Lepas anatifera, Linnaeus, 1758
|
publication ID |
https://doi.org/10.1111/zoj.12373 |
|
persistent identifier |
https://treatment.plazi.org/id/666987EE-CF43-F215-FBF1-FB23FD16FBE8 |
|
treatment provided by |
Marcus |
|
scientific name |
Lepas anatifera |
| status |
|
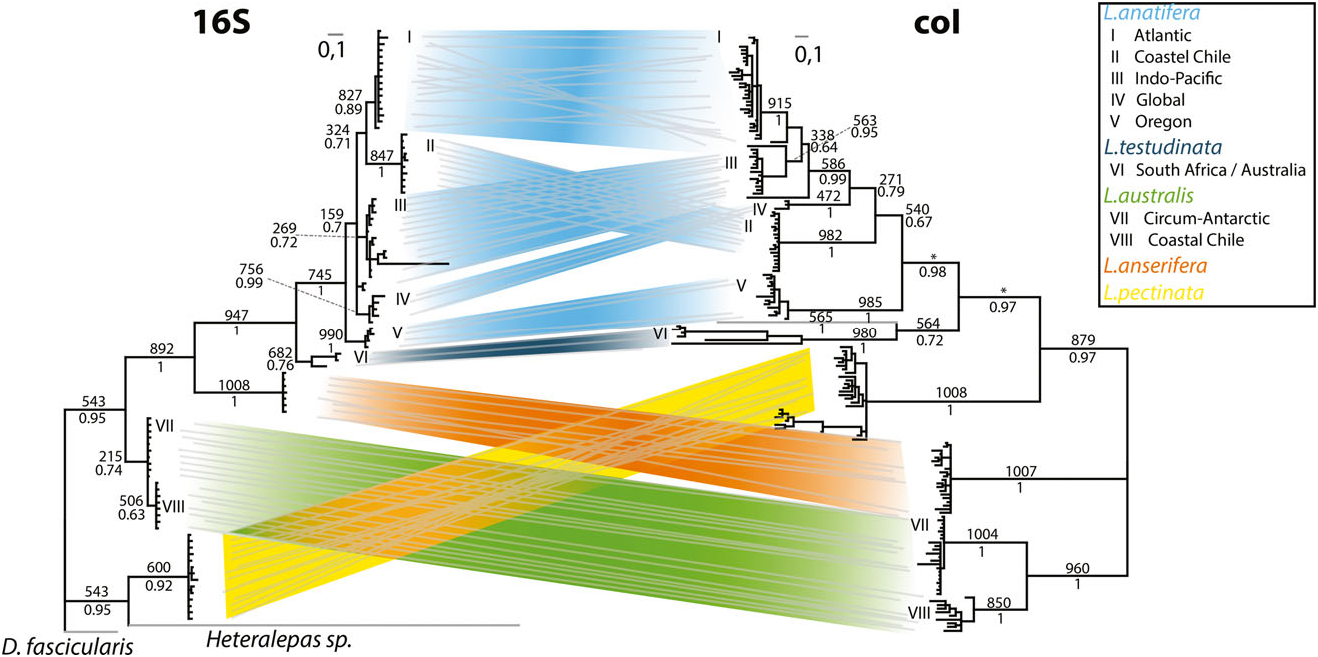
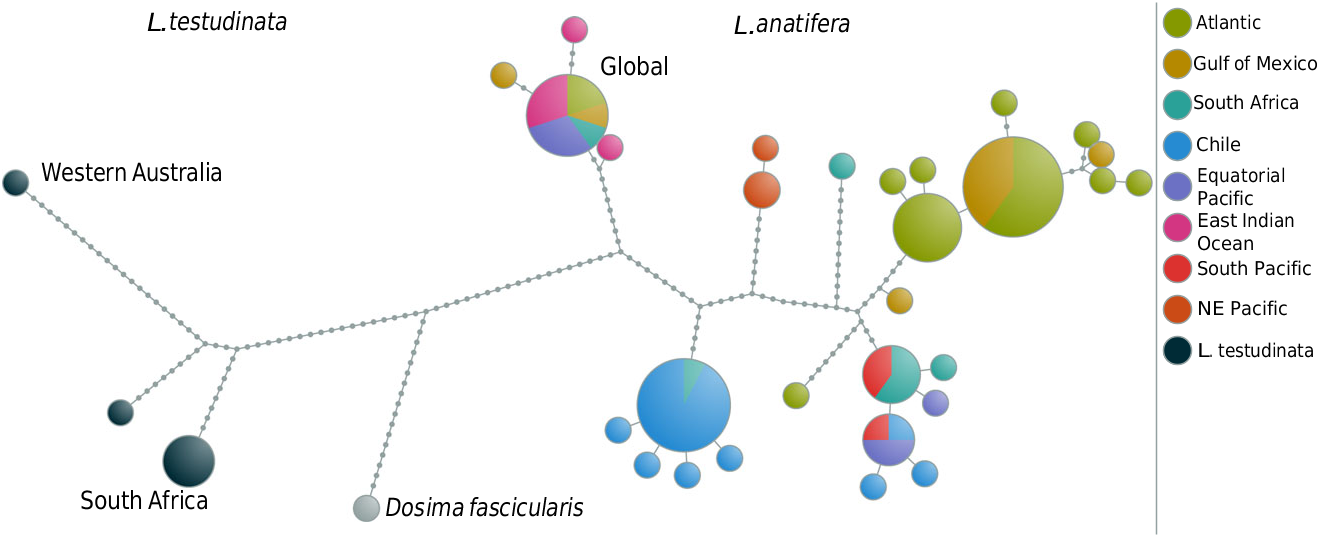
Based on mitochondrial DNA we found five distinct subgroups, which can be clustered into three major divisions.
1. A subgroup collected off the Chilean coast during several sampling campaigns is set apart from all other globally sampled specimens by divergence in mitochondrial and nuclear markers, except for a single specimen. It shares the global nuclear genotype, but is retrieved inside the Chilean group based on its mitochondrial genes. This specimen might have been introduced by human activity.
2. Distinct Atlantic, Indo-Pacific, and Northeast Pacific subgroups based on differing mitochondrial loci within one nuclear genotype.
3. A global subgroup containing specimens from dispersed sampling sites.
Division 1: the coastal Chilean subgroup
The coastal Chilean subgroup is equivalent to our findings in L. australis ( Fig. 6). Obviously, the coldwater system of the Humboldt Current and the asso- ciated upwelling system along the Chilean margin prevent exchange between confined populations of certain organisms, and induce a Chilean phylogeographic province of strongly restricted permeability. Internally it is subdivided by increasing SSTs (see above). The influence of the Humboldt Current is obvious, as the warmer water species L. anatifera is not recorded south of 33 ° S ( Hinojosa et al., 2006).
The divergence of L. australis and L. anatifera in both mitochondrial and nuclear markers indicates an ancient origin of that ‘Chilean waterpocket’, which might be also seen in the present-day tendency of floating debris to accumulate in the South Pacific, west of Chile ( Lebreton, Greer & Borrero, 2012).
Division 2: regional populations with mitochondrial differences
The well-individualized Atlantic subgroup of L. anatifera ( Fig. 6) includes specimens from the Gulf of Mexico and from the Spanish Atlantic coast (Galicia, Gulf of Cadiz), with the Gulf Stream apparently mediating genetic exchange across the Atlantic. Specimens further south from Madeira and from the Cape Verde Islands demonstrate continued rafting within the Canary Current, and thus show wellexpressed circling within the North Atlantic gyre. Finally, specimens from the Brazilian coast (Recife) demonstrate trans-equatorial exchange with the Southern Hemisphere, and thus provide evidence of a single large trans-equatorial Atlantic population. In the relatively narrow, north – south-orientated Atlantic Ocean, the North Equatorial Countercurrent is seasonally less pronounced, and water masses of both hemispheres are exchanged by the western, north-flowing Guiana Current in boreal winter and spring, although some through-flow also occurs in summer ( Csanady, 1990; Condie, 1991). That current system is also in line with data on the distribution of L. pectinata in the Atlantic (see below). A similar pan-Atlantic population has also been observed in the ocean skater Halobates micans Eschscholtz, 1822 ( Andersen et al., 2000), and in the scyphozoan Pelagia noctiluca (Forsskal, 1775) ( Miller, von der Heyden & Gibbons, 2012) .
The most favourable tropical gateway between Pacific and Atlantic marine populations was blocked by the formation of the Panama land bridge about 3.5 – 3.1 Mya ( Duque-Caro, 1990). The resulting genetic divergence in marine taxa has previously been described by Knowlton & Weigt (1998), and more recently by Bacon et al. (2015). As a result of SSTs as low as 8 ° C ( Boyer et al., 2005), drifting around Cape Horn is most unlikely for the warmer water species L. anatifera . In fact, along the Chilean coast L. anatifera dominates at 23 ° S, at a mean SST of 21.9 ° C, but fades towards 33 ° S, at an SST of 18.2 ° C ( Hinojosa et al., 2006). This southern limit at the Chilean coast is in accord with the GBIF database ( GBIF, 2015), which lists only a single specimen, without collecting data further south, at 43.88 ° S. Although somewhat variable, SSTs around the southern tip of South America have not been substantially higher through geological time ( Mashiotta, Lea & Spero, 1999; King & Howard, 2000; Feldberg & Mix, 2002), and therefore this passage has apparently been blocked continuously.
The only feasible remaining genetic exchange between populations from the Indo-Pacific and Atlantic would be around the Cape of Good Hope. The warm Agulhas Current transports organisms from the Indian Ocean to the southern tip of Africa, but is then reflected back eastwards, thus impeding the transport of rafting organisms into the Atlantic. The retroflection of the Agulhas Current is caused by West Wind drift and the Benguela Current, which transports cold water masses across the southern Atlantic towards the south-west African coast, feeding the upwelling system along the south-west African (Namibian) coast ( Lange et al., 1999). The SST of the Benguela Current close to the coast is around 15 ° C ( Clement & Gordon, 1995). Towards the open ocean, this rises up to 19 – 20 ° C during summer, but does not exceed 17 – 18 ° C in winter (e.g. GES DISC, 2012). These relatively cold waters might be outside of the preferential ecospace of L. anatifera , or warmwater seasons might be too short for successful rafting across the area. Besides the general current reflection these seem to be additional factors impeding gene flow. In fact, L. anatifera is reported in a diversified assemblage of goose barnacles from the Cape Town peninsula ( Whitehead, Biccard & Griffiths, 2011), at the westernmost waning of the Agulhas Current, but no record from the Namibian coast is known to us from literature or databases; only three specimens from offshore Luanda ( Angola) are listed in the GBIF database ( GBIF, 2015), and are the only ones found from along the African coast south of Dakar ( Senegal), and south of our samples from approximately the same latitude from the Cape Verde Islands, which are plotted in the global subgroup (see below). The formation of Indo-Pacific and Atlantic echinoid species within the genus Tripneustes was also related to the Benguela Current ( Lessios et al., 2003). Although direct comparison with Lepas might be somewhat problematic, as the pantropical shallow-water echinoids and their planktonic larvae are more sensitive to cold water, the current systems appears to be an ancient and impermeable barrier to other organisms, like the ocean skater H. micans ( Andersen et al., 2000) , the nudibranch G. atlanticus ( Churchill et al., 2014) , and warm – temperate fish ( Henriques et al., 2014). The general bottleneck for marine plankton, caused by the complicated current systems at the southern tip of Africa, was recently elucidated by Villar et al. (2015).
The Benguela Current System is ancient. It was established about 10 – 12 Mya ( Diester-Haass, Meyers & Vidal, 2002; Heinrich et al., 2011). During the Last Glacial Maximum the southern 15 ° C SST isotherm, herein considered as the minimum threshold temperature for L. anatifera , was considerably farther north than it is today, effectively touching the African and West Australian Coasts ( Williams & Benzie, 1998; for present-day SST isotherms, see Fig. 1 View Figure 1 ). Similar shifts have to be assumed for the preceding Pleistocene glacials. These extended intervals of globally decreased SSTs provided first-order barriers to gene flow in L. anatifera : the ‘Algulhas Leakage’ during Pleistocene interglacials that allowed certain species to enter the Atlantic from the Indic ( Vermeij, 2012) obviously did not work for L. anatifera , and nor for G. atlanticus ( Churchill et al., 2014) . In summary, the present-day Atlantic subpopulation of the species ( Brazil, Gulf of Mexico, Atlantic Spain, and Madeira) is related to different ancient vicariance events, namely: (1) plate tectonics (closure of the Panama land bridge); (2) installation and modern persistence of current systems (Benguela Upwelling System); (3) climate variability in time, resulting in shifting ecospace limits (i.e. the extension of low SSTs towards the north during glacial periods) as well as permanent climate barriers (Cape Horn passage).
The Oregon coast subgroup of L. anatifera appears to be related to the North Pacific gyre ( Fig. 6). It seems to be effectively separated from the Southern Pacific and Indic regions by the Equatorial Countercurrent ( F ST 0.93 and 0.94). Notably, the endemic Northeast Pacific species Lepas pacifica Henry, 1940 appears to be very similar to L. anatifera ( Newman & Abbott, 1980, and references therein). As molecular data firmly establish a separate Northeast Pacific subgroup inside L. anatifera , we suggest that L. pacifica might be regarded as a subspecies, L. anatifera pacifica ; however, as our specimens (conserved in in a solution containing dimethyl sulphoxide, disodium EDTA, and saturated NaCl: ‘DESS’, Yoder et al., 2006) were not suitable for in-depth anatomical comparisons, further studies are needed to resolve the taxonomy.
Unfortunately, samples of L. anatifera from Japan are missing, and we cannot comment on relationships between north-western and north-eastern Pacific populations. The separate Oregon coast subgroup supports the importance of the Pacific gyre systems for L. anatifera , however, just opposite to L. anserifera (see below). Species of the ocean skater Halobates , as well as genetically discriminated populations of H. micans and Halobates sericeus Eschscholtz, 1822 ( Andersen et al., 2000; Leo, Cheng & Sperling, 2012), also prove the existence of disjunct northern and southern populations in the Pacific. The rafting bryozoan Membranipora shows a much higher degree of regionalization, but the northern and the southern Pacific clades are clearly separated ( Schwaninger, 2008). Like Membranipora , eucalanid copepods display strong regional diversification ( Goetze, 2003, 2005). Rhincalanus nasutus has five genetically distinct populations in the Pacific ( Goetze, 2003). As for L. anatifera , a north-eastern Pacific population is discerned, but additionally a north-western Pacific population is present. In the southern Pacific, R. nasutus shows separate south-eastern and south-western Pacific populations supplemented by a Sulu Sea population. In contrast to the different R. nasutus populations, L. anatifera forms a spatially extended, huge Indo-South Pacific subgroup, with specimens found from the Juan Fernandez Islands, off Chile, to South Africa, similar to the nudibranch G. atlanticus ( Churchill et al., 2014) . Oceanic surface flow in the ‘Southern Hemisphere supergyre’ ( Speich, Blanke & Cai, 2007) was also evidenced by surface drifting tracer buoys ( Van Sebille, England & Froyland, 2012), and this supports our observations.
Division 3: the global group – a dispersed L. anatifera stemgroup?
Most puzzling is a globally distributed subgroup with specimens from the Gulf of Mexico, the Cape Verde Islands, South Africa ( Cape Town region), Western Australia, and Tonga ( Figs 4 View Figure 4 , 5 View Figure 5 ). One could speculate that this is the most ancestral group, originating from the circumequatorial Tethys before its disruption by Miocene plate tectonic collisions in the Middle East and later closure of the Isthmus of Panama, in the early Pliocene, about 3.5 – 3.1 Mya (Duque- Caro, 1990). Vicariance through both events is also discussed for other taxa, such as the ocean skater Halobates micans ( Andersen et al., 2000) and the green alga Halimeda ( Hillis, 2001) . The same appears to be supported by our F ST values from the rapidly evolving COI gene, indicating a lack of gene flow between the subgroup and other L. anatifera subgroups. Interestingly, the specimens acquired from the Western Australian Museum were putatively determined as Lepas indica Annandale, 1909 (A. Hosie, pers. comm.). As they share the global nuclear L. anatifera genotype, they do not belong to a distinct species. The original determination of Annandale (1909) as L. anatifera indica might thus be taxonomically correct, but further in-depth analysis of type material is needed for clarification, as is the case for ‘ L. anatifera pacifica ’ discussed above.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.