Rulyrana saxiscandens ( Duellman & Schulte 1993 )
|
publication ID |
https://doi.org/10.11646/zootaxa.3851.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:9840D64B-F08C-44E7-B2DC-4818F8FFDD4F |
|
DOI |
https://doi.org/10.5281/zenodo.6136445 |
|
persistent identifier |
https://treatment.plazi.org/id/664887B1-FFE9-FF8B-FF7C-FD85D382FA66 |
|
treatment provided by |
Plazi |
|
scientific name |
Rulyrana saxiscandens ( Duellman & Schulte 1993 ) |
| status |
|
Rulyrana saxiscandens ( Duellman & Schulte 1993) View in CoL
Figures 28 View FIGURE 28. a, b , 29 View FIGURE 29
Cochranella saxiscandens Duellman and Schulte, 1993 View in CoL . Holotype KU 211779, an adult male from “Catarata Ahuashiyacu, ( 06°30’ S, 76°20’ W, 730 m), 14 km (by road) northeast of Tarapoto, Provincia San Martín, Departamento San Martín, Perú ”
Cochranella tangarana Duellman and Schulte, 1993 View in CoL . New synonym.
Cochranella croceopodes Duellman and Schulte, 1993 View in CoL . New synonym.
“ Cochranella View in CoL ” croceopodes— Guayasamin, Castroviejo-Fisher, Trueb, Ayarzagüena, and Vilà 2009.
Rulyrana View in CoL saxiscandens— Guayasamin, Castroviejo-Fisher, Trueb, Ayarzagüena, and Vilà, 2009.
Rulyrana View in CoL tangarana— Guayasamin, Castroviejo-Fisher, Trueb, Ayarzagüena, and Vilà, 2009.
Background information. Cochranella saxiscandens View in CoL was described from a series of 30 adult males collected from Ahuashiyacu waterfalls ( 730 m), 14 km NE from Tarapoto, San Martín, Peru ( Duellman & Schulte 1993). Cochranella tangarana View in CoL was described on the basis of two adult males and one adult female, all collected from Abra Tangarana ( 1080 m) View in CoL , San Martín, Peru, a site roughly 50.8 km W from Ahuashiyacu. Both species were allocated to the Cochranella ocellata View in CoL group due to the combination of white parietal peritonea with clear visceral peritonea. Additional characters of these two species include vomerine teeth present, bones green, dorsal skin finely spiculate, humeral spine absent, and color in life dark green to black. Duellman & Schulte (1993) distinguished these two species from each other based on color in preservative (dark grey to black in R. saxiscandens View in CoL vs. lavender in R. tangarana View in CoL ), presence/absence of melanophores on ventral surface of shanks and tarsi (melanophores present in R. saxiscandens View in CoL vs. absent in R. tangarana View in CoL ), and presence/absence of inner tarsal fold (fold present in R. saxiscandens View in CoL vs. absent in R. tangarana View in CoL ). Both species were removed from Cochranella View in CoL and placed in the genus Rulyrana View in CoL by Guayasamin et al. (2009) on the basis of overall similarity with other species of the genus.
Cochranella croceopodes View in CoL was described on the basis of two adult females, the holotype collected from km. 23.2 on the Tarapoto-Yurimaguas road and the paratype collected from Ahuashiyacu waterfalls, both San Martín, Peru ( Duellman & Schulte 1993). This species was assigned to the Co. granulosa View in CoL group due to the combination of white parietal and visceral peritonea. Cochranella croceopodes View in CoL was described as having a “diffuse yellow line on flanks from axilla to groin” ( Duellman & Schulte 1993) and diagnosed only against species having dorsolateral stripes; i.e., Centrolene hesperium View in CoL and Ce. lemniscatum View in CoL , both of which lack vomerine teeth and have first finger shorter than the second. Cochranella croceopodes View in CoL was moved from the granulosa View in CoL group to the spinosa group (Ruiz-Carranza & Lynch 1995), then back to the granulosa View in CoL group ( Guayasamin & Bonnacorso 2004). Cisneros- Heredia and McDiarmid (2007) removed it from the granulosa View in CoL group but did not assign it to any group. Guayasamin et al. (2009) left Co. croceopodes View in CoL as incertae sedis within the subfamily Centroleninae View in CoL .
New data. In 2010 and 2011 we collected a series of 10 R. saxiscandens View in CoL from two sites: three males (CORBIDI-HE-2012-14150, CORBIDI-HE-2012-14151, and CORBIDI-HE-2012-14153) and three females (CORBIDI-HE-2012-14149, CORBIDI-HE-2012-14152, and ET-10-124) from the type locality of Ahuashiyacu ( 6°27'19.69"S, 76°18'32.33"W, 797 m); and four males (ET-10-126, ET-10-127, CORBIDI 14668, CORBIDI 10464) from 2.3 km E of the type locality ( 6°27'30.28"S, 76°17'14.35"W, 988 m, Figs. 7 View FIGURE 7 , 28 View FIGURE 28. a, b ). In addition, we collected four R. tangarana View in CoL males, three of which were from the type locality ( 6°16'52.86"S, 76°43'57.86"W, 1047 m), and one from 6 km SE from the type locality ( 6°19'22.40"S, 76°41'43.55"W, 1003 m, Figs. 7 View FIGURE 7 , 28 View FIGURE 28. a, b ). Morphological, bioacoustic, and genetic data all strongly suggest the existence of a single species. With respect to color in preservative, of the 10 R. saxiscandens View in CoL , two (CORBIDI 10464 and CORBIDI 14668) were lavender in preservative; seven (CORBIDI-HE-2012-14149 through CORBIDI-HE-2012-14153, ET-10-124, ET-10-126, and ET-10-127) ranged from bright green or yellowish green to dark or dull green in preservative, and one individual (CORBIDI-HE-2012-14151) was completely black in preservative. In contrast with Duellman and Schulte (1993), none of the R. tangarana View in CoL were lavender in preservative but rather dull green. With respect to melanophore presence/absence on the ventral surface of the tarsi and shanks, of the 10 R. saxiscandens View in CoL , two (ET-10-126 and ET- 10-127) entirely lacked melanophores in this area, six (CORBIDI-HE-2012-14149 through CORBIDI-HE-2012- 14151, ET-10-124, MNCN 45962, and CORBIDI 14668) had faint melanophores restricted to the edges of the leg (barely extending to ventral surface), and two (CORBIDI-HE-2012-14152 and CORBIDI-HE-2012-14153) had distinct melanophores present. Of the four R. tangarana View in CoL , two individuals (CORBIDI 14149 and CORBIDI 10476) completely lacked melanophores in this area. The remaining two individuals (CORBIDI 10478 and CORBIDI 14151) had melanophores present only on the leg edges, similar to six of the R. saxiscandens View in CoL mentioned above. Finally, with respect to presence/absence of the inner tarsal fold, of the 10 R. saxiscandens View in CoL , six individuals (CORBIDI-HE-2012-14151, CORBIDI-HE-2012-14153, ET-10-124, ET-10-127, CORBIDI 10464, and CORBIDI 14668) had a distinct inner tarsal fold, while one individual (ET-10-126) lacked a fold completely. The remaining three individuals (CORBIDI-HE-2012-14149 through CORBIDI-HE-2012-14151) have very faint folds, mainly present as a row of yellowish discoloration extending from the base of the foot along the inner surface of the tarsus. In the series of four R. tangarana View in CoL , one individual from 6 km SE from the type locality (CORBIDI 10478) clearly has an inner tarsal fold. Two individuals (CORBIDI 10476 and CORBIDI 14151) from the type locality do not. One individual (CORBIDI 14149) from the type locality has a discoloration along the inner tarsus, similar to three of the R. saxiscandens View in CoL , but lacks a fold.
Specimens from the type locality of R. saxiscandens View in CoL display considerable variation in dorsal coloration in life. In a series of five individuals collected in a single night, one individual (CORBIDI-HE-2012-14153) was black ( Fig. 28a View FIGURE 28. a, b ), one was bluish-gray (ET-10-124), one dull green (CORBIDI-HE-2012-14152), and two lime-green (CORBIDI-HE-2012-14149 and CORBIDI-HE-2012-14151) (e.g., Fig. 28 View FIGURE 28. a, b c). Four individuals (ET-10-126, ET-10- 127, CORBIDI 10464, and CORBIDI 14668) found 2.3 km E of the type locality ranged from black to bright green; furthermore, two of these individuals (CORBIDI 10464 and CORBIDI 14668) had faint lime-green flecks on the dorsum. The four specimens collected in or near the type locality of R. tangarana View in CoL , dorsal coloration ranged from dull to bright green. Two (CORBIDI 14149 and CORBIDI 10476) had prominent lime-green flecks on the dorsum, which appear to vary in number from just a few to consistently scattered throughout the dorsum. Much of this variation may be due to the apparent ability for individuals to change color. Two individuals (CORBIDI 10464 and CORBIDI 14668) from 2.3 km E of Ahuashiyacu were lime green upon collection but turned nearly black by the following morning. Iris color also varies from dark bronze to light bronze ( Fig. 28 View FIGURE 28. a, b ), and appears to be related to dorsal color (with darker individuals tending to have darker irises). Skin texture of R. saxiscandens View in CoL from the type locality varies from spiculate to shagreened to nearly smooth. The two specimens from the R. tangarana View in CoL locality both had spiculate skin.
There is variation in the extent to which iridophores cover the parietal peritoneum among R. saxiscandens View in CoL specimens and those collected from the type locality of R. tangarana View in CoL . In some individuals the iridophores cover nearly the entire venter (e.g., Fig. 28b View FIGURE 28. a, b ) whereas in others they are restricted to only the anterior ~1/2 of the venter (e.g., Fig. 28 View FIGURE 28. a, b f). Additionally, there is some variation in the prominence of the inner tarsal fold (see above) and extent of melanophores on ventral surfaces of limbs (see above).
Calls obtained from both the type locality of R. tangarana View in CoL and R. saxiscandens View in CoL are nearly identical. The call consists of a short, high-pitched “zip” given every few seconds. We obtained a series of six calls from a single male at the type locality of R. tangarana View in CoL , recorded 6 June 2011 at 21.5°C. Note length was 0.038– 0.05 s (mean = 0.046 s), each note was composed of 7 or 8 pulses (mean = 7.8 pulses), pulse rate 160–184 pulses/s (mean = 170.3 pulses/ s), dominant frequency 6103–7106 Hz (mean = 6599 Hz). We also recorded calls of three R. saxiscandens View in CoL (two from Ahuashiyacu and one from 2.3 km E Ahuashiyacu) at temperatures ranging from 20–23°C. Calls were similar to R. tangarana View in CoL calls; note length 0.028– 0.056 s (mean 0.041 s), notes composed of 7–12 pulses (mean = 8.2 pulses), pulse rate 179–250 pulses/s (mean = 205.6 pulses/s), dominant frequency 6568–6915 Hz (mean = 6833 Hz). While there are slight differences between the two nominal taxa (e.g., when comparing means), the amount of interspecific variation is in most cases is equal to or lower than intraspecific or even intra-individual variation ( i.e., different notes given by a single male). Thus, there are no fixed, discernible differences in the calls of these two taxa.
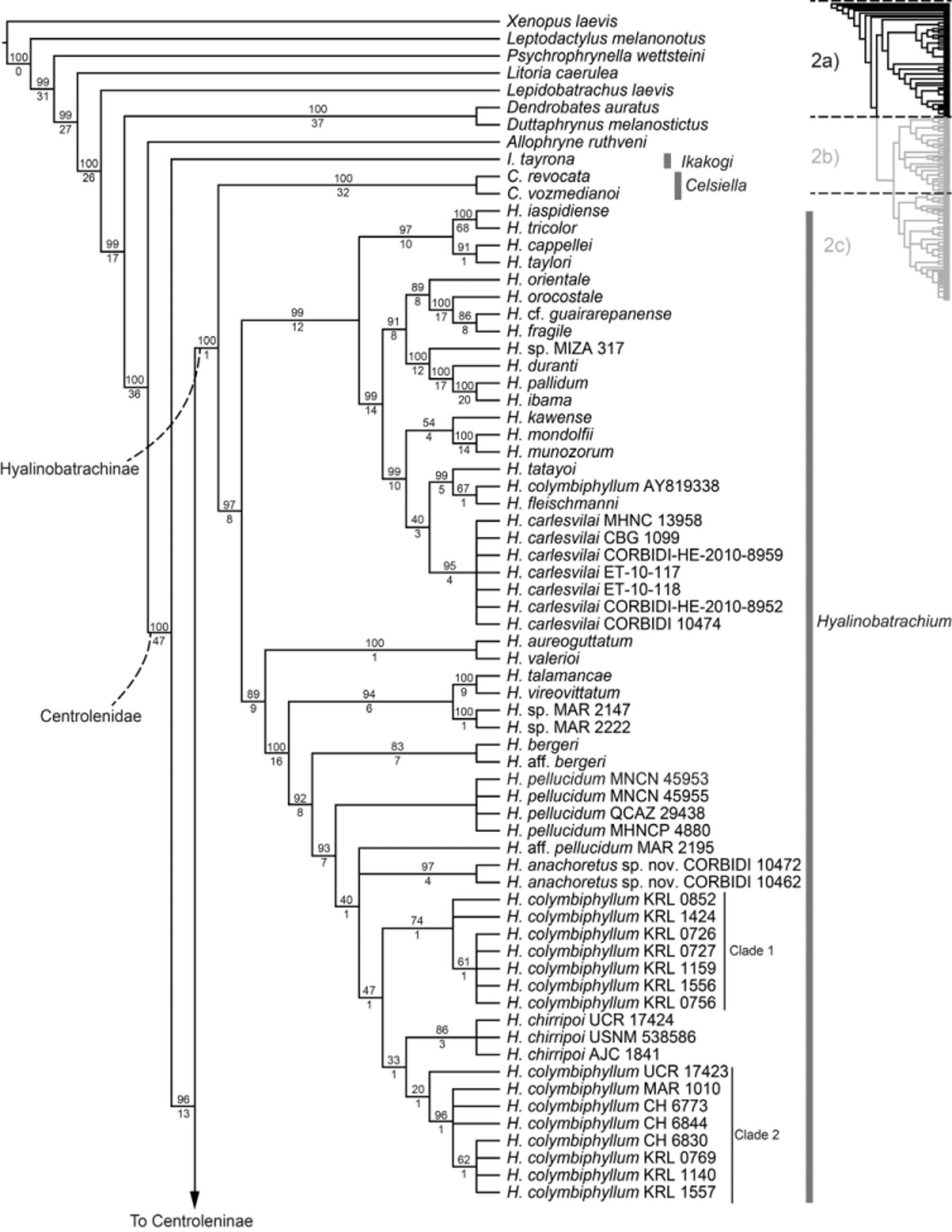
Our new molecular data reveals that Rulyrana mcdiarmidi View in CoL , R. tangarana View in CoL , R. saxiscandens View in CoL , and an individual referred to as R. flavopunctata ( Guayasamin et al. 2008) View in CoL from Provincia Morona-Santiago, Ecuador are not reciprocally monophyletic ( Fig. 2c View FIGURE 2 a View FIGURE 2 c ). The specimen R. tangarana View in CoL CORBIDI 14149 is retrieved as sister to all other specimens, which are grouped in a polytomy. Within this polytomy, there are eight single terminals corresponding to specimens of R. saxiscandens View in CoL and R. tangarana View in CoL , and three clades. The first clade groups two specimens of R. saxiscandens View in CoL (CORBIDI-HE-2012-14150 and CORBIDI-HE-2012-14151), the second clade groups another three individuals of R. saxiscandens View in CoL (ET-10-124, CORBIDI 14668, and CORBIDI-HE-2012-14152) in a polytomy, and the third clade groups three specimens of R. mcdiarmidi View in CoL (CORBIDI-HE-2010-6848, CORBIDI 10470, and CORBIDI 10473) with the specimen of R. flavopunctata View in CoL QCAZ 32265. Intra- and interspecific genetic distances among these terminals are low (p-distances = 0.0–0.6%) and overlapping ( Table 6 View TABLE 6 ).
Comparisons. Based on the above data, none of the original diagnostic characters between these two species are supported. Color in preservative is variable both within and among populations. Furthermore, none of the Rulyrana tangarana View in CoL specimens turned lavender in preservative (as mentioned in the original diagnosis, although our specimens were fixed in ethanol and not in formalin, but this did not prevent other specimens from turning lavender). Presence/absence of melanophores on the ventral surfaces of the tarsi and shanks is variable within populations and is not fixed in either type locality of the nominal taxa. Finally, the inner tarsal fold appears to vary within populations and is also likely influenced by preservation. For example, CORBIDI 14668, (a R. saxiscandens View in CoL from the type locality), has the most prominent inner tarsal fold of any of the specimens mentioned above. This individual also appears to be the most desiccated and also has prominent dorsolateral ridges, which were not visible in life. Thus, the prominence of the inner tarsal fold was likely exaggerated in this specimen due to desiccation. The modification on ulnar and tarsal folds due to preservation artifacts in centrolenids was already mentioned by Kok and Castroviejo-Fisher (2008). Uncorrected pairwise genetic distances among DNA sequences of a fragment of 16S also indicate that the two populations are genetically similar (p-distances 0.0–0.4%, see Table 6 View TABLE 6 ). Furthermore, as they were not recovered as reciprocally monophyletic in the phylogenetic analysis ( Fig. 2c View FIGURE 2 a View FIGURE 2 c ), we consider them to be conspecific.
In the original description, Duellman and Schulte (1993) did not compare Cochranella croceopodes View in CoL with either Rulyrana saxiscandens View in CoL or R. tangarana View in CoL . We studied in detail the types of Co. croceopodes View in CoL ( Fig. 29 View FIGURE 29 ), as well as all available photos of the live types deposited at KU (see Duellman & Schulte 1993). We found that: (1) there are problems in the original description of C. croceopodes View in CoL and (2) the types are extremely similar to the syntopic R. saxiscandens View in CoL , which is similar to R. tangarana View in CoL (see above). Below, we list and discuss the ways in which the original description of Co. croceopodes View in CoL is inconsistent with our own observations on the type specimens and how they fall within the variability observed in R. saxiscandens View in CoL and R. tangarana View in CoL .
The visceral peritoneum of Cochranella croceopodes View in CoL was described as white, but our examination of the holotype revealed that the visceral peritoneum is transparent ( Fig. 29 View FIGURE 29 ), which is also the character state in all specimens of R. saxiscandens View in CoL and R. tangarana View in CoL examined. Cochranella croceopodes View in CoL was described as having a "diffuse yellow line present on flanks from axila to groin" Duellman and Schulte (1993: 21). The diffuse yellow line on flanks is not appreciable in the preserved types as described in Duellman and Schulte (1993: 21). Based on the examination of the photos of the live types, we interpret that this diffuse yellow line is formed by the absence of melanophores on the pigments of the flanks (which are a continuation of the dorsal pigments). A similar diffuse yellow line is also present in two (CORBIDI-HE-2012-14149 and CORBIDI-HE-2012-14151) of the newly collected specimens of R. saxiscandens View in CoL ( Fig. 28 View FIGURE 28. a, b c). We interpret this character as variability in coloration. Cochranella croceopodes View in CoL had shagreened dorsal skin in the description ( Duellman & Schulte 1993, Fig. 2 View FIGURE 2 a , upper left); however, it appears smooth after 23 years in ethanol ( Fig. 29 View FIGURE 29 ). Most specimens of Rulyrana saxiscandens View in CoL have spicules in dorsal surfaces but their distribution and density varies substantially to the point that females of our newly collected specimens have shagreened skin with no spicules. We interpret differences in this character as variability in dorsal skin texture, sexual dimorphism, and effects of preservation. Cochranella croceopodes View in CoL was described as having a snout “nearly truncate in profile” but examination of the holotype reveals that it is round ( Fig. 29 View FIGURE 29 ) as in R. saxiscandens View in CoL . Lastly, examination of photos of the live types of Co. croceopodes View in CoL taken in situ by the authors revealed that those specimens show the same overall coloration pattern, skin texture, and habitus as the newly collected specimens of R. saxiscandens View in CoL and R. tangarana View in CoL .
Taxonomic remarks. Based on available morphological, bioacoustic, and phylogenetic evidence, we hereby consider Cochranella croceopodes View in CoL , Rulyrana saxiscandens View in CoL , and Rulyrana tangarana View in CoL synonymous. Under the Principle of the First Reviser of the ICZN (article 24.2.2) we give precedence to saxiscandens View in CoL , rendering Rulyrana tangarana View in CoL and Cochranella croceopodes View in CoL junior synonyms of Rulyrana saxiscandens View in CoL . We also recognize that Cisneros-Heredia & Guayasamin (in press) arrived at a similar conclusion regarding the synonymy of R. saxiscandens View in CoL and R. tangarana View in CoL .
Distribution and ecology. In addition to the four localities mentioned above, we have also obtained photographic records of this species from two sites in the Cainarachi valley north of Tarapoto ( Fig. 7 View FIGURE 7 ): near the village of Santa Rosa ( 6°24'44.79"S, 76°19'2.09"W, 685 m) and San Jose ( 6°25'14.67"S, 76°17'28.47"W, 517 m).
Considering these nominal taxa as a single species (see ‘Taxonomic remarks’), this species is distributed in the Cordillera Escalera, roughly between the cities of Tarapoto and Moyobamba, at elevations of 517–1047 m. This species occurs in habitats ranging from small creeks to torrential streams (e.g., Ahuashiyacu) to spray zones of waterfalls. This species has never been registered from heavily disturbed habitat, but does occur in secondary forest.
At the type locality of Ahuashiyacu, we have noticed that individuals encountered in the streamside vegetation tend to be bright green whereas those collected from the rocks (particularly near the spray zone of the waterfall) tend to be dark green or black. Members of the genus Rulyrana are thought to be primarily rock-breeding species (Cisneros-Heredia et al. 2008), but we found that they often call from vegetation along rocky streams ( i.e., our observations in both R. mcdiarmidi and R. saxiscandens ). Observed variation in coloration among individuals reflected microhabitat use when collected (vegetation vs. rocks), suggesting that the ability to change color may be a form of crypsis to aid in dynamic microhabitat use.
Neither eggs nor tadpoles have been found from this species. Adults are active in a variety of microhabitats such as small trees (calling males have been heard as high as ~ 4 m), streamside vegetation, and rocks along streams.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Rulyrana saxiscandens ( Duellman & Schulte 1993 )
| Twomey, Evan, Delia, Jesse & Castroviejo-Fisher, Santiago 2014 |
R. flavopunctata (
| Guayasamin et al. 2008 |
Cochranella saxiscandens
| Duellman and Schulte 1993 |
Cochranella tangarana
| Duellman and Schulte 1993 |
Cochranella croceopodes
| Duellman and Schulte 1993 |