly shaped, giving it a rough appearance ( Fig. 14 View FIGURE 14 B). Older bracts frequently, but not always, had diverticula penetrating into the mesoglea ( Fig. 14 View FIGURE 14 D). Mature bracts had a distinct keel ( Fig. 14 View FIGURE 14 E). Bracts were associated either with a palpon or a gastrozooid (see below). We found no morphological differences between the bracts attached at these different locations.
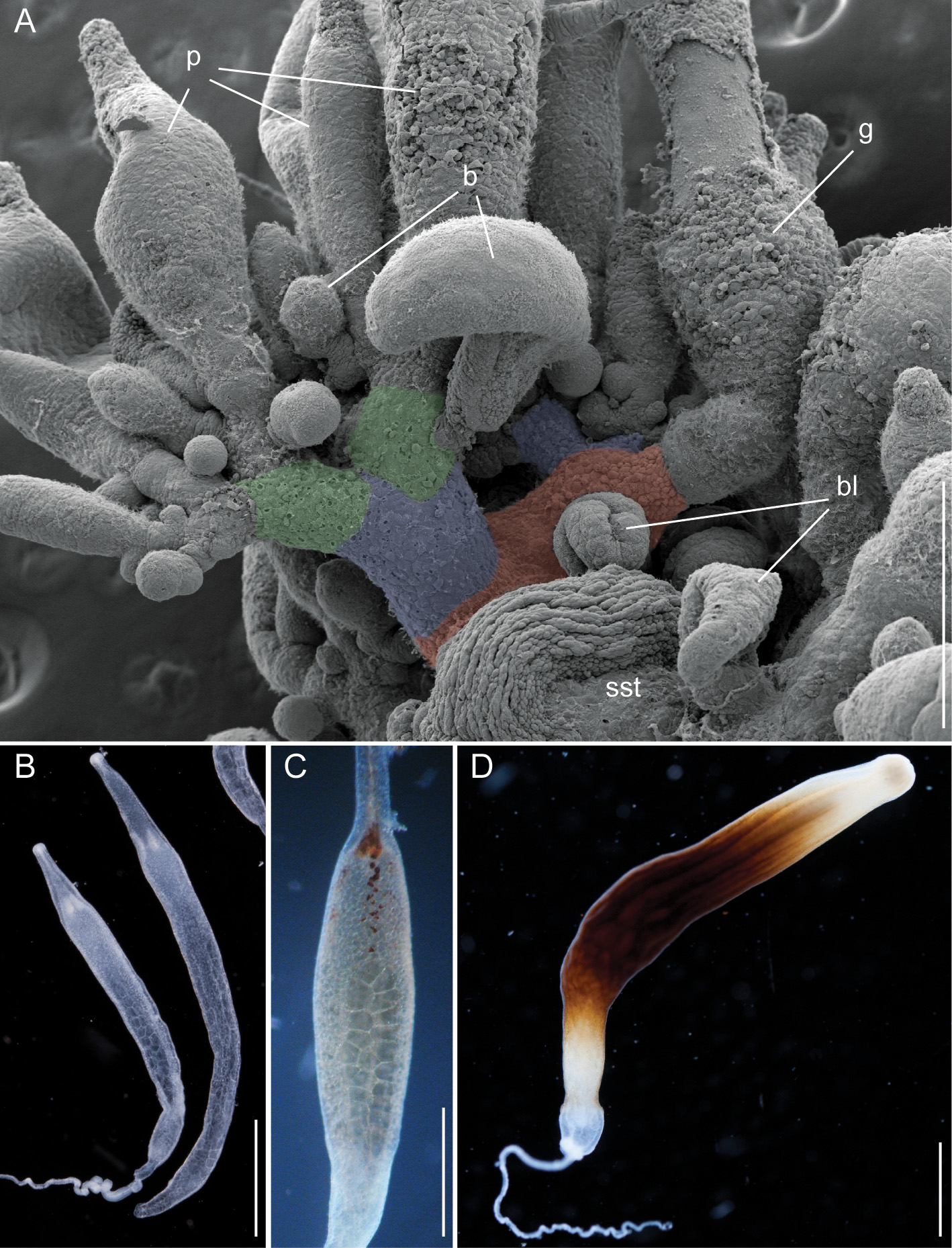
Palpons and gastrozooids: The body column of gastrozooids was colored by a deep red pigment. This pigment gradually faded out close to the distal end so that the oral region was white. Multiple clusters of zooids were attached to the gastrozooid peduncle ( Fig. 15 View FIGURE 15 A). At the base of each gastrozooid peduncle a single curled lamella frequently could be observed, indicating the point of attachment of a single gastrozooidal bract ( Fig. 15 View FIGURE 15 A). The secondary branches themselves were branched. Palpons sat on and budded off these secondary branches. Each palpon had a palpacle and was accompanied by a bract. Mature palpons were translucent with a distinct white tip. The palpacle originated at the very base of the palpon ( Fig. 15 View FIGURE 15 B). A distinct basigaster region could not be observed. In the holotype, cells with red pigment were visible in live or freshly fixed tissue in the distal region where the obvious gastric cavity ended, and the proboscis region began ( Fig. 15 View FIGURE 15 C). A region with very large cells could be frequently observed along one side of the palpons ( Fig. 15 View FIGURE 15 B,C). Tentacles associated with gastrozooids could be found in fixed tissue, but these tentacles were difficult to distinguish in live tissue ( Fig. 15 View FIGURE 15 D).
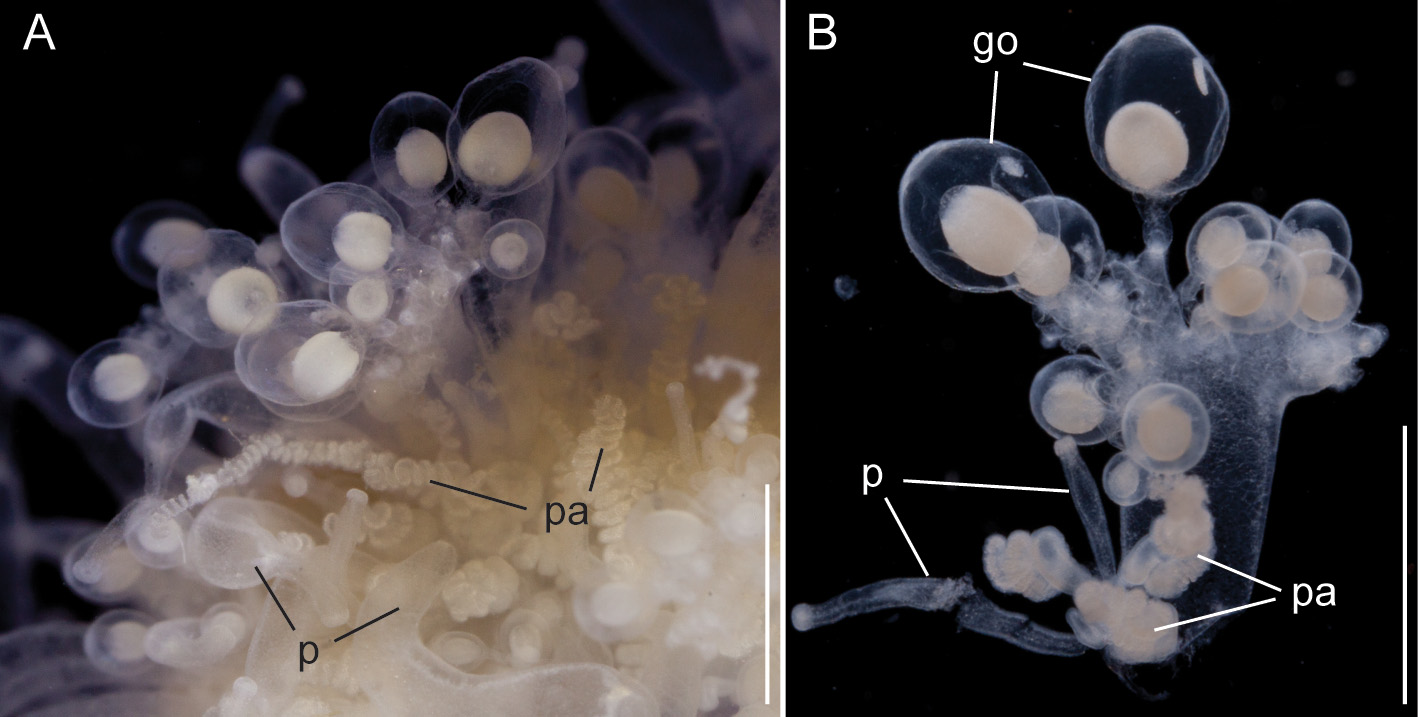
Gonodendra: Male gonodendra were found along the siphosomal stem of specimen V908 ( Fig. 16 View FIGURE 16 ). The tissue was highly contracted and detailed studies of cormidial organization could not be conducted. However, branches exclusively bearing gonophores, attached by thin peduncles, could be removed from the colony. Young palpons at the base of these branches indicated palpon bearing secondary branches as the point of their attachment ( Fig. 16 View FIGURE 16 B).
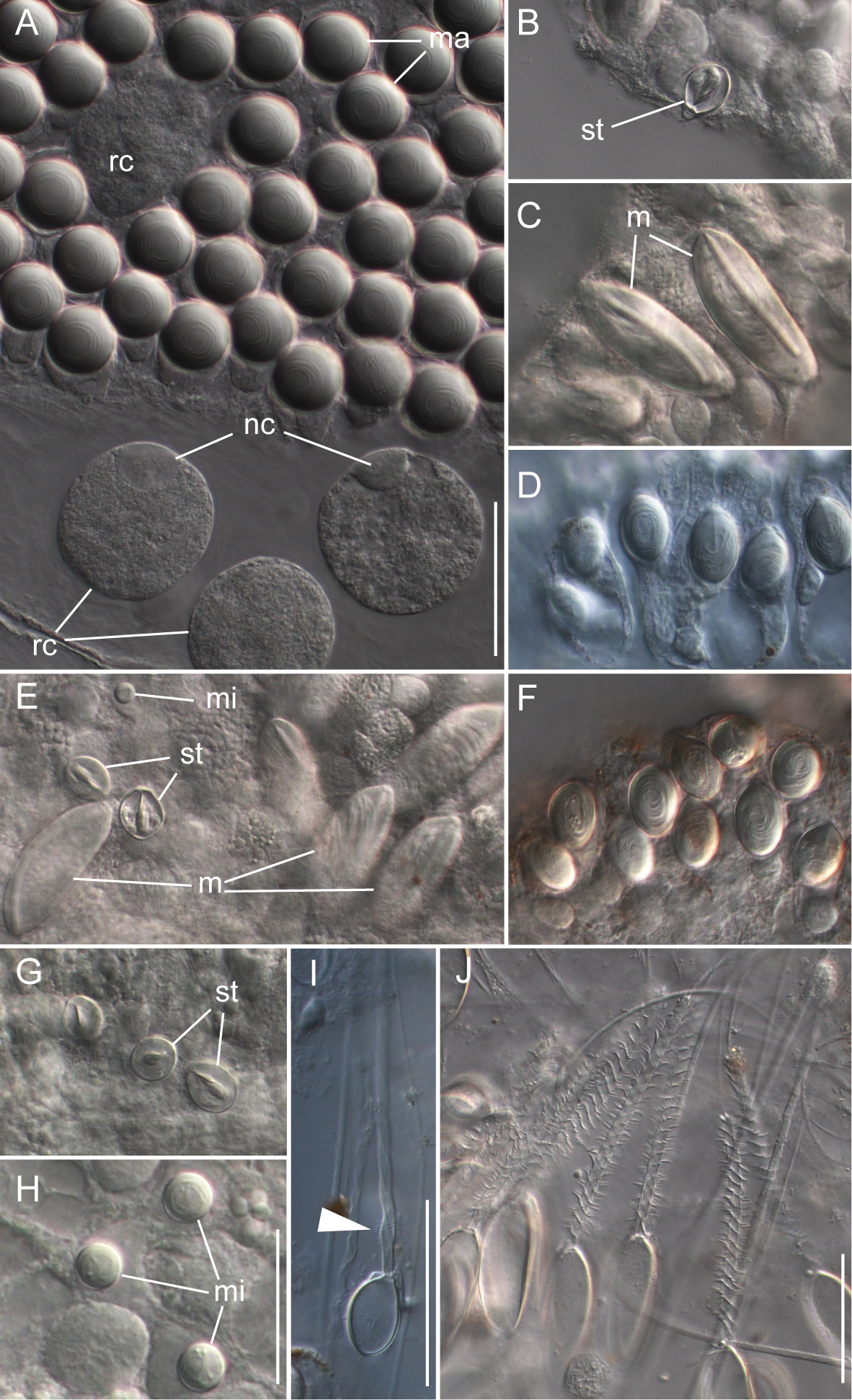
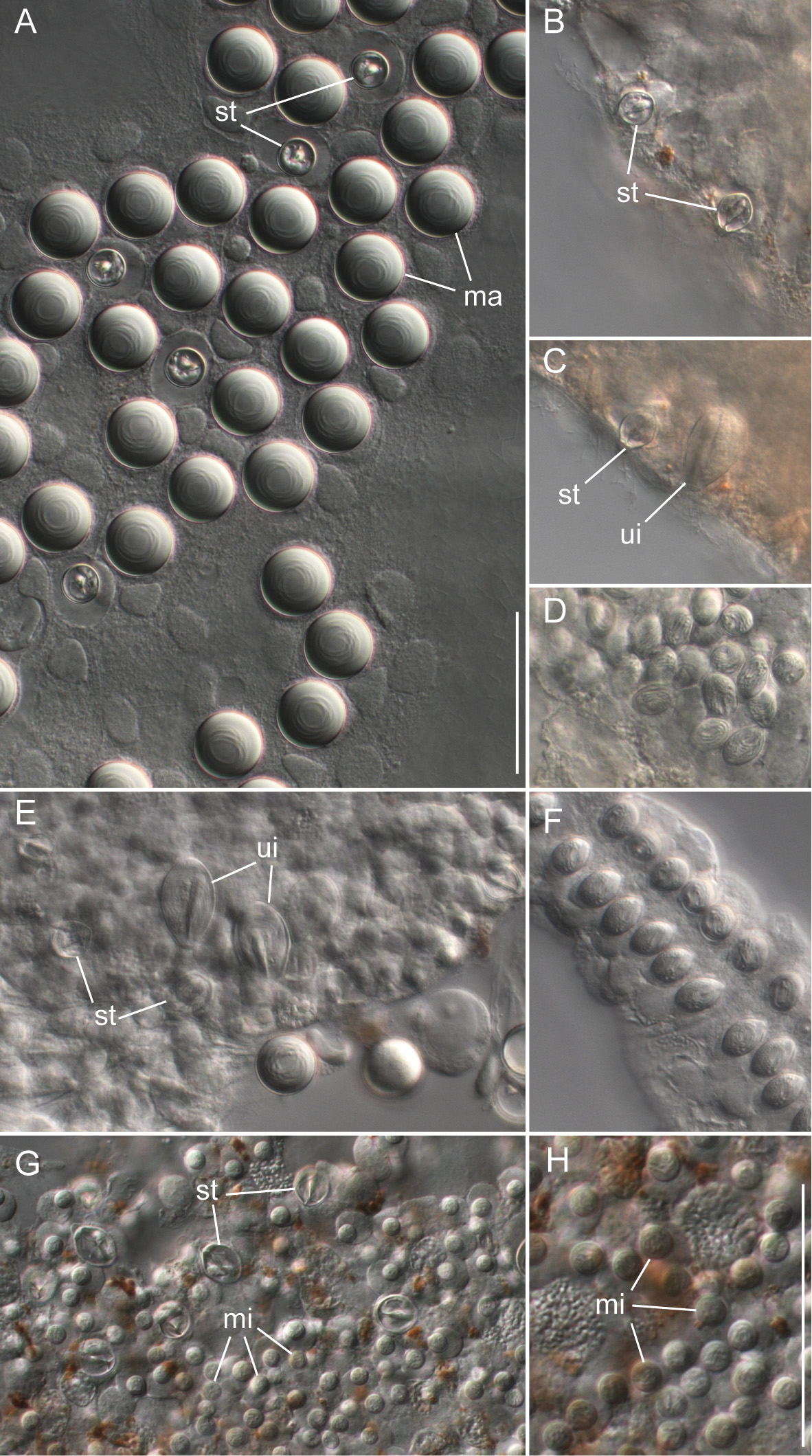
Nematocyst complement: Four different types of nematocysts were found on the different zooids of the colony ( Fig. 17 View FIGURE 17 ).
Spherical isorhizas of two distinct sizes.
Macroisorhizas, with a mean diameter of 21.0 µm ( Fig. 17 View FIGURE 17 A) found exclusively in nematocyst patches on nectophores and bracts. Refractile cells were not observed within these patches.
Microisorhizas, with a mean diameter of 5.8 µm. These nematocysts were found predominantly at the distal ends of gastrozooids, palpons and nectophoral palpons and scattered along the body columns of these zooids and in very high densities at the tip of the nectophoral palpons ( Fig. 17 View FIGURE 17 G,H).
b) Stenoteles, with a mean length of 14.1 µm and a mean width of 11.2 µm. These were regularly found interspersed between the macroisorhizas of the bracts and nectophores ( Fig. 15 View FIGURE 15 A). Stenoteles were also found predominantly at the distal ends of gastrozooids, palpons and nectophoral palpons and scattered along the body columns of these zooids ( Fig. 17 View FIGURE 17 B,E,G).
c) Unknown type, with a mean length of 26.7 µm and a mean width of 16.1 µm.
Microbasic mastigophores as described for Apolemia lanosa ( Fig. 10 View FIGURE 10 C,E) were not found. The unknown capsule type was, however, found in corresponding locations at the tips of gastrozooids and palpons ( Fig. 17 View FIGURE 17 C,E). It was smaller than the mastigophores of A. lanosa and was ovoid in shape. Unfortunately, no discharged capsules of this type were found.
d) Capsules of tentacles and palpacles, mean length 15 µm in length and mean width 9.1 µm ( Fig. 17 View FIGURE 17 D,F).
The ovoid capsules of the tentacles and palpacles were similar in appearance to the rhopaloids found on the palpacles and tentacles of Apolemia lanosa but, unfortunately, their true identity could not be established as no discharged capsules were found. For the palpons, the base of the palpacle was found to be a nematogenic region. No mature capsules were found there, whereas they could easily be identified more distally, but developing nematocysts were identified.
Distribution. The holotype and paratype specimens all came from the vicinity of Monterey Bay, California. Several other specimens from the same region have either been collected or identified from in situ photographs, as listed below in Table 2 View TABLE 2 . One specimen, however, was collected at the southern end of the Gulf of California, Mexico. The mean depth for all these specimens was 649 ± 151 m. Similar morphology has been reported for nectophores collected off Vancouver Island ( Mapstone 2009, p. 83, Fig. 13 View FIGURE 13 ) and described as “ Apolemia sp.”, raising the possibility that they are the same species. Mapstone (2009) also reported more findings of this particular Apolemia sp. from Bahamian waters taken by manned submersibles JSL I and JSL II, which would extend the geographic distribution of A. rubriversa if they prove to belong to that species. There are, however, critical differences between the nectophores of these samples and those of A. rubriversa . The nectophores described by Mapstone (2009) did not have diverticula on the lateral canals or nematocyst patches. Thus it will be necessary to have more complete material in order to make a definitive statement about the identity of those samples.
Etymology. The specific name rubriversa is derived from the Latin for red furrow, indicating the red pigmentation in the lower furrow of the nectophore.
| USNM |
Smithsonian Institution, National Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |