Trichophoromyia peixotoi, Rodrigues & Pinto & Galati, 2023
|
publication ID |
https://doi.org/ 10.1186/s13071-023-05850-w |
|
persistent identifier |
https://treatment.plazi.org/id/67495434-FFF9-950E-997C-FE15FE45B868 |
|
treatment provided by |
Felipe |
|
scientific name |
Trichophoromyia peixotoi |
| status |
|
Genus Trichophoromyia Barretto, 1962 View in CoL
Trichophoromyia peixotoi n. sp. Rodrigues, Pinto & Galati ( Figs. 1 View Fig , 2 View Fig , 3 View Fig , 4 View Fig , and 5)
Diagnosis: Te male of this new species can be distinguished from the others of the genus Trichophoromyia by characters of the terminalia, including the paramere with a triangular median lobe in the dorsal margin, and a rectangular paramere apex covered with fine bristles, in addition to the presence in the gonocoxite of a median cluster with about 12 thick and long setae.
Holotype description (male)
Insect predominantly brown in colour, with the pleurae pale. Pronotum, mesonotum, and metanotum dark brown. Katepisternum and katepimeron brown. Paratergite, upper anepisternum light brown. Medium-sized sand fly, total body length (from the head to the apex of the gonostylus) 2883 (3028.6; 88.71; n = 9).
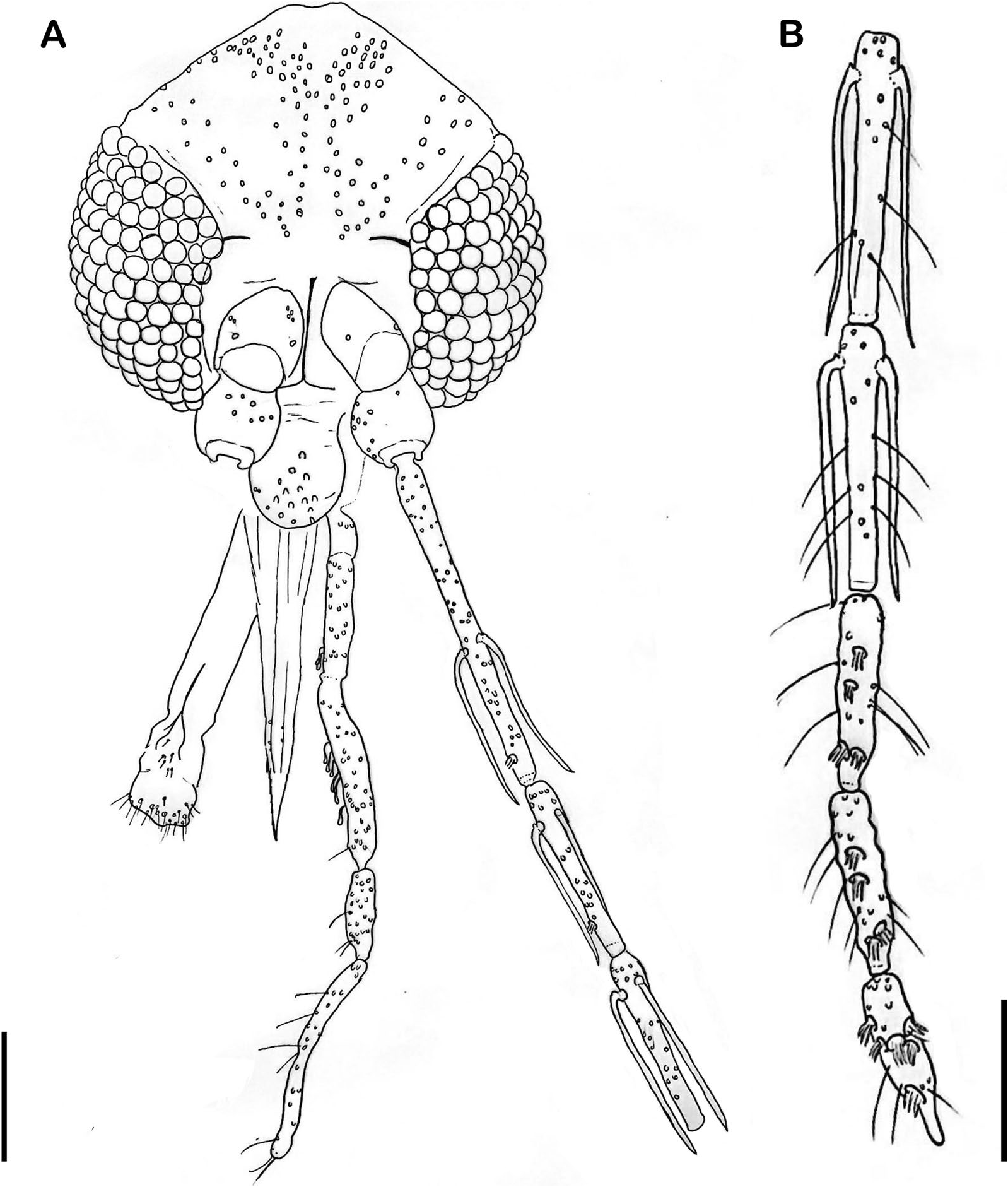
Head ( Fig. 1 View Fig ): length 373 (364.5; 9.9; n = 10), width 333 (331.1; 9; n = 9). Clypeus length 104 (100.2; 3.8; n = 10). Eye length 212 (206.7; 10.3; n = 10), width 101 (103.3; 4.9; n = 9). Interocular distance 132 (129.3; 4.3; n = 9). Interocular and interantennal sutures unconnected. Flagellomere lengths: FI 242 (222.7; 11; n = 9) FII 127 (121.2; 6.5; n = 9), FIII 124 (122.2; 4.6; n = 9), FXIII 69 (66.3; 2.9; n = 9), FXIV 58 (56.6; 4; n = 9). Labroepipharynx 221 (212; 8; n = 9). Preapical papilla present in FI and FII, but absent in FIII. Presence of apical, median, and basal papillae in FXII – FXIV. Simple setae present in FV– FXIV. Ascoidal formula FI–FXI 2, FXII – FXIV 0; ascoids long with rudimentary posterior spur, and the anterior projection reaching the basis of the subsequent flagellomere; internal/external ascoids implanted at nearly the same level; for FI, the external is slightly more basal than the internal. Labial sutures united. Palpal formula: 1.4.2.3.5. Newstead’s sensilla present in second palpal segment, and scattered in the third palpal segment. Length of the palpal segments: PI 33 (34.9; 3.1; n = 10), PII 85 (88.7; 3; n = 10), PIII 136 (129.4; 4.2; n = 9), PIV 58 (55.7; 3.3; n = 9), PV 159 (155.8; 8.7; n = 9).
Cervix: Ventro-cervical sensilla absent.
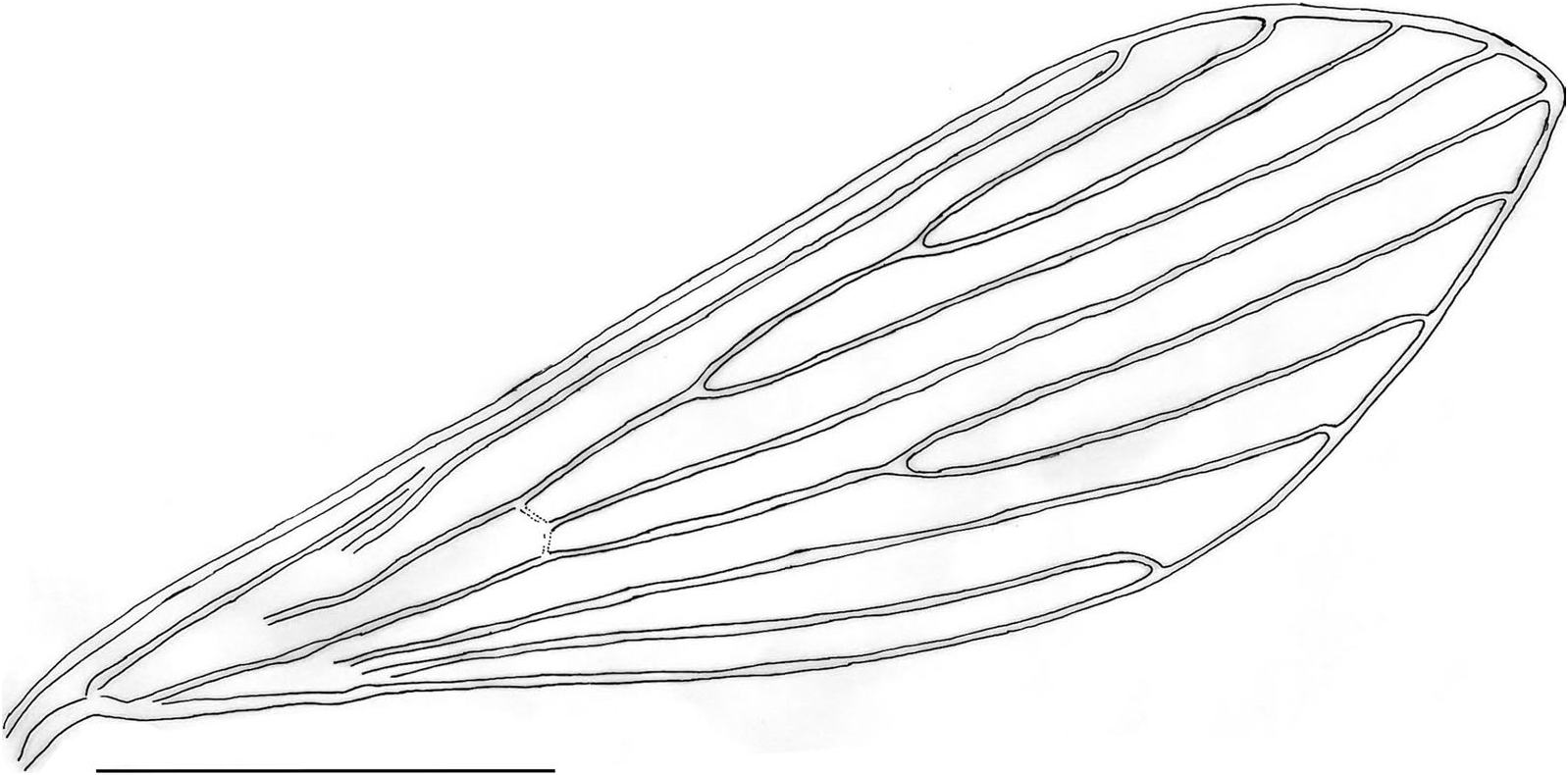
Torax: length 525 (538.8; 21.2; n = 10), mesonotum length 501 (498.9; 18.8; n = 10). Pleurae with four proepimeral setae (3–4; n = 10) and 10 upper anepisternal setae (9–15; n = 10). Cervical sclerite with two sensilla. Wing ( Fig. 2 View Fig ), length 2062 (2058.3; 58.5; n = 10), width 561 (573.9; 35.4; n = 10). Length of vein sections: R 5 1261 (1289; 44.7; n = 10), R 2 466 (539.4; 51.3; n = 10), R 2+3 292 (268.9; 19.5; n = 10), R 2+3+4 244 (238.6; 16.6; n = 10), delta 298 (365.2; 42.8; n = 10), pi 181 (150.1; 24.8; n = 10). Leg length: anterior, median, and posterior, respectively: coxa 330 (334.6; 11.6; n = 10), 319 (331.2; 6.3; n = 10), 332 (343.3; 10.9; n = 10). Te remaining leg segments—femur, tibia, and tarsomeres—are not available for the holotype as they were processed for DNA extraction; however, we provide these measurements for some of the paratypes: femur (811; 32.1; n = 3), (784.3; 15.6; n = 3), (879.3; 25.5; n = 3); tibia (1053.7; 60.3; n = 3), (1318.3; 22.2; n = 3), (1502.3; 27.7; n = 3); tarsomere I (643; 16.8; n = 3), (775.3; 22.7; n = 3), (844.3; 22; n = 3); tarsomeres II+III+IV+ V (692; 13; n = 3), (756; 28.6; n = 3), (824; 20; n = 3). Metatarsomere III (two verticils with spines, one median and one apical).
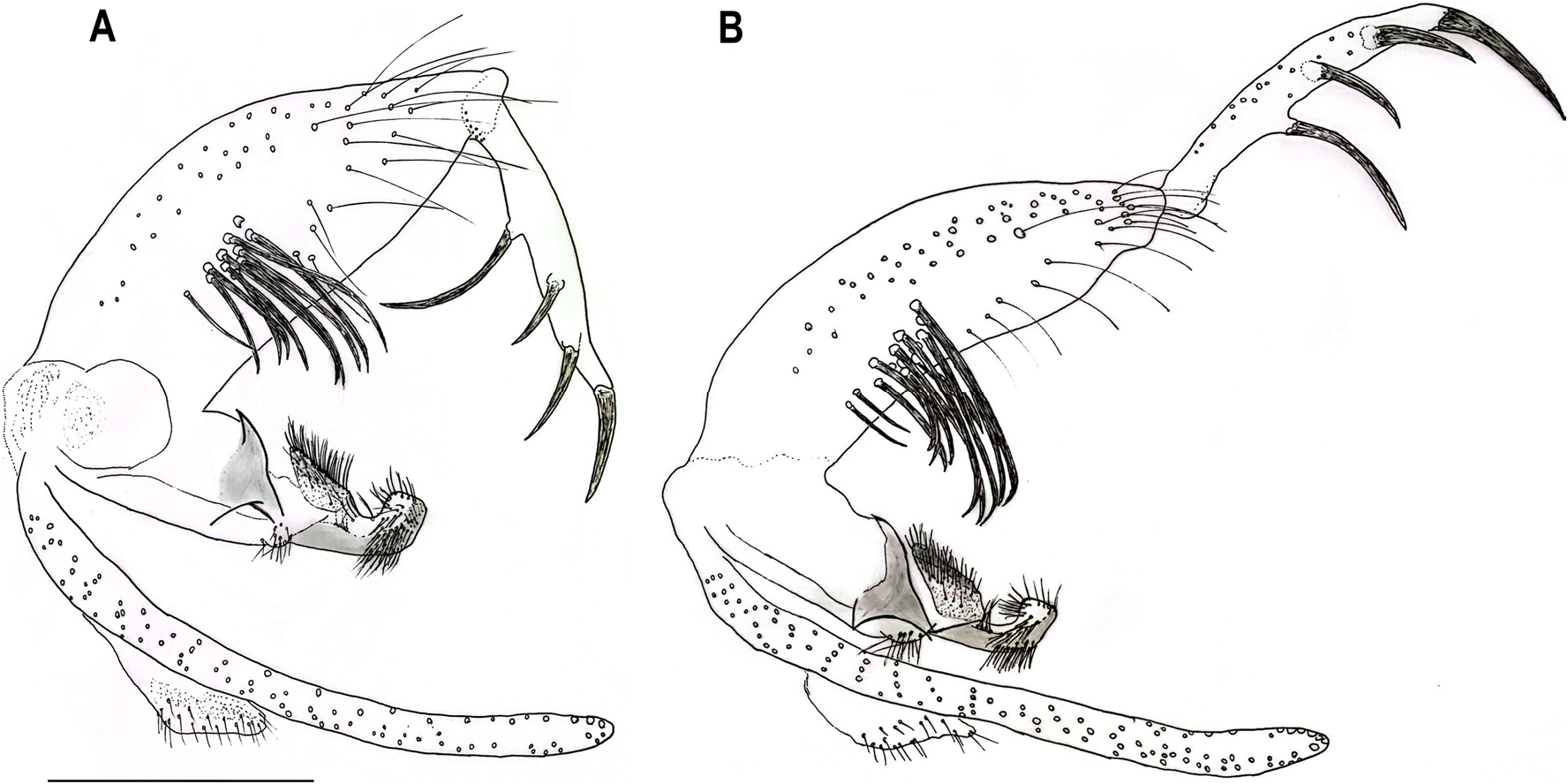
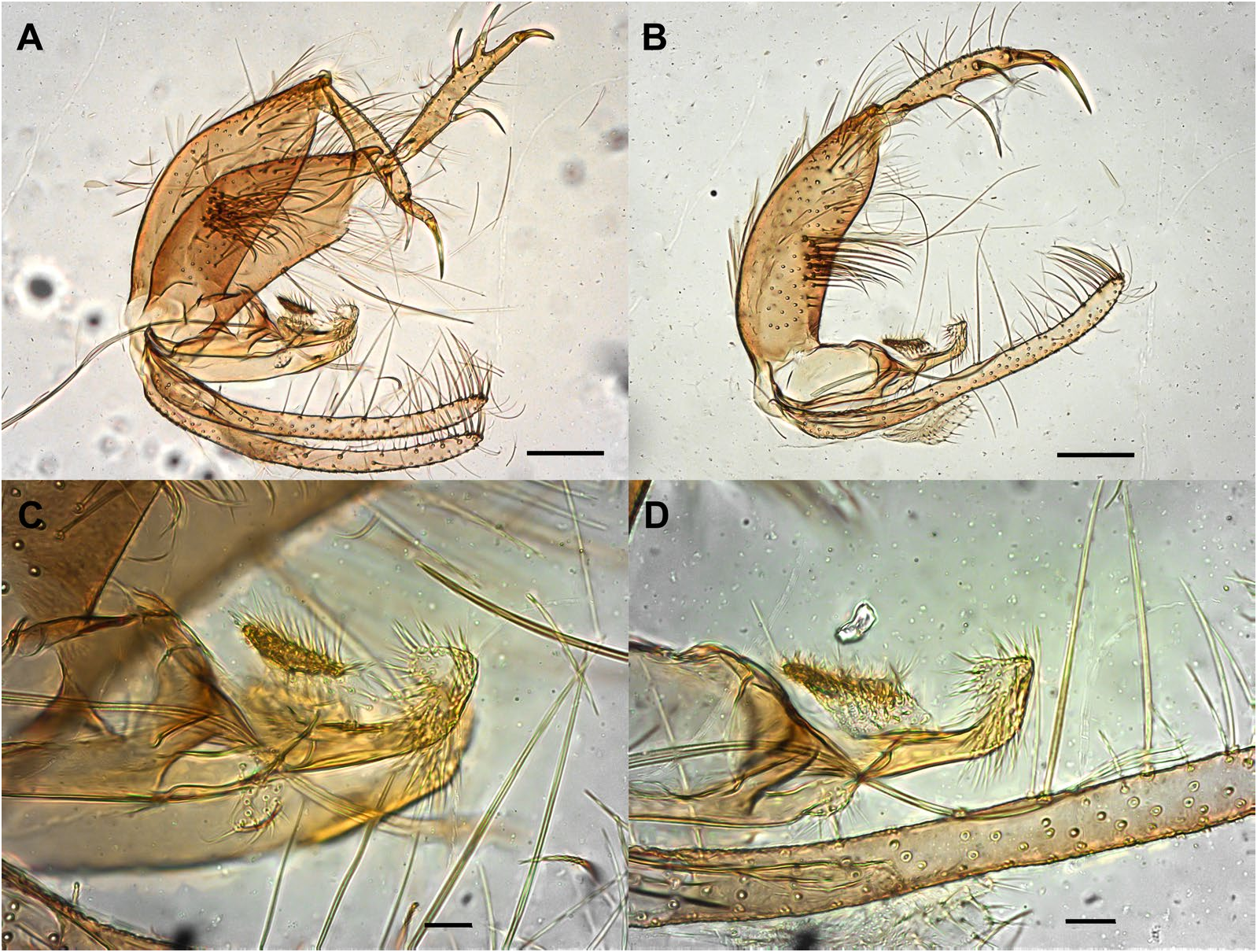
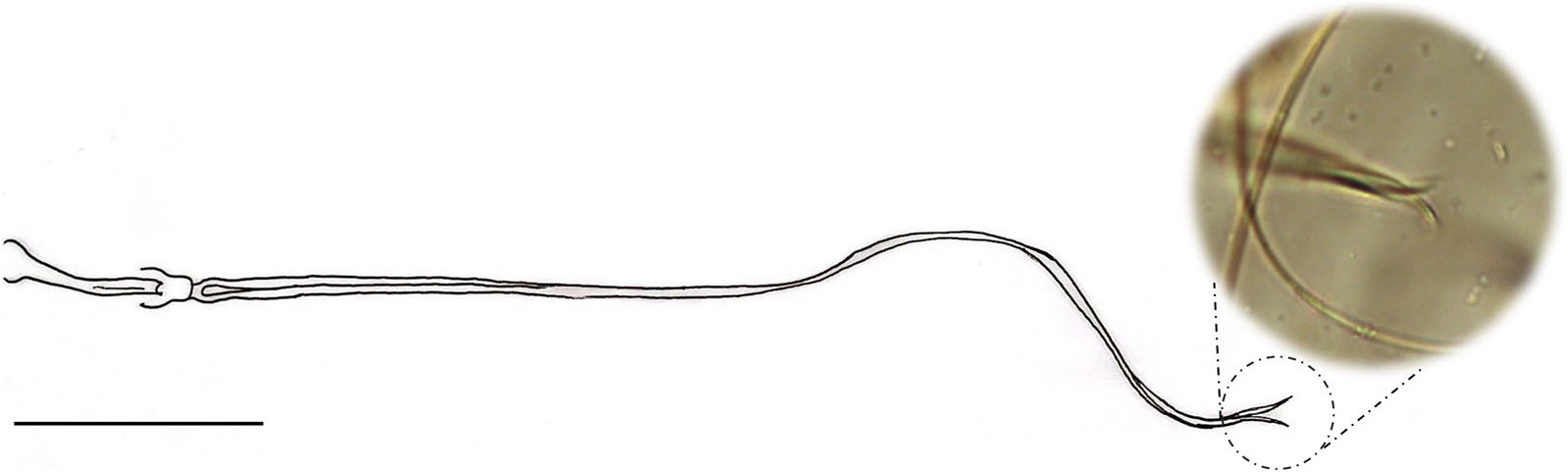
Abdomen: length 1394 (1494.7; 80; n = 10). Absence of tergal papillae from second to seventh tergites. Terminalia ( Figs. 3 View Fig and 4 View Fig ): gonocoxite length 365 (382.4; 19.1; n = 10), width 124 (132.2; 17.7; n = 10); presence of a median cluster with about 12 (10–14; n = 10) thick and long setae, and more dispersed and thin bristles in the median-apical region of the gonocoxite, the most apical being thicker than the median portion. Gonostyle 226 (233; 9.8; n = 10) long, with four spines, having the following disposition: one apical 72 (85.3; 7; n = 10), the upper external preapical 55 (62.8; 8.6; n = 10), the lower external implanted after the medium region, so that it is closer to the upper than the internal one, which is implanted at the basal third of the gonostyle. Preapical spiniform setae absent. Paramere simple; dorsal margin length 189 (187.3; 7.8; n = 10) forming a triangular median lobe covered with fine bristles; apex of paramere rectangular, covered with fine bristles; ventral margin length of paramere 258 (246.4; 10.2; n = 10) with a small cluster of about 12 short setae. Parameral sheath triangular, dorsal margin length 89 (89.6; 5.6; n = 10), ventral 52 (55; 5.1; n = 10), and basal 72 (76.3; 5.3; n = 10). Epandrial lobe length 468 (464.6; 11.5; n = 10), width 29 (32; 1.9; n = 10). Cercus length 212 (219.2; 13.9; n = 10). Sperm pump ( Fig. 5 View Fig ) length 172 (171.2; 3.7; n = 10); ejaculatory apodeme length 131 (137.1; 5.7; n = 10); sperm sac length 56 (52.2; 2.4; n = 10), width (26; 26.6; n = 10); pavillion width 40 (35.6; 5.5; n = 10); aedeagal duct 1020 (1034.2; 66; n = 9); ratio of aedeagal duct/sperm pump 5.9 (6; 0.3; n = 9); apex of the aedeagal duct tapered ( Figs. 4D View Fig and 5 View Fig ).
DNA barcoding
Te sequencing and analysis of the COI DNA barcoding region resulted in fragments ranging from 584 to 658 base pairs. Of the 10 analysed specimens (one holotype and nine paratypes), COI sequences of five paratypes were obtained. Te visual inspection of the alignment indicates the absence of stop codons in the middle of sequences, pseudogenes, and/or nuclear copies of mitochondrial origin ( NUMT) .
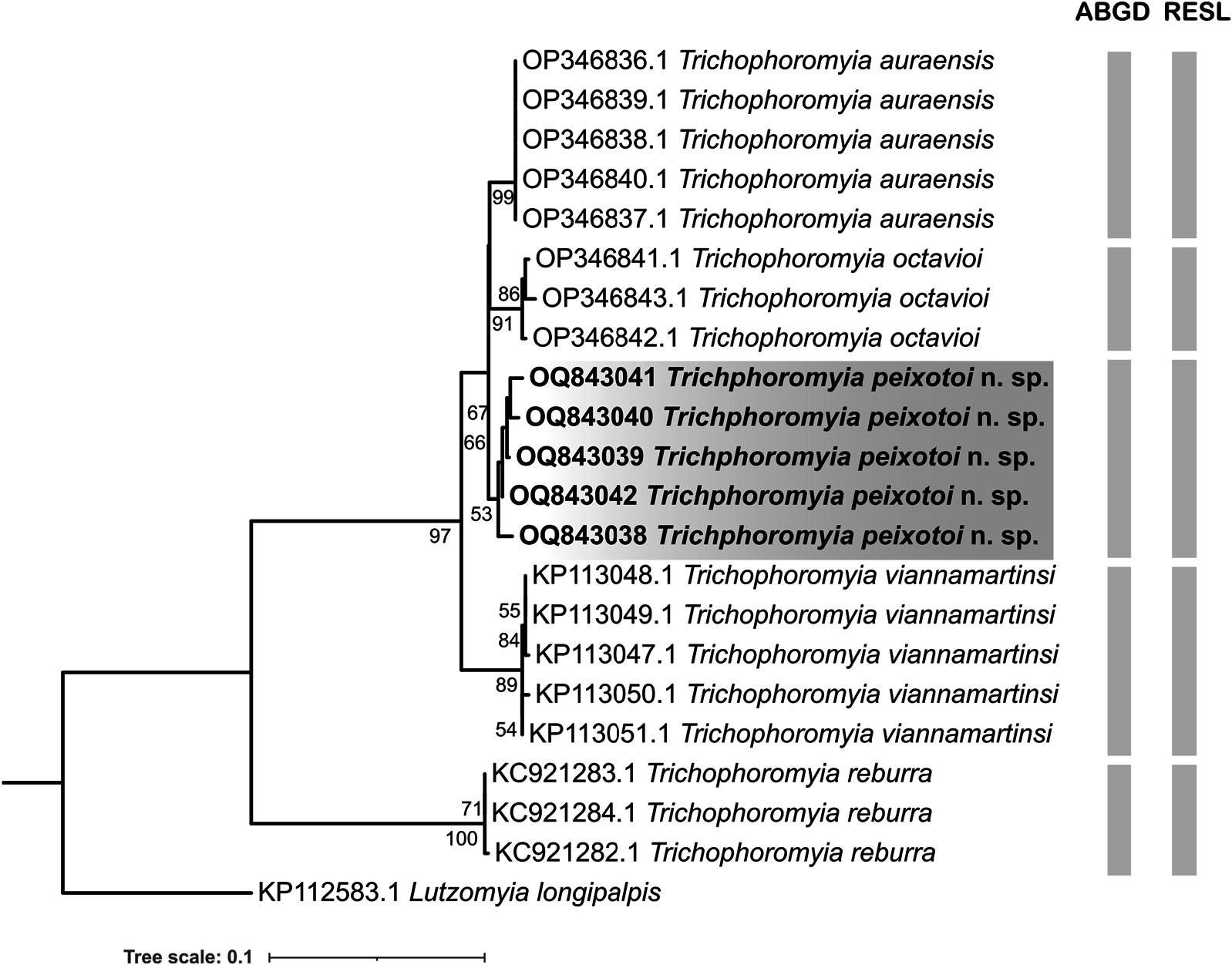
In total, the five new COI sequences of T. peixotoi n. sp. were analysed with 16 publicly available barcode sequences of T. auraensis (Mangabeira [33]), T. octavioi (Vargas [34]), T. reburra (Fairchild & Hertig [35]), and T. viannamartinsi (Sherlock & Guitton [36]), totalling an alignment of 21 COI sequences and five species ( Table 1). Te maximum intraspecific K2P distance ranged from 0 to 1.39%, and the minimum distance to the nearest neighbour ranged from 1.92 to 13.3% ( Table 1). In general, the interspecific distance was low, but T. reburra reached the highest value (13.3%), in discordance with the relationships of the other four species ( Table 1).
Te phylogenetic inference recovered five well-supported clades that are in agreement with the analysed nominal species. In the same sense, the species delimitation algorithms sorted the COI sequences in MOTUs that agree with morphological identification ( Fig. 6 View Fig ). Also, the ML analysis revealed a single well-supported clade containing the species T. auraensis , T. octavioi , T. peixotoi n. sp., and T. viannamartinsi , which seem to be more related to each other than to T. reburra ( Fig. 6 View Fig ).
Type material
Te male holotype and paratypes were collected in the PNA using CDC-type light traps operated overnight (Pinto et al. Col.). Holotype and three paratypes male were collected on 12 November 2022 near the Uruá base of the PNA. Four paratypes were collected on 7 July 2022, also near the Uruá base, and two were collected on 10 September 2022 near the Tracoá base of the PNA. Tese two latter specimens were collected in the tree canopy and were slide-mounted using Enecê resin, while the others were above the ground level and mounted using Canada balsam.
Te five sand flies processed for molecular analysis correspond to the paratypes which were collected on 10 September 2022 (GenBank accession numbers OQ843041–OQ843042) and 12 November 2022 ( OQ843038 –OQ843040) .
Te holotype and paratypes are deposited in the Coleção de Referência da Faculdade de Saúde Pública ( FSP – USP) at the Universidade de São Paulo, São Paulo, Brazil under museum numbers E-16393–E-16402 .
ZooBank registration
To comply with the regulations set out in Article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature [49], details of the new species have been submitted to ZooBank. Te Life Science Identifier ( LSID) of the article is urn:lsid:zoobank.org:pub:43B5EC2C-239A-4D15-9969-A4C28726C575 . Te LSID for the new species name Trichophoromyia peixotoi is urn:lsid:zoobank.org:act:EF1DD733-4958–4669-BEE8-E35B92788B21 .
Etymology
Te name Trichophoromyia peixotoi is a tribute to Professor Alexandre Afranio Peixoto, for his great contribution to the knowledge of sand flies, especially in studies on population genetics.
| PI |
Paleontological Institute |
| R |
Departamento de Geologia, Universidad de Chile |
| V |
Royal British Columbia Museum - Herbarium |
| ML |
Musee de Lectoure |
| USP |
University of the South Pacific |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |