Calliophis castoe, Smith, Eric N., Ogale, Hemant & Giri, Varad B., 2012
|
publication ID |
https://doi.org/ 10.5281/zenodo.211535 |
|
DOI |
https://doi.org/10.5281/zenodo.6176704 |
|
persistent identifier |
https://treatment.plazi.org/id/666D878B-2A50-2B78-2DB3-7092FE6C2770 |
|
treatment provided by |
Plazi |
|
scientific name |
Calliophis castoe |
| status |
sp. nov. |
Calliophis castoe sp. nov.
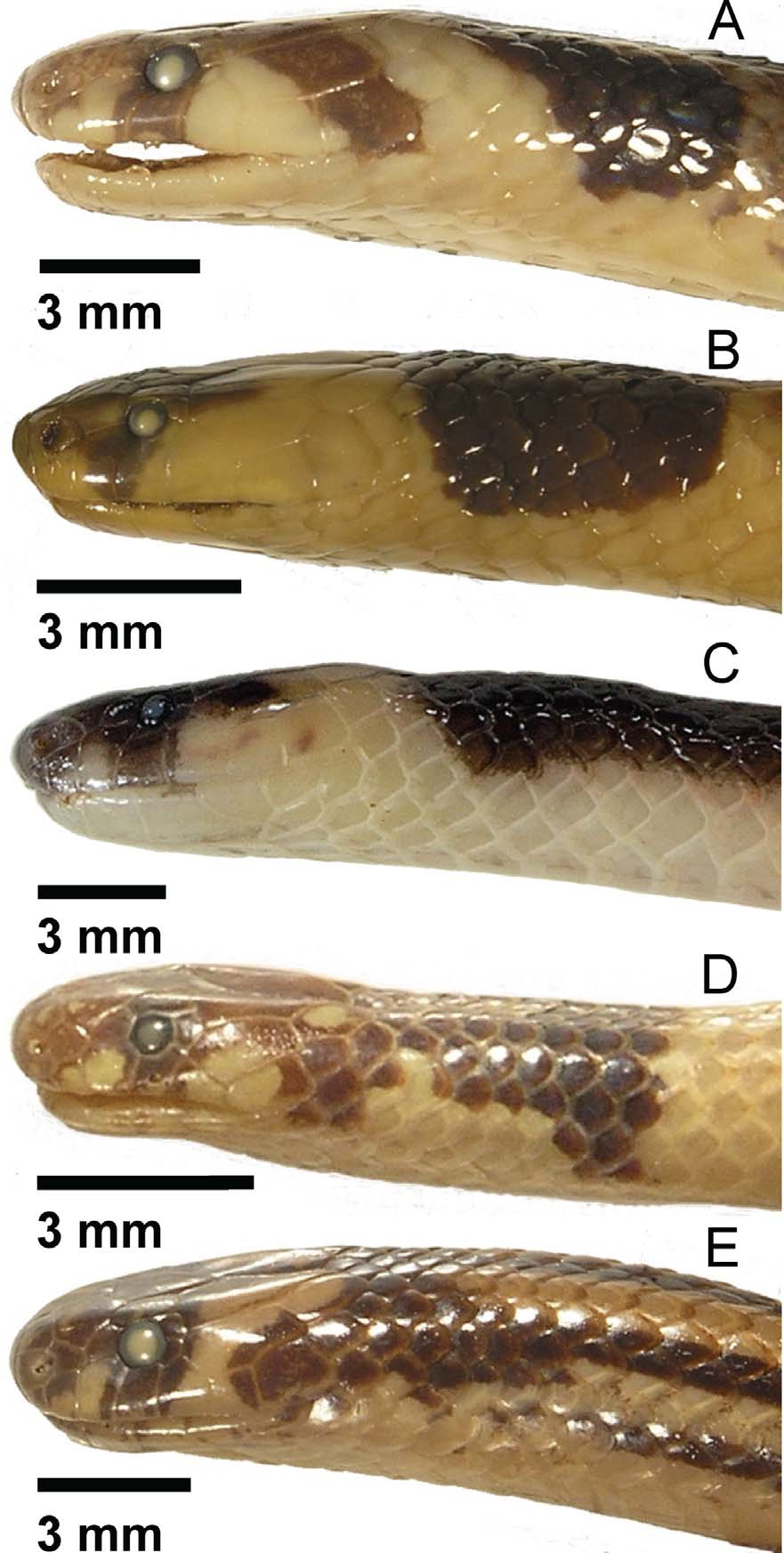
( Figs. 1–7 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 )
Callophis nigrescens View in CoL ( Phipson 1887: 245, 248, Carwar [Karwar], Bombay Presidency specimen; Vidal 1890: 65 –66, in part, North Kanara specimen)
Hemibungarus nigrescens variety khandallensis ( Wall 1913: 638, in part, Karwar specimen)
Hemibungarus nigrescens ( Wall 1928: 22, 35, in part)
Hemibungarus nigrescens variety A ( Wall 1928: 36, Karwar specimen)
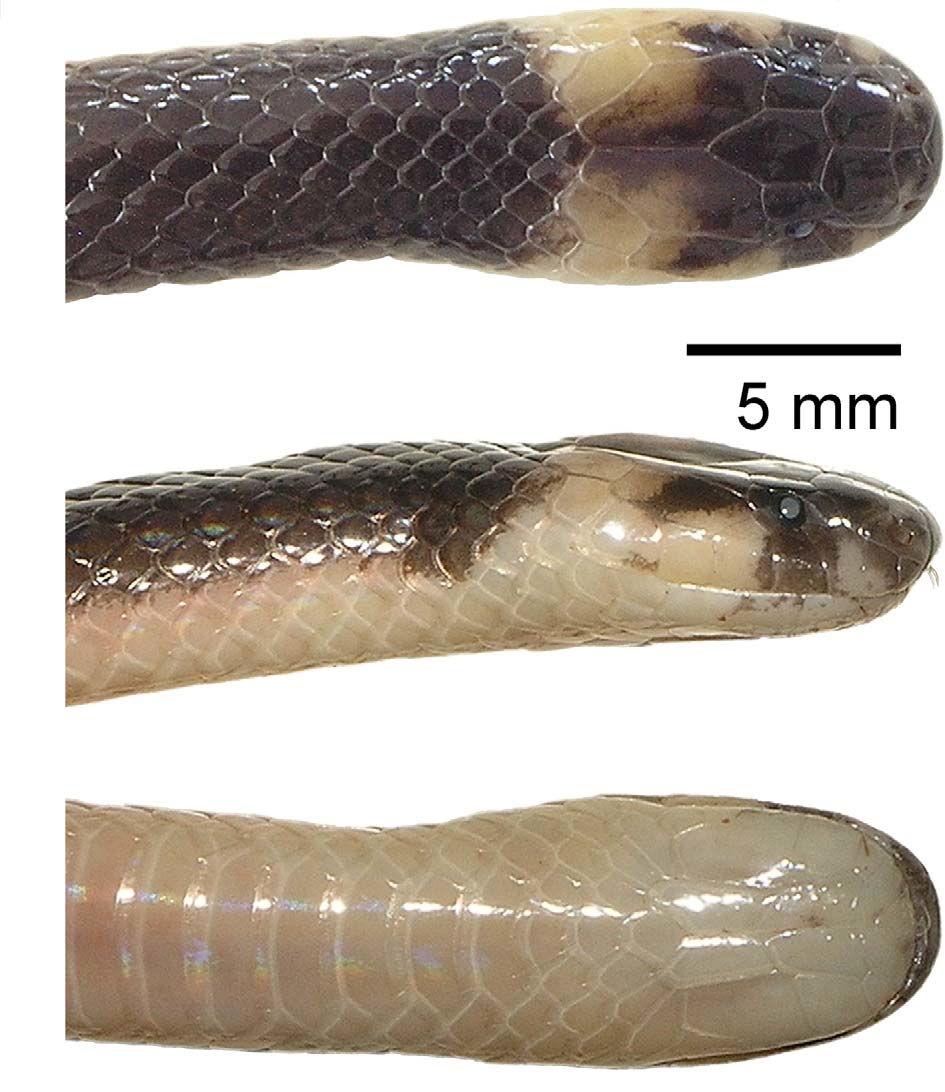
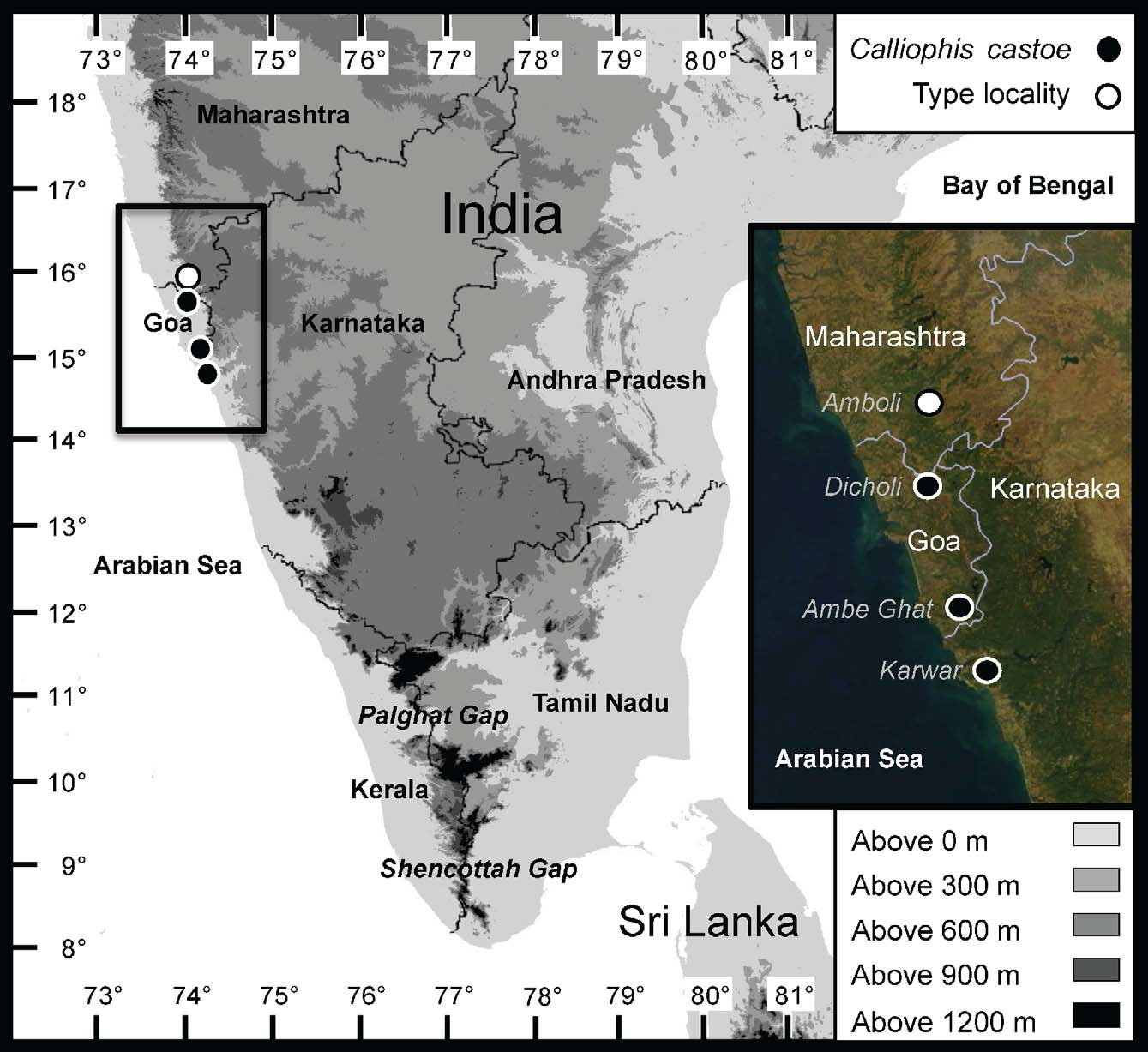
Holotype. BNHS ( Bombay Natural History Society, Bombay, Maharashtra, India) 3461, an adult male from Amboli, Sindhudurg district, Maharashtra, India, [ca. 715 m] (ca. 15.958790° N 73.994686° E), collected 12 September 2009 by Hemant Ogale ( Figs. 3–4 View FIGURE 3 View FIGURE 4 , 6–7 View FIGURE 6 View FIGURE 7 ).
Paratypes (2). BNHS 2191, an adult male from Karwar, Karwar [Uttara Kannada district], Karnataka, India, [ca. 15 m] (ca. 14.804947° N 74.133317° E), collected between 1880 and 1887 by G. Vidal (1907 date of collection in BNHS catalogue in error, specimen reported by Phipson in 1887 and Vidal in 1890) ( Fig. 5 View FIGURE 5 ). BNHS 3474, a subadult female from Ambe Ghat, South Goa district, Goa, India, 295 m (15.06400° N 74.16578° E), collected 30 June 2010 by Ravindra Bhambure, Harish Kulkarni, and Varad B. Giri ( Fig. 2 View FIGURE 2 ).
Referred digital photo (1). UTADC (Digital Collection, The University of Texas at Arlington, Arlington, Texas, United States of America) 6738–45, photos of adult male from Dicholi (Bicholim), North Goa district, Goa, India, ca. 10 m (ca. 15.59° N 73.95° E), photographed on 29 July 2009 by Hemant Ogale ( Fig. 1 View FIGURE 1 ).
Diagnosis. A medium (536–540 mm TL, mature males), brownish, terrestrial coralsnake in which the tail comprises 12.4–14.0% of the TL in the two known male vouchered specimens and 12.0% in the known female. The maxilla bears 4 maxillary teeth behind the fang, the dentary 10, the palatine 9 and the pterygoid 2. It has sublabial-chin-shield contact variable, usually 7 supralabials (8 on one side of one specimen), 6/6 infralabials, two postoculars, 240–254 ventrals, a divided anal, 45–53 divided subcaudals, dorsal scale rows arranged in 13 rows along entire body, and a color pattern of a wide parietal orange band, an unpatterned vinaceous-brown dorsum, a white lower lip, and Salmon Color to Flame Scarlet ventral and lateral areas (from neck to tail).
The new species can be distinguished from all Asian and American coralsnakes, except for Sinomicrurus japonicus ( S. j. boettgeri [ Fritze, 1894], S. j. japonicus [ Günther, 1868], S. j. takarai [ Ota, Ito & Lin, 1999]) and some Calliophis maculiceps ( Günther, 1868) , in having the highest number of maxillary teeth behind the fang, four on each side. The species of coralsnakes with the next highest counts are C. beddomei Smith, 1943 and C.
nigrescens Günther, 1862 with two or three, S. hatori ( Takahashi, 1930) and S. sauteri ( Steindachner, 1913) with two, C. maculiceps with one, two and rarely four, and some populations of S. macclellandii ( Reinhardt, 1844) with none, one, or two teeth. Sinomicrurus japonicus can have from 3 to 5 maxillary teeth behind the fang. The new species can additionally be distinguished from all species of Asian coralsnakes ( Calliophis Gray, 1835 [ Maticora Gray, 1835 ] and Sinomicrurus Slowinski, Boundy & Lawson 2001 ), except some C. beddomei , in lacking contact between the preocular and nasal, allowing the prefrontal and third supralabial to touch. Calliophis bibroni ( Jan, 1858) also has the prefrontals touching the supralabials but lacks preoculars and has a banded body pattern. Calliophis castoe also differs from all other Indian coralsnakes, including C. beddomei and with the exception of some individuals of S. macclellandi , in having an unpatterned body, no dark pigmentation on the last supralabial, and a wide post-temporal light band. Calliophis beddomei has a pattern of dorsal spots adjacent to a middorsal stripe, never being dorsally unicolored. Calliophis nigrescens usually has a striped pattern but occasionally (variety C. n, khandallensis [ Wall, 1913]) has an obscured pattern that seems unicolored black. Nevertheless, these always have a dark stripe or spot covering part of the last supralabial and a dark nuchal band fused or very close to the head cap, both never the case in C. castoe . The underside of the tail differs between C. castoe and C. nigrescens , being uniformly orange in the former and red with white-bordered scales in the later. Some specimens of C. melanurus ( Shaw, 1802) have a nearly unicolored dorsal pattern, but always possess two tail bands, absent in the new species, and the nuchal band and the head cap are broadly fused (widely separated in the new species).
The new species can be further distinguished from other coralsnakes. From species in the C. melanurus group, according to Smith et al. (2008) ( C. haematoetron Smith, Manamendra-Arachchi & Somaweera, 2008 , C. melanurus , and C. maculiceps ), the new species differs in lacking a bluish ventral tail color and melanized tail base muscles and associated tissues. It can also be distinguished from C. melanurus , in having more supralabials (6 vs. 7 or 8) and subcaudal scales (24–37 vs. 45–53). From C. maculiceps it can also be distinguished by its high number of ventrals (240–254 vs. 169–222) and subcaudals (45–53 vs. 20–31). From C. bibroni it can be distinguished by having a preocular (vs. no preocular), two postoculars (vs. 1), no tail bands (vs. 3–9), a divided anal (vs. single), and higher ventral (240–254 vs. 220–234) and subcaudal counts (45–53 vs. 26–37). Besides differing in dentition, color pattern and head scalation the new species differs from C. nigrescens in having relatively higher subcaudal counts (45–53 vs. 29–48) and fewer pterygoid teeth (2 vs. 5–8). Calliophis beddomei also has more pterygoid teeth (2 vs. 4). Calliophis castoe differs from C. gracilis Gray, 1835 in possessing fewer ventral scales (240–254 vs. 303–320), more subcaudal scales (45–53 vs. 21–23), a unicolored dorsal pattern (vs. large and paired paravertebral spots and 5–7 well-defined stripes), and a venter with no bands (vs. numerous regularly spaced wide bands).
From the long-glanded coralsnakes Calliophis bivirgata ( Boie, 1827) and C. intestinalis ( Laurenti 1768) , previously in the genus Maticora (see Slowinski et al. 2001), the new species differs in having a venom gland that is confined to the temporal region (vs. extending behind the head), a Harderian gland with a moderately developed posterior extension (vs. enlarged posterior extension, larger than the eyeball), and a unicolored dorsum (vs. striped).
From species in the genus Sinomicrurus , i.e., S. hatori , S. japonicus , S. kelloggi ( Pope, 1928) , S. macclellandi , and S. sauteri (sensu Slowinski et al. 2001) , the new taxon differs in possessing no protuberant sclerified tail tip, and a Harderian gland with a moderately developed posterior extension (vs. no extension). It can further be distinguished from S. hatori , S. japonicus , and S. sauteri in having no pattern of stripes, and from S. kelloggi and S. macclellandi in having no white band anterior to the nuchal band.
Etymology. It is a pleasure for us to name this beautiful coralsnake after Todd A. Castoe , a talented and prolific scientist, and a partner in the study of coralsnake and pitviper systematics. The first author has worked on venomous snakes with him and shared “coralsnake trips” to Colombia, México and India. During a trip to India, we first examined and realized the uniqueness of the species herein described. Because the Latin word castus means pure, the specific epithet is also reminiscent of the unmarked dorsum characteristic of the species.
Suggested English name. Castoe’s coralsnake
Description of holotype and variation. Features of the adult male holotype are followed in parentheses by variation of the adult male and subadult female paratypes. Total length 536 mm (540, 313); tail length 75 mm (67, 38); head length 8.0 mm (10.0, 6.5) from anterior edge of rostral to posterior end of mandible; head width 6.1 mm (5.6, 3.7) at broadest point; head slightly distinct from neck; snout 3.4 mm (3.4, 2.3) from front of rostral to anterior edge of eye; eye 0.2 (0.2, 0.2) times length of snout; pupil round; rostral 1.3 (1.4, 1.2) times wider than high; internasals 1.1 (1.1, 1.4) times wider than long, contacting only the nasals laterally; length of internasal suture 1.1 times diameter of eye (1.3, 0.9); prefrontals slightly wider than long (as wide as long, slightly wider than long), in contact laterally with nasal, third supralabial, preocular, and supraocular; prefrontal suture 1.8 (2.0, 1.5) times diameter of eye; frontal 1.6 (1.5, 1.4) times longer than wide; supraoculars 1.6 (1.4, 1.6) times longer than wide; parietals 2.1 (2.1, 2.3) times longer than wide; parietal suture 0.6 (0.7, 0.6) times length of parietals, 0.9 (1.1, 1.1) times longer than frontal; 1+0 temporals and one posttemporal, shields touching parietal laterally, large and elongated; temporal 2.3 (2.1, 2.5) times longer than wide; single preocular, 1.0 (1.4, 1.2) times longer than wide, lanceolate (rhomboidal), with apex rostrally, located mostly above line between center of eye and posterior border of naris; two postoculars, of about the same size, upper and lower, reaching beyond upper (or just reaching) and lower borders of eye, respectively; no loreal, preocular and nasal not in contact, prefrontal touching third supralabial; 7/7 (8/7 [higher count due to what appears to be a scale split due to injury], 7/7) supralabials, seventh largest and longest, first in contact with anterior nasal, second in contact with both nasal plates; third in contact with posterior nasal, prefrontal, preocular, and fraction of orbit; fourth below orbit and contacting lower postocular, fifth in contact with lower postocular and temporal, sixth in contact with temporal, and seventh in contact with temporal and posttemporal; mental 1.5 (1.4, 1.2) times as broad as long; anterior chin-shields 2.0 (2.1, 1.8) times longer than wide; posterior chin-shields 2.0 (2.6, 2.6) times longer than wide; 6/6 infralabials, first pair in contact behind mental, second small, second and third touching anterior chin-shields, fourth largest and contacting anterior and posterior chin-shields and first sublabial, fifth and sixth contacting sublabials; first sublabial touching chinshields (or not, in adult paratype); 2 (2, 2) gulars and 1 (1, 2) preventrals at midline between posterior chin-shields and first ventral; with few tubercles on head scales, concentrated anteriorly; dorsals in 13 rows, smooth, unreduced; apical pits absent; ventrals 240 (254, 254); anal divided; preanal single; subcaudals 53 (45, 46), paired; tail complete, tip round; no anal ridges or tubercles; no umbilical scar noticeable.
Dentition of paratype BNHS 2191 examined in detail: maxillae bearing one fang 1.1 mm long, arising below supralabials 2 and 3, slanted backward; four posterior maxillary teeth on each side, first at about one fang length behind fang and largest, caudally smaller, slanted backward, below supralabials 3–5; 9/9 palatine teeth; 2/2 pterygoid teeth; 10/10 dentary teeth, decreasing in size from front to rear. Holotype also with four posterior maxillary teeth on right side, other teeth bearing bones not examined.
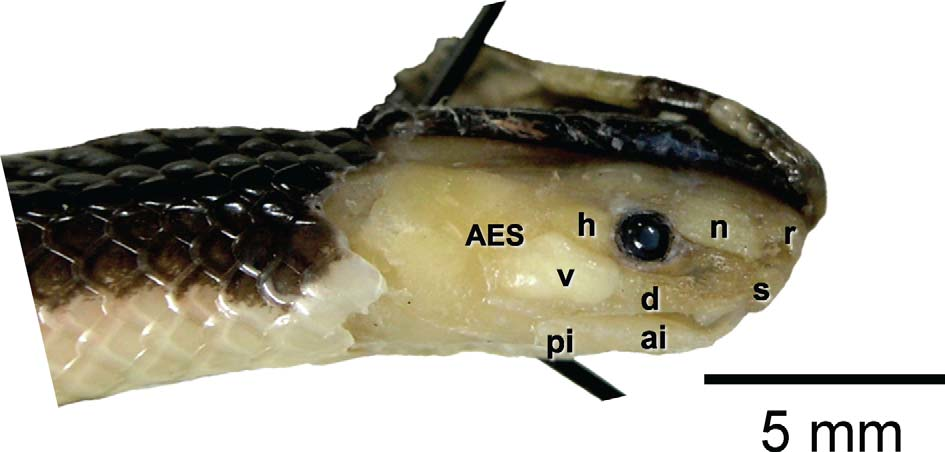
Head glands examined on right side of holotype by reflecting head skin (UTADC 6733–34, Fig. 6 View FIGURE 6 ): granular gland situated under rostral shield; salivary gland developed under supralabials 1–3; nasal gland occupying area below prefrontal shields (prefrontal shields, posterior nasal, and preocular), 1.18 mm wide, 1.93 mm long, rhomboid; Harderian gland under anterolateral portion of parietal (and posterior of supraocular, upper postocular, and anterior area of temporal), 1.41 mm long, 0.80 mm wide, triangular, apex caudal, with a moderate posterior extension; venom gland triangular (rounded anteriorly and posteriorly), corners at middle of border between fourth and fifth supralabial, middle and back of sixth supralabial, and middle of temporal at level of middle of eye, 1.45 mm wide, 2.70 mm long, not inflected ventrally and confined to head; venom duct 2.73 mm in length; infralabial gland bordering mouth under lateral tips of mental to middle of fifth infralabial, with two areas differentiated, one anterior and longer, and one posterior under the fourth and fifth infralabials; the anterior infralabial area is 3.11 mm in length, overlapping with the posterior infralabial area of 1.59 mm in length; salivary, nasal, Harderian and infralabial glands yellowish and of irregular texture (granular), venom gland whitish and smooth; m. adductor mandibulae externus superficialis (AES) forming a continuous loop, from upper parietal surface above and behind Harderian gland to insertion on compound bone.
Left hemipenis of holotype exposed in situ, slightly bifurcated, spinous, reaching level of subcaudal 6; hemipenis and associated muscles (m. retractor penis magnus, m. propulsor, and subvertebral and medial hypaxial musculature) and m. constrictor sacculi ani not covered by melanic epymisium; spines numerous, present throughout the length of the organ, from base (level of subcaudal 1) to tip, spines slightly larger at level of third and fourth subcaudal; cloacal scent glands oval, ending at level of subcaudal 2.
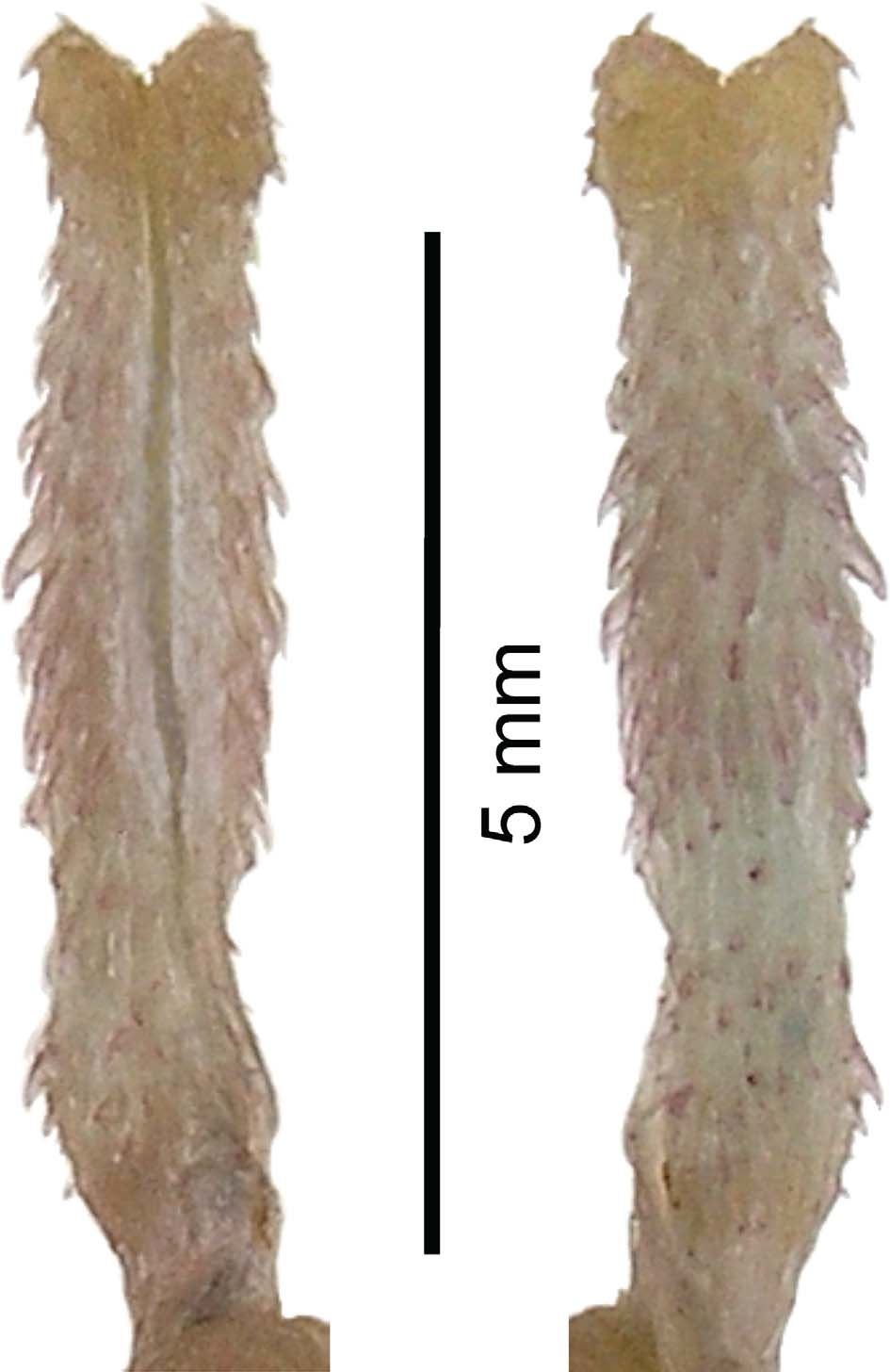
Right hemipenis of holotype, removed, fully everted and partially expanded ( Fig. 7 View FIGURE 7 , UTADC 6733–34), is bilobed, about 6.2 mm in length, and 1.3 mm in width, at apices. The organ includes a pedicel with tiny spines for the first 1.5–2 mm. Between 1.5 mm and the tips of the lobes there are spines all around the hemipenis. At midhemipenis there are 17 spines around the organ. Spines diminish in size distally, from about 0.4 mm at about 2 mm from the base to about 0.1 mm near the tips. The hemipenis bifurcates 0.3 mm before the terminus and the sulcus spermaticus bifurcates approximately 0.1 mm before the hemipenial furcation. The sulcus spermaticus is bordered at the base (sinistrally) by a flap-like fold, is centripetal, and terminates distally on each lobe. There are no grooves, flounces, papillae, or calyces.
Hemipenes of male paratype BNHS 2191exposed in situ and dissected, differ from those of holotype in being relatively larger, reaching level of subcaudal 7 and also bifurcating at this level; also without any melanic epymisium; level of insertion of m. retractor penis magnus not examined; spines also numerous, throughout the length of the organ; cloacal scent glands slender and dessicated, difficult to examine.
Color ( Figs. 1–5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 ). Holotype, coloration of recently killed specimen as recorded with a Nikon D90 digital camera, UTADC 6724–31: Dorsum of head and body Dusky Brown (19), turning Burnt Sienna (132) towards sides of body, with no dorsal bands or blotches over body; dark color extending to upper half of scale row 2, on body, and one half scale more in neck area; Dusky Brown (19) color on top of head extends as suborbital and temporal markings, restricted to rostronasal, circumorbital, frontal, and anterior and medial parietal surfaces; small dark markings covering part of lower edge of seventh supralabial, on right side, and upper edge of fourth infralabials; chin and area between supralabials 2 and 3 Pale Horn Color (92); subtemporal surface Chamois (123D) to Yellow Ocher (123C), turning whitish Pale Horn ventrally; head band interrupted by interparietal Dusky Brown (19) coloration, band occupying posterolateral surface of parietal, posttemporal, posterior labials, and first two rows of lower neck scales, Spectrum Orange (17) above, Chamois (123D) to Yellow Ocher (123C) laterally, whitish Pale Horn ventrally; body flanks, first two scale rows, Salmon Color (106) anteriorly to Flame Scarlet (15) posteriorly; no spots on ventral scales; body venter Salmon Color (106), anteriorly, to Flame Scarlet (15), towards anal plate; underside of tail and first row of dorsal scales on tail Burnt Orange (116), slightly darker near vent.
Referred specimen, in life, as recorded with a Nikon D90 digital camera, UTADC 6738–45 ( Fig. 1 View FIGURE 1 ): Similar to holotype; Dorsum of head, neck, and tail Dusky Brown (19) to Warm Sepia (221A); dorsum of body Deep Vinaceous (4), slightly darker and brownish middorsally, with no dorsal bands or blotches; dark color extending to upper half of scale row 2, on body, and one more scale on tail area; Warm Sepia (221A) color on top of head extends as suborbital and temporal markings, restricted to rostronasal, circumorbital, frontal, and anterior and medial parietal surfaces; chin and area between supralabials 2 and 3 pale Flesh Color (5); Dusky Brown (19) interparietal mark not interrupting posterior half of head band; subtemporal and posttemporal surfaces Spectrum Orange (17), turning Vinaceous (3) ventrally; body flanks, first two scale rows, Vinaceous (3) anteriorly to Flame Scarlet (15) posteriorly; no spots on ventral scales; first row of dorsal scales on tail Burnt Orange (110), slightly darker near vent.
Subadult female paratype (BNHS 3474) coloration, in life, as recorded with a Nikon D300S digital camera, UTADC 6831–32 ( Fig. 2 View FIGURE 2 ): Dorsum of head and body Burnt Sienna (132), turning Raw Umber (223) towards sides of body, with no dorsal bands or blotches over body; dark color extending to upper half of scale row 2, on body, and one half scale more in neck area; Burnt Sienna (132) color on top of head extends as suborbital and temporal markings, restricted to rostronasal, circumorbital, frontal, and anterior and medial parietal surfaces; small dark markings covering part of upper edge of third and fourth infralabials, on right side; underside of head Pink (7); subtemporal and middle of third infralabial surface Chamois (123D), turning Pale Horn ventrally; head band interrupted partially by interparietal Burnt Sienna (132) coloration, band occupying posterolateral surface of parietal, posterior surface of temporal, posttemporal, posterior labials, and first two rows of lower neck scales, Chamois (123D) above, Pale Horn ventrally; body flanks, first two scale rows, Warm Buff (118) to Vinaceous (3), towards venter; no spots on ventral scales; body venter Vinaceous (3) anteriorly, Deep Vinaceous (4) at midbody, Salmon Color (106) at posterior third of body, to Spectrum Orange (17), before anal plate; anal plate Pale Horn Color (92); tail venter Spectrum Orange (17).
Holotype in preservative ( Figs. 3–4 View FIGURE 3 View FIGURE 4 ): Dorsum Sepia (119), with Pale Horn Color (92) markings on head; chin Pearl Gray (81); body venter Beige (219D) anteriorly, turning Vinaceous Pink (221C) posteriorly; underside of tail Vinaceous Pink (221C), anal plates slightly lighter.
Male paratype, after more than 100 years in preservative ( Fig. 5 View FIGURE 5 ): Dorsum Antique Brown (37), darker above, with Pale Horn Color (92) markings on head; chin Pearl Gray (81); body venter Drab-Gray (119D) anteriorly, turning Pale Horn Color (92) posteriorly and under tail.
Habitat, distribution and natural history ( Figs. 8–11 View FIGURE 8 View FIGURE 9 View FIGURE 10 View FIGURE 11 ). The four known localities for Calliophis castoe lie in three different states along the Malabar Coast and adjacent Western Ghats of India, in Goa, Karnataka and Maharashtra. The altitudinal range for C. castoe is from near sea level (ca. 10–15 m) in Goa and Karnataka to about 715 m in southern Maharashtra. The mean annual precipitation near the lowland localities has been recorded between 2900 and 3500 mm, and at the type locality of Amboli it is known to reach 7445 mm ( Yadav & Sardesai 2002; Jog 2009). The region experiences precipitation between 215 and 265 days per year ( Champion & Seth 1968), with most rain (about 80%) falling between June and August ( India Meteorologial Department 2010). According to the forest type classifications by Champion (1936) and Champion and Seth (1968) the two lowland sites are within Tropical Semi-Evergreen Forest and the upland locality within Tropical Wet Evergreen Forest. Presently the Amboli and Karwar localities support dense forests and/or secondary growth, but the Goa locality of the referred specimen lies in the periphery of the heavily populated town of Dicholi and is only covered by subsistence crops dominated by coconut groves. The referred specimen from Goa was found at the house of Amrut S. Singh, a local from the Animal Rescue Squad of Dicholi, who reported to us that this distinctive species is very common in the area. The holotype was found freshly killed on a road, at 1815 h. The small female paratype comes from Ambe Ghat, Goa, a locality near the south border of the state. This specimen was found at a distance of less than 4 m from a specimen of C. nigrescens (BNHS 3475).
Relationships. Calliophis castoe is probably closely related to highland Calliophis with longitudinal stripes or stripes and spots that also inhabit wet forests in peninsular India, namely, C. nigrescens and C. beddomei . These species share a small and spinous hemipenis with only slight terminal bilobation, short sulcus furcation, and no associated basal pocket. Some C. beddomei specimens share with C. castoe a contact between the prefrontal and third supralabial, but C. castoe has an unpatterned body, no dark pigmentation on the last supralabial, and a wide post-temporal light band. Calliophis beddomei has a pattern of dorsal spots adjacent to a middorsal stripe, never being dorsally unicolored, and has lower ventral (Males 228–242 vs. 240–254; Females 216–222 vs. 254) and subcaudal (Males 41–46 vs. 45–53; Females 34 vs. 46) scale counts. When compared to C. castoe , C. nigrescens has a pattern of stripes or stripes and spots along the body (no patterns in C. castoe ) and lower subcaudal counts (Males 33–48 vs. 45–53; Females 29–39 vs. 46). In life, specimens of C. nigrescens we have seen, have bright-red or coral-red subcaudal scales with white borders. These white borders are more prominent at the midline suture, forming a zigzag midventral white stripe. The underside of the tail of C. castoe is uniformly orange, with no white borders. As mentioned above, Calliophis castoe has more maxillary teeth behind the fang and less pterygoid teeth than either C. beddomei or C. nigrescens . Hemipenial morphology clearly relates the new species to C. beddomei and C. nigrescens , but no exclusive sister relationship between any two of these species is clear from the characters presented.
Comparison of the DNA sequences obtained for one specimen of C. castoe (Paratype BNHS 3474, Genbank accession number JQ282155 View Materials ) and the specimen of C. nigrescens ( var. khandallensis ) found in close proximity to the first (BNHS 3475, Genbank accession number JQ282156 View Materials ) shows high genetic differentiation, supporting our new species description. The sequences were 12% different, having 705 bp of identical sequence on an 807 bp alignment. The ND4 protein-coding segment (666 bp) showed no indels between the two taxa, and differed in 14 non-synonymous and 76 synonymous substitutions. The C. nigrescens sequence had five deletions and seven other changes in its tRNA coding segment, as compared to that of C. castoe . One deletion is in the D-loop of the tRNA- His and four occur in the Acceptor-stem (two 1-bp deletions) and contiguous D-Arm (2 bp, probable adjacent 2-bp deletion) of the tRNA-Ser(AGY). The striped coralsnakes of peninsular India exhibit remarkable variation in color pattern and lepidosis, deserving a more thorough analysis comparing morphology and molecules when more material becomes available.
| BNHS |
Bombay Natural History Society |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.