Hylomyscus heinrichorum, Carleton, Michael D., Banasiak, Rebecca A. & Stanley, William T., 2015
|
publication ID |
https://doi.org/10.11646/zootaxa.4040.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:BBCBBFFA-D62C-4278-BF3E-2E860C70182B |
|
DOI |
https://doi.org/10.5281/zenodo.6118774 |
|
persistent identifier |
https://treatment.plazi.org/id/776AA715-FF8F-6224-8785-CF9EF8EF19F7 |
|
treatment provided by |
Plazi |
|
scientific name |
Hylomyscus heinrichorum |
| status |
sp. nov. |
Hylomyscus heinrichorum , new species
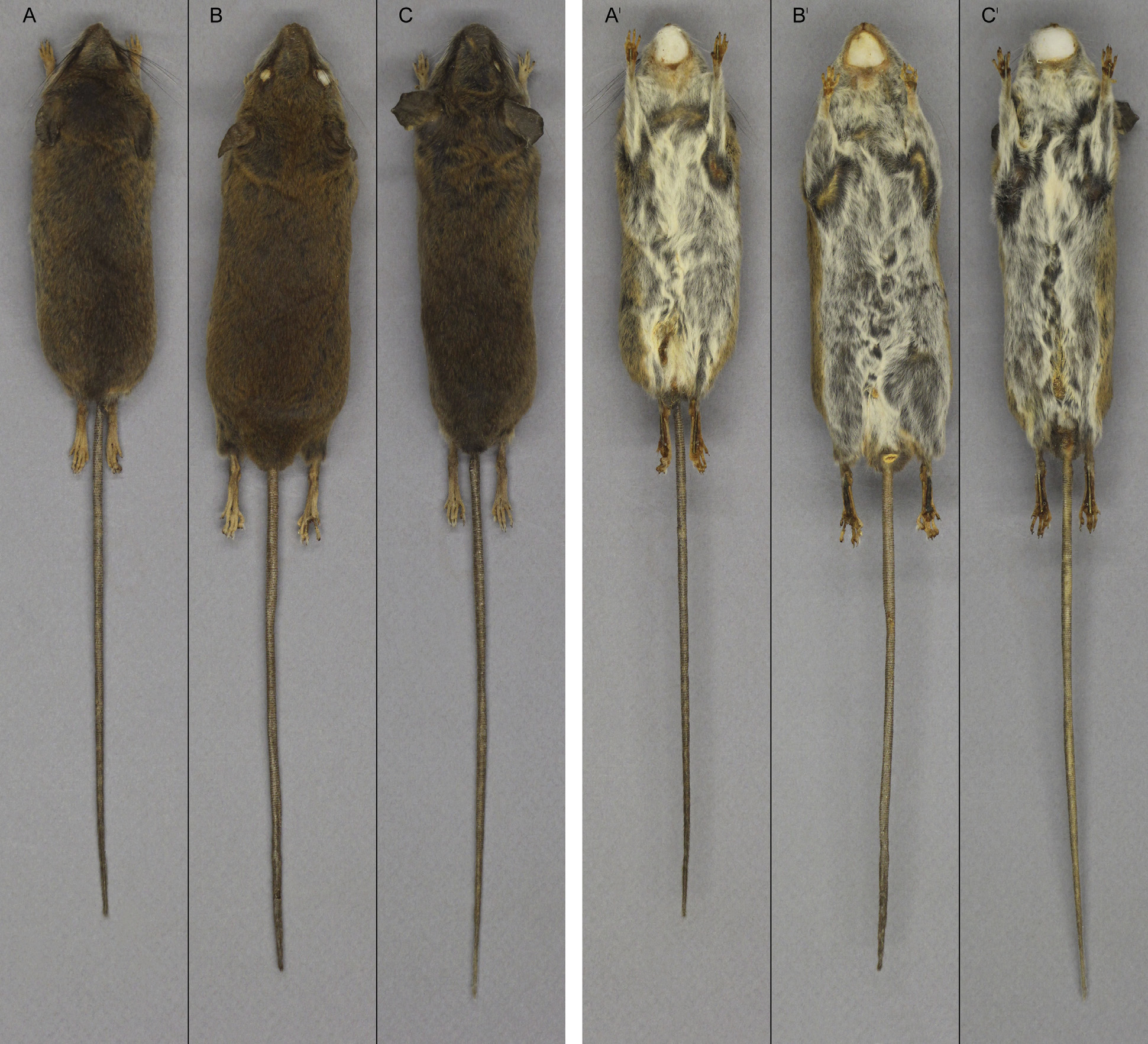
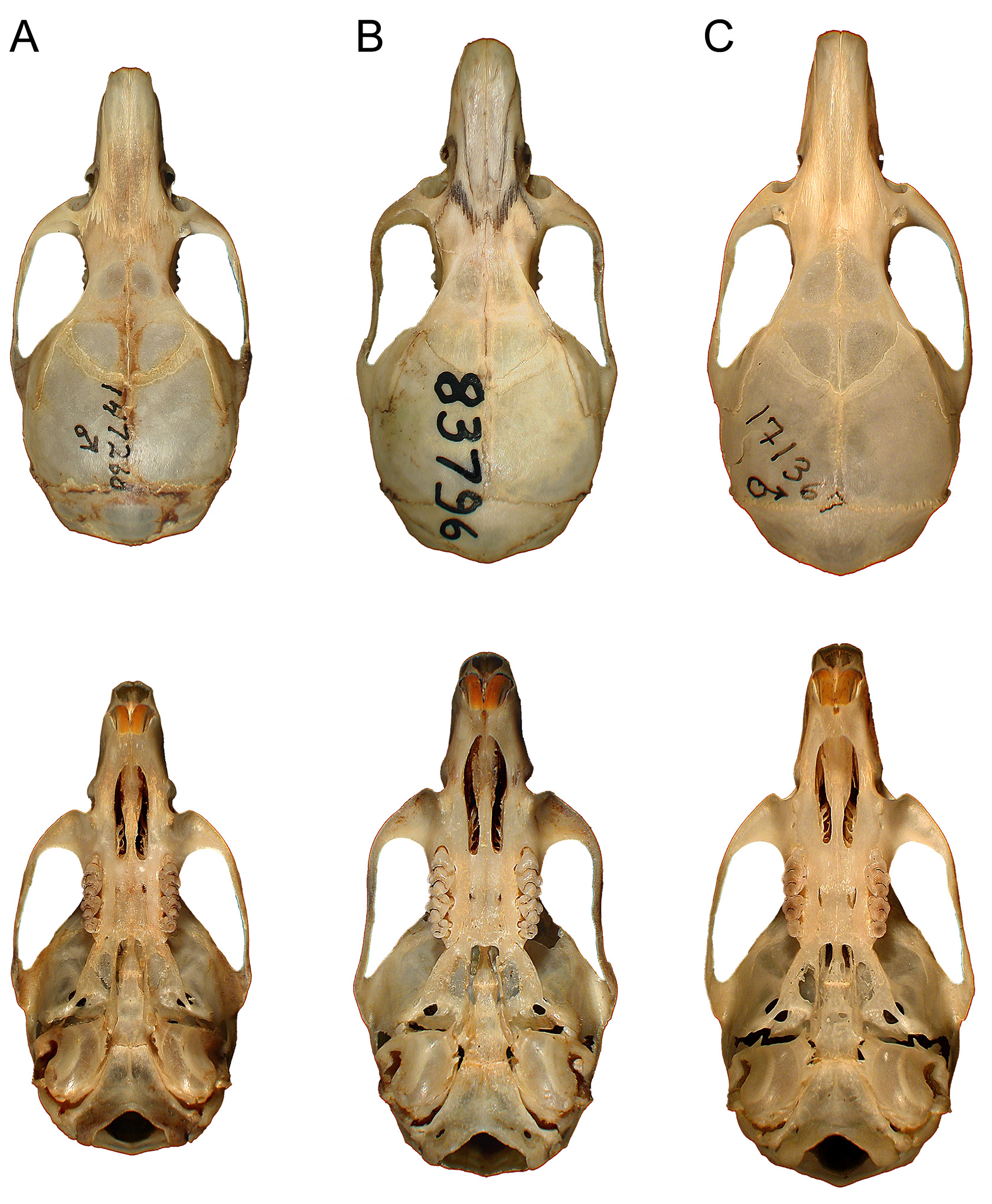
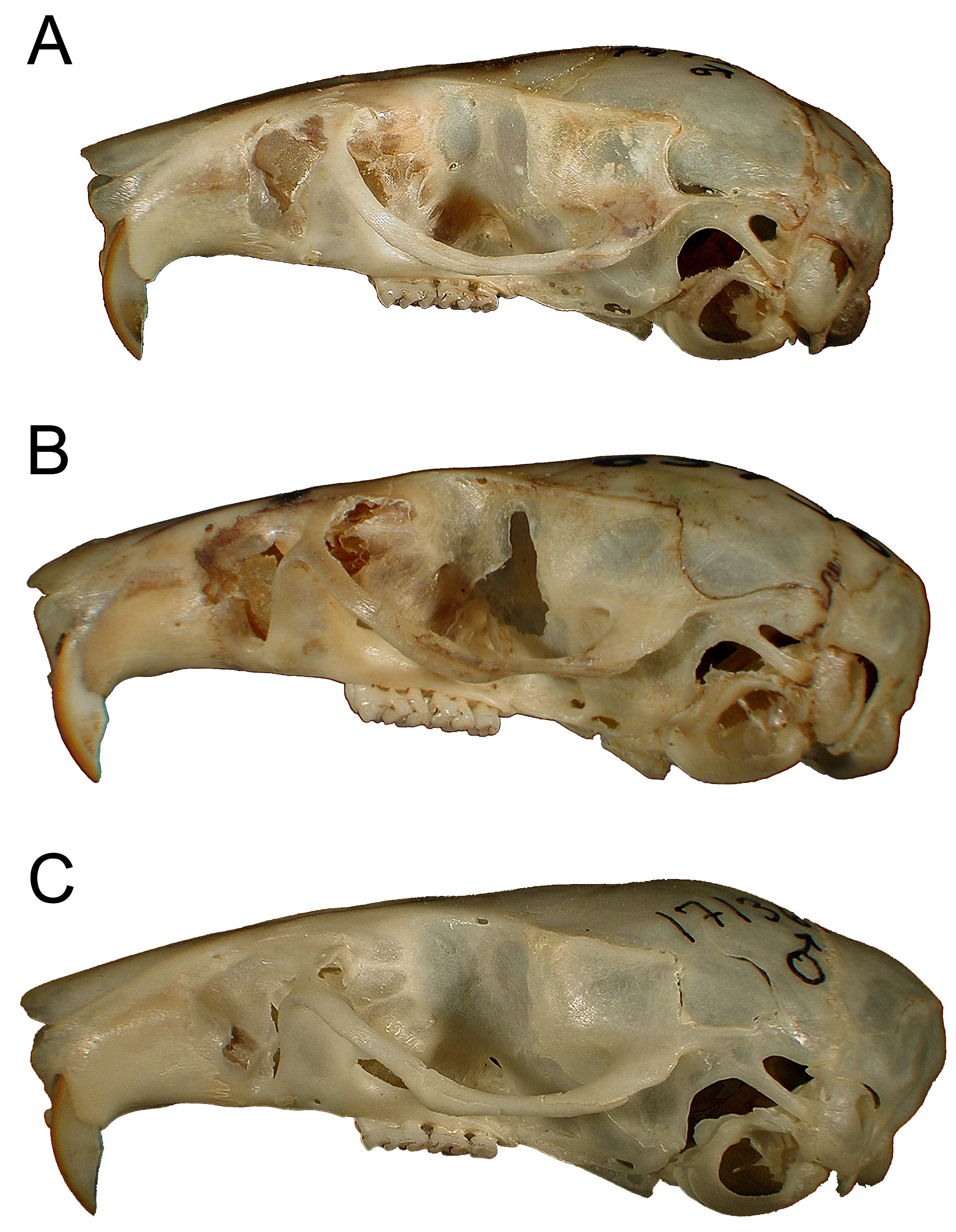
( Figs. 5–7 View FIGURE 5 View FIGURE 6 View FIGURE 7 ; Table 6 View TABLE 6 )
Hylomyscus carillus, Hill & Carter, 1941: 97 View in CoL (part, faunal report); Crawford-Cabral, 1998: 79 (part, faunal report).
Praomys carillus, Crawford-Cabral, 1986: 163 (part, name combination, zoogeography).
Hylomyscus denniae, Musser & Carleton, 1993: 599, 2005: 1336 View in CoL View Cited Treatment (part, systematic checklists, referenced as isolated population of indeterminate status).— Dieterlen, 2013: 435 (part, faunal report, distribution).
H [ lomyscus]. cf. anselli group, Carleton & Stanley, 2005: 629 (taxonomic revision, definition of the H. anselli species group and its contents).
Holotype. FMNH 83796, an adult male prepared as skin and skull, captured 7 October 1954 (skin tag reads “ 7 X 1954 ”) by Gerd H. Heinrich; the field number is recorded on the skin tag as 8778 and as GH 8778 on the now loose skull tag. Machine-printed on back of the tag is “Angolan Zoological Expedition, 1954.” Written in black pen in the collector’s script are the external measurements (“T.L.: 245, Tail: 145, H.F.: 22, Ear: 19”) and habitat (“Evergreen wood. – High mountain region”); the testes were noted as scrotal in position. See Table 6 View TABLE 6 for cranial measurements of the holotype. The skin is well prepared, in fine condition, and the skull is intact, in good condition with minor damage confined to the left orbital wall.
Type locality. Angola, Provincia Huambo, Mount Moco, ca. 12°27.712ʹS, 15°10.600ʹE (per the U.S. National Geospatial-Intelligence Agency).
The locality as recorded on the skin tag reads only “ Angola, Mount Moco.” Heinrich collected at two localities on Mt Moco, as evidenced by locality modifiers recorded in the field catalog and by an annotated, X-marked field map accessioned with that catalog (both documents maintained in the FMNH Mammal Division). One X-marked site is centered near the peak of Mt Moco, and a second X is located nearby to the southeast. Specimens obtained at the latter place were labeled “foot” and collected 14–20 Sep 1954. Heinrch then relocated to a higher elevation and settled in a “high mountain region,” presumably corresponding to the former X, where he remained for over three weeks, 22 Sep–16 Oct 1954. The type specimen and referred specimens from Mt Moco all originated from the high mountain locality.
Paratypes. All material here assigned to H. heinrichorum originates from two localities in Angola, Provincia Huambo. These include 14 additional specimens from Mt Moco ( FMNH 83793–83795, 83797–83799, 83801– 83807, 83895), collected from 5–10 Oct 1954 by G. Heinrich; and 10 specimens from Mount Soque, 42 km WSW Luimbale ( FMNH 83783–83792), collected 24–28 Aug 1954 by G. Heinrich.
Heinrich’s Mount Soque (also as Serra Ussoque) is a ridge-shaped inselberg (12°17.450ʹS, 15°08. 633ʹE), located approximately 20 km NNW Mt Moco and 20 km WSW Luimbale, its peak elevation ca. 2165 m. We presume that Heinrich’s locality modifier, “ 42 km WSW Luimbale,” was a by-road calculation.
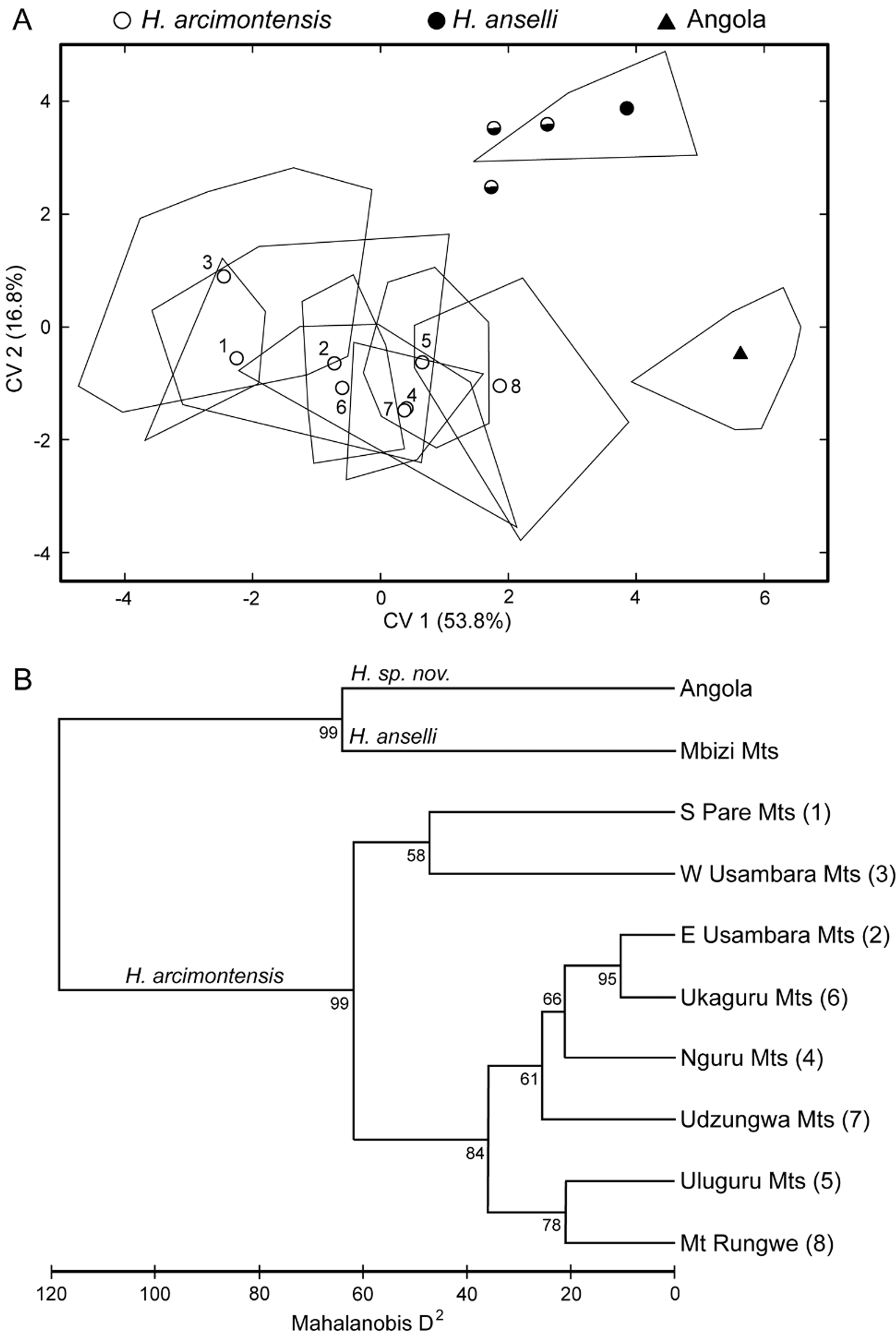
Diagnosis. A species of the Hylomyscus anselli group characterized by larger size (ONL ≈ 26.0– 27.5 mm; CLM ≈ 4.1–4.3 mm) compared with examples of H. arcimontensis and H. kerbispeterhansi (ONL ≈ 24.5–26.5 mm; CLM ≈ 3.5–4.0 mm), as reflected in most craniodental dimensions recorded ( Table 6 View TABLE 6 ). Compared with examples of H. anselli , size comparable, but bony palate notably shorter with absolutely and proportionally longer incisive foramina (LIF ≈ 75–80% of LD) that penetrate between the anterior roots of the first molars; molars slightly more robust and zygomatic plate broader; head-and-body and tail average shorter in length, and rostral dimensions (LR, LD) average smaller.
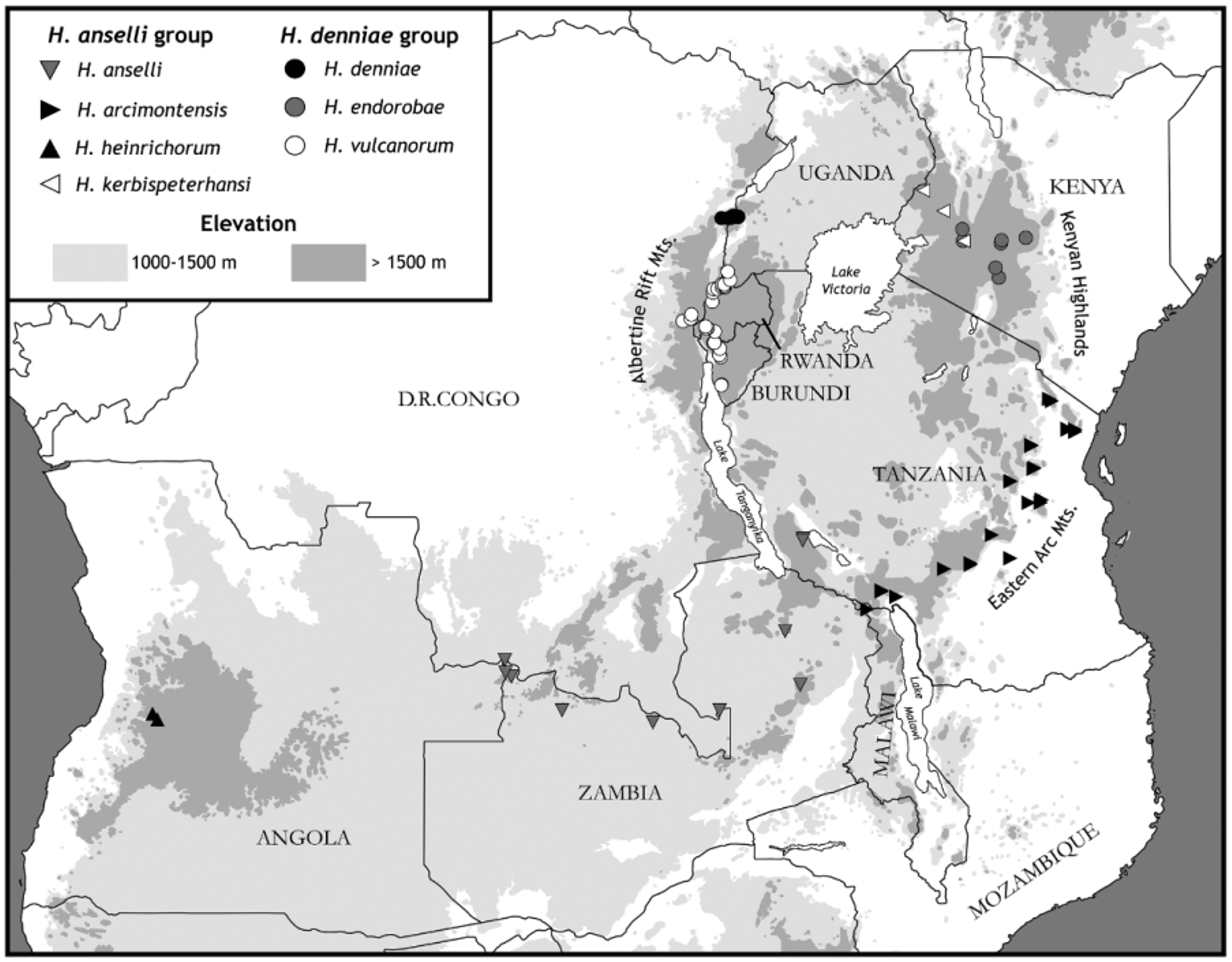
Distribution. Restricted to high mountainous region of westcentral Angola as so far known ( Fig. 4 View FIGURE 4 ).
Description and comparisons. Hylomyscus heinrichorum can be associated with the other species so far recognized in the H. anselli group ( H. anselli , H. arcimontensis , H. kerbispeterhansi ) based on certain qualitative traits. Foremost among these are the lack of pectoral mammae; the large expanse of the subsquamosal fenestrum, which together with the postglenoid foramen defines a slender and long hamular process (see Carleton & Stanley, 2005: Fig. 6 View FIGURE 6 ); and its relatively shorter incisive foramina (namely, shorter in comparison with members of the H. denniae group). The number of mammary glands in H. heinrichorum —six, distributed as one post-axial pair and two inguinal pairs—was verified on several prepared round skins (FMNH 83793, 83795, 83802–83804), all females that were apparently in late-term pregnancy or lactating.
Although we excluded external variables in our multivariate analyses, mean differences were highly significant for TOTL, TL, and HBL in one-way ANOVAs that compared our tabulated samples of H. heinrichorum and H. anselli (F values = 13.8–14.4, P <0.001, df 1, 35), but not for HFL (F = 0.02, P = 0.884, df 1, 35). The sample of H. heinrichorum averaged smaller than that of H. anselli for all three significantly different skin variables ( Table 6 View TABLE 6 ). Our samples of the two species were obtained by different collectors, and such external variables are notoriously variable depending upon investigator habit and field experience. Even so, the disparity in means seems suitably large to convey real taxonomic differences.
The pelage of H. heinrichorum resembles that observed in other members of the H. anselli group, being soft and fine in texture, short and closely adpressed to the body. The most notable pelage contrast of the Angolan form involves dorsal pelage color. Individual dorsal hairs can be characterized as medium plumbeous gray tipped with bright buff; the overall effect, however, is not so dark and somber a brown as in examples of H. anselli and H. arcimontensis ( Fig. 5 View FIGURE 5 ), but rather an even toned, dull yellowish brown (buckthorn brown), light to moderate in saturation. Guard hairs are brown with hyaline tips, only a little longer than the dorsal fur except over the rump. There is little tendency toward darker over the middle dorsum or conspicuously brighter flanks as is common in the other two species; color is generally evenly graded in expression over the entire dorsum, from the middle back to the flanks. A few individuals of H. heinrichorum exhibit brighter ochraceous-tipped hairs along the upper leg and shoulder and side of the head; nevertheless, the dorsal pelage color is predominantly even toned in appearance. Dorsal-ventral pelage contrast is well marked but not accentuated by a brighter lateral line. In all three species, the ventral hairs are basally gray and tipped with white, imparting a grayish-white effect. The three can be crudely sorted as bright gray ( H. arcimontensis ), medium gray ( H. heinrichorum ), and dark gray ( H. anselli ), with much variation and overlap among them. The tail of H. heinrichorum , as in other members of the H. anselli group, is notably longer than the head and body (TL ≈ 142–146% of HBL), its color dusky-brown and wholly dark around the circumference; caudal scales are finely textured and hairs short, imparting a naked appearance over most of its length, the fine caudal hairs becoming macroscopically visible toward the tip. The hind foot is short and narrow, as per the genus, with digit 5 nearly as long as digits 2–4; the naked plantar surface bears six well-formed, cushiony pads. Pale brown hairs of the limb usually continue onto the ankle and proximal metatarsum, forming a dusky medial metatarsal streak in some individuals, replaced by white hairs over the distal metatarsum and phalanges; glistening white ungual tufts are present. Pinnae are pale brown to dark tan in the Angolan species, compared with darker, more blackish brown ears in the other two.
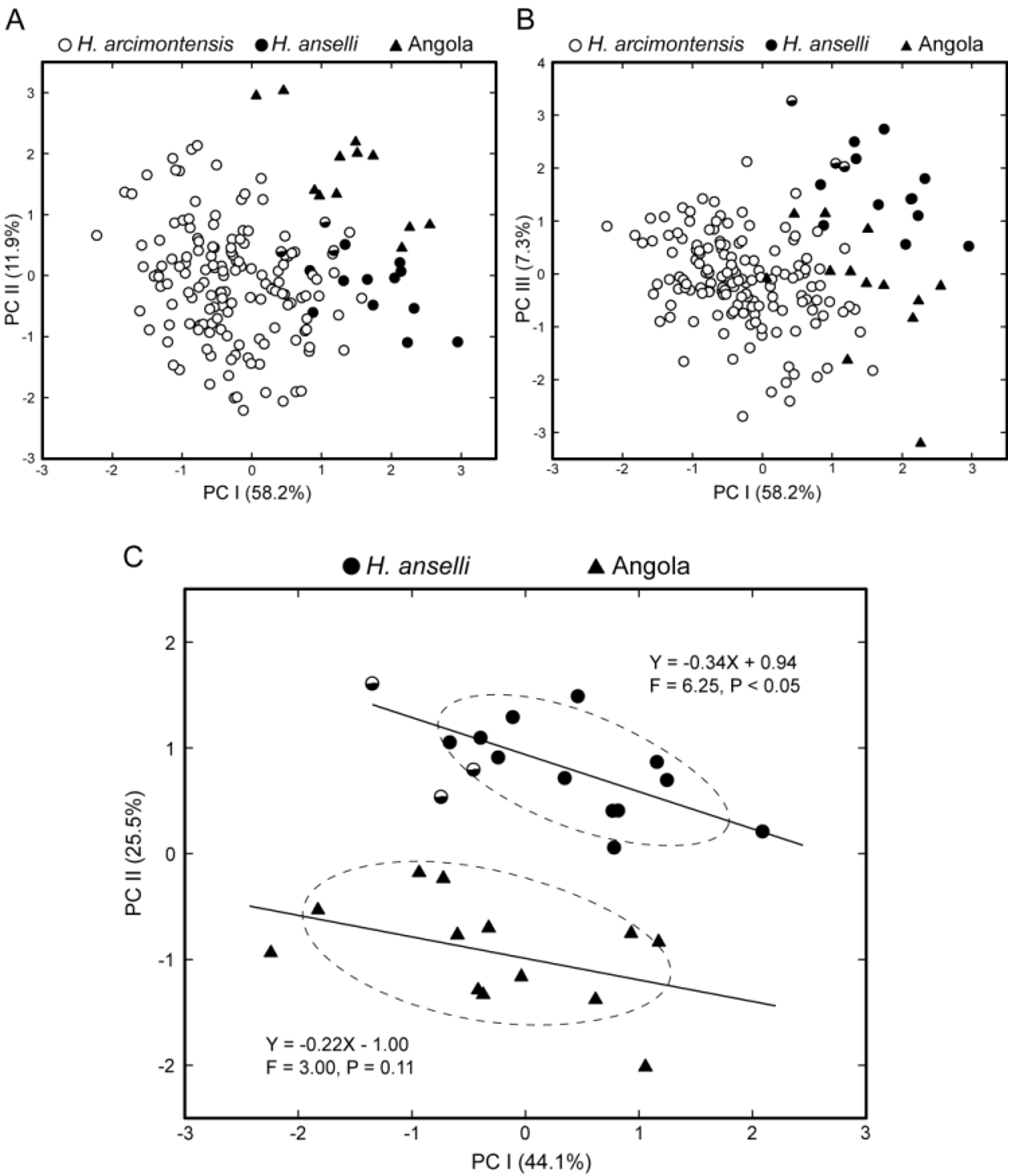
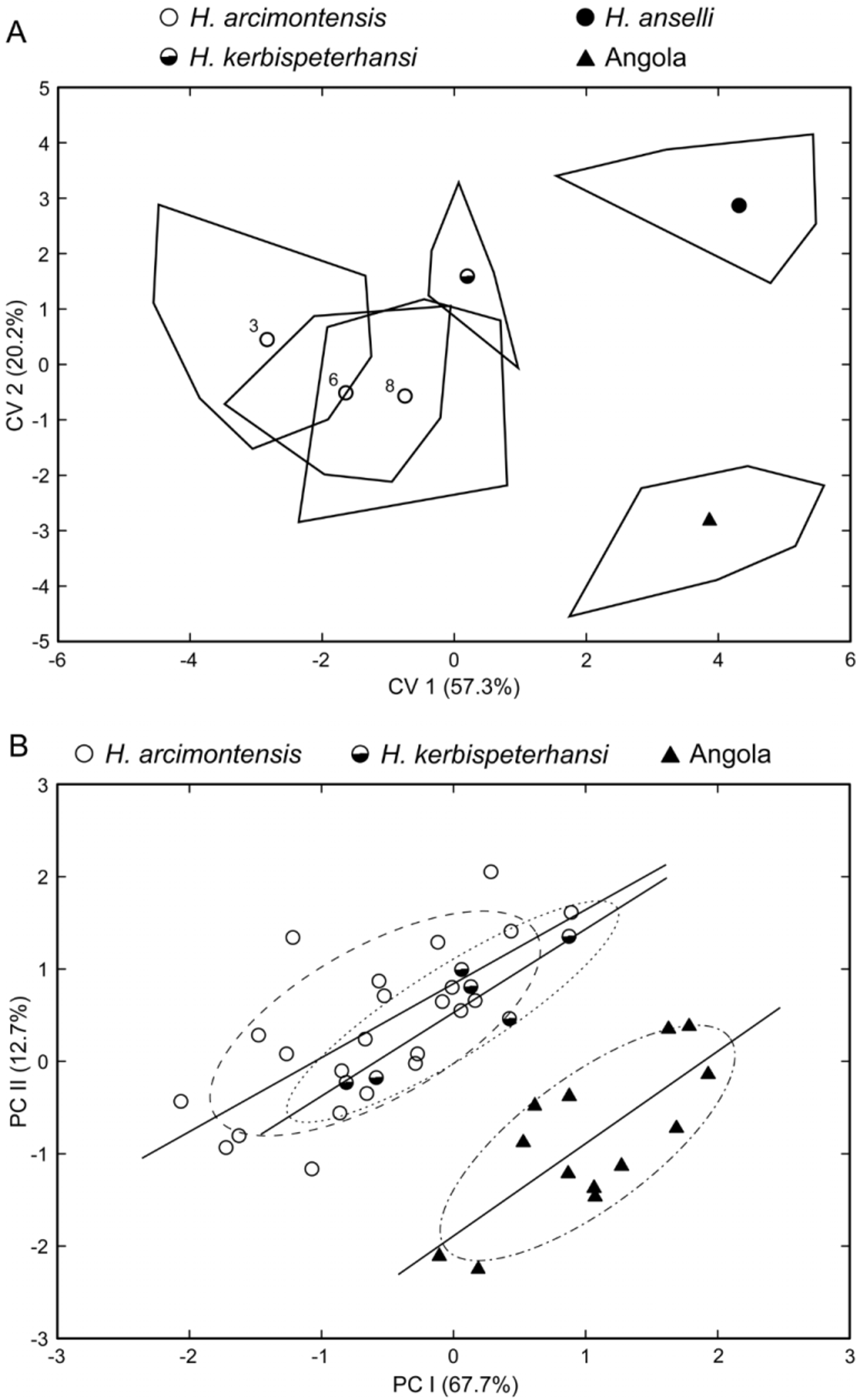
As a gestalt observation, the skull of H. heinrichorum is approximately equal to or only slightly smaller than H. anselli , albeit heavier in build, but both are substantially larger than H. arcimontensis ( Figs. 6 View FIGURE 6 , 7 View FIGURE 7 ). Thus, standard external measurements and those we recorded for the skull and molar rows easily serve to distinguish examples of H. heinrichorum from those of the smaller H. arcimontensis and H. kerbispeterhansi ( Table 6 View TABLE 6 ); size routinely emerged as the preeminent factor accounting for their separation in multivariate space ( Figs. 1 View FIGURE 1 , 3 View FIGURE 3 ). Mensural differences between H. heinrichorum and H. anselli , on the other hand, are not readily grasped when eyeballing series of skulls, yet specimens of each did conform to distinct morphometric footprints based on the cranial measurements we obtained (see Figs. 1 View FIGURE 1 , 2 View FIGURE 2 C). However, fewer variables contributed to their discrimination, rendering their cranial separation correspondingly more difficult, based on subtle distinctions. Configuration of the hard palate relative to the length of the incisive foramina offers particularly important contrasts, those variables (BBP, HPL, LIF) loading strongly on that principal component which captured their separation in multivariate space ( Fig. 2 View FIGURE 2 C, Table 4 View TABLE 4 ). As practical anatomical landmarks when viewing skulls, these loadings translate as absolutely shorter incisive foramina (LIF ca. 69–73% of LD) whose posterior end falls just short of or about level with the anterior border of the M1 anterior root in H. anselli , versus longer incisive foramina (LIF ca. 75–80% of LD) that extend beyond the anterior roots about to the rim of the first lamina of M 1 in H. heinrichorum . Therefore, the incisive foramina in H. heinrichorum are proportionately the longest within the H. anselli group, albeit not so “long” as members of the H. denniae group. The absolutely longer bony palate documented in H. anselli , compared with a shorter palate in H. heinrichorum , is a reciprocal geometric corollary of the different foraminal lengths ( Table 6 View TABLE 6 ). The shape of the incisive foramina, however, is similar in both: broader over the anterior portion, narrowing slightly over the posterior half. The dorsal notch formed between the upper zygomatic plate and rostrum appears slightly deeper and wider in specimens of H. heinrichorum compared with crania of H. anselli and H. arcimontensis ( Fig. 6 View FIGURE 6 ), a visual impression consistent with the actually broader zygomatic plate recorded in the former ( Table 6 View TABLE 6 ) and the important contribution of this variable (BZP) to between-group discrimination ( Table 4 View TABLE 4 ). The interorbital region of H. heinrichorum conforms to that described for the H. anselli group: relatively narrow and amphoral in shape over its anterior portion, with post-orbital shelving weakly expressed in full and old adults, its edges lacking supraorbital ridging or beading. When examining series of skulls arrayed side-by-side ( Figs. 6 View FIGURE 6 , 7 View FIGURE 7 ), examples of H. anselli exhibit the longest, more attenuate rostrum, those of H. arcimontensis the shortest and most truncate, and those of H. heinrichorum appear intermediate in rostral length and shape. These macroscopic impressions find some statistical precision in the sample statistics ( Table 6 View TABLE 6 ), variable loadings of the ordinations performed ( Tables 1 View TABLE 1 , 2 View TABLE 2 , 5 View TABLE 5 ), and/or one-way ANOVAs ( Table 4 View TABLE 4 ).
Specimens of H. heinrichorum dentally resemble those of H. anselli and H. arcimontensis in the slight curvature of the upper incisors (nearly orthodont or weakly opisthodont), molar proportions, and cusp development. Pigmentation of the enamel face of the upper incisors in H. heinrichorum is a more saturated, medium orange compared with pale yellow-orange in H. anselli and H. arcimontensis ; the tone is drab in all three.
Ecological notes. Other species of the Hylomyscus anselli group are known to be closely associated with Afromontane forest, typically found within an elevational belt of 1000–2500 m ( Carleton & Stanley, 2005; Carleton et al., 2006; Demos et al., 2014b). The habitat information recorded on the skin tag of all Hylomyscus specimens collected at Mt Moco—“Evergreen wood. – High mountain region”—fits this ecological and topographical setting. Also, a photograph, taken by some member of Heinrich’s field team and labeled only Mt Moco, captures a substantial stand of closed-canopy, high forest in the vicinity of a campsite that we believe to represent Heinrich’s “High mountain” collecting locality ( Fig. 8 View FIGURE 8 ).
Mills et al. (2011) documented the condition of Afromontane forest on Mt Moco as part of their ongoing studies of the endangered Swierstra’s Francolin ( Francolinus swierstrai Roberts ), a partridge-like bird endemic to Angola. High forest persists as small patches, the largest about 25 hectares, restricted to narrow ravines and steep valleys in the remotest parts of the mountain; these isolated fragments are distributed over an elevation of 2000 to 2400 m, a range that falls within the 1400 mm annual rainfall isohyet. Characteristic genera include the Gondwanan conifer Podocarpus along with other evergreen, flowering trees and shrubs (species of Apodytes , Ficus , Halleria , Ilex , Olea , Pittosporum , Polyscias , and Syzygium — Huntley & Matos, 1994; Mills et al., 2011). Canopy height tends to be irregular, conforming to the steep slopes and rugged valleys that retain forest coverage. Montane grasslands, both natural and anthropogenic, and Miombo woodlands, dominated by Brachystegia , Isoberlinia , and Julbernardia , sprawl in between the high-forest patches; the latter woodlands, a relatively mesic savanna association (Zambezian Woodland Biotic Zone), cover most of the mountain. Presumably, tracts of Afromontane forest were more extensive when Heinrich visited Mt Moco in 1954.
Most skin tags of H. heinrichorum from Mt Soque indicate their capture “Along mountain brook through tall grass. – Below mt. top.” Whether remnant Afromontane forest existed in proximity to Heinrich’s trap line is indeterminate from this scant description. When Mills canvassed Mt Soque for Swierstra’s Francolin in 2005, he ( Mills et al., 2011: 6) recorded that Afromontane forest no longer existed, but a few pairs of the endangered francolin were discovered in “dense herbaceous and shrubby growth in gullies and around the mountain summit [peak at 2165 m].”
Other rodents collected with Hylomyscus heinrichorum over the same range of dates at both Mt Soque ( 24–28 Aug 1954) and Mt Moco ( 5–10 Oct 1954) include Funisciurus congicus Kuhl , Graphiurus murinus Desmarest , Dendromus nyikae Wroughton , Grammomys dolichurus Smuts , Lophuromys angolensis Verheyen, Dierckx , & Huselmans, Myomyscus angolensis Bocage , Oenomys hypoxanthus Pucheran , and Pelomys campanae Huet. Examples of Aethomys namaquensis A. Smith , Graphiurus rupicola Thomas & Hinton , Mus triton Thomas , Otomys cuanzensis Hill & Carter , and Cryptomys mechowi Peters were captured in sympatry with H. heinrichorum only at Mt Soque, but not Mt Moco; specimens of Mus minutoides Smith , Cryptomys hottentotus Lesson , and Thryonomys gregorianus Thomas were also obtained at Mt Moco, but not at Mt Soque. Certain species in some of these genera have been documented in moist forest at high elevations (e.g., Graphiurus , Dendromus , Grammomys , Lophuromys — Stanley et al., 1998); still more of them are commonly associated with non-forest habitatsgrasslands, open woodlands, marshes and riverine vegetation, thickets and forest edges—environments that are also represented on Mt Moco and were around Heinrich’s campsite at the time of their survey ( Fig. 8 View FIGURE 8 ).
At this stage of understanding, Hylomyscus heinrichorum may be reasonably characterized as endemic to Afromontane forest based on the habitat recorded for the Mt Moco series and on the known affinity of its speciesgroup relatives for highland forest. However, without knowing the exact placement of Heinrich’s trap line and traps, its strict ecological reliance upon this biome must remain inferential pending renewed field surveys. Fresh autecological study is obviously in order.
Remarks. To our knowledge, specimens of Hylomyscus heinrichorum have not been collected since the 1954 Heinrich expedition. Until new field assessments are conducted, the conservation status of H. heinrichorum is plausibly considered Near Threatened, if not Vulnerable, in view of the continuing decline in extent and quality of Afromontane forest in Angola ( Huntley & Matos, 1994; Mills et al., 2011). Unlike the Endangered classification of Swiestra’s Francolin (IUCN Red List, accessed Jul 2014), a comestible, partridge-sized bird subjected to hunting pressure compounded by disappearance of its preferred habitat, populations of a small murid rodent like H. heinrichorum should remain resilient to habitat loss so long as even minimal forest cover, primary or secondary, persists. The recent documentation of substantial tracts of Afromontane forest in the Namba Mts ( Mills et al., 2013), situated in southern Cuanza Sul Province about 80 km northwest of Mt Moco, augurs that ample populations of H. heinrichorum still exist.
Etymology. Our specific epithet honors both Gerd Hermann Heinrich ( 1896–1984) and Hildegarde Maria Buruvna ( 1917–2012), husband and wife ( Fig. 9 View FIGURE 9 ), to belatedly acknowledge their important field work in Africa conducted in the middle 1900s. Notwithstanding the enduring scientific relevance of their bird and mammal collections, these museum expeditions—to Angola ( 1953–1955, 1957–1958), Tanzania ( 1961–1963), and South Africa (1963)—unfolded as a means to an end, that end being to afford Heinrich yet another opportunity to pursue his zoological first love, the natural history and taxonomic diversity of parasitic wasps or ichneumon flies (Insecta: Hymenoptera : Ichneumonidae ). While just a teenager, Heinrich devoted himself to understanding this exceedingly diverse, systematically intractable family, and it remained a lifelong passion, somehow sustained during, between, and after the World Wars that sundered normalcy in Europe. He opportunistically promoted his service as professional collector, obtaining vertebrate specimens under the auspices of large, well funded museums in order to visit exotic faraway geographies and collect never-before-seen ichneumons. His body of publications and prolific taxonomic descriptions were foundational for understanding major groups of Ichneumonidae over vast areas of their distribution (e.g., G. Heinrich, 1934, 1938, 1961 –1962, 1967–1968). For more about Gerd Heinrich, his scientific contributions and improbable life, readers should consult Weems (1986), B. Heinrich (2007), and Collar (2009).
Although Gerd Heinrich’s personal energy in the field surpassed indefatigable, the successes of his many expeditions owed as much to the abilities and equally unflagging dedication of his female companions. Prior to World War II, his wife Anneliese Machatchek and her younger sister Liselotte accompanied him to Sulawesi ( 1930–1932) and Myanmar ( 1937–1938), laboring as indispensable field assistants. After emigrating to the U.S. following World War II, Heinrich resumed fieldwork in Mexico ( 1952–1953) and those various countries of Sub- Saharan Africa ( 1953–1963), where he was joined by his second spouse Hildegarde (Hilde) Buruvna, who shared in all aspects of camp maintenance, data collection, and specimen preparation. Gerd Heinrich’s efforts as mammal collector have been individually recognized for his earlier fieldwork in Sulawesi ( Hyosciurus heinrichi Archbold and Tate, 1935 ; Rattus penitus heinrichi Tate and Archbold, 1935 [= Bunomys andrewsi J. A. Allen ]) and Myanmar ( Callosciurus griseimanus heinrichi Tate, 1954 [= C. phayrei Blyth ]). Our specific patronym acknowledges both Gerd and Hilde and their complementary roles in generating these significant African collections. The species name heinrichorum thus combines the family surname Heinrich, a proper noun, and the Latin genitive plural suffix -orum.
TABLE 6. Measurements of the holotype (FMNH 83796) of Hylomyscus heinrichorum, new species, and descriptive statistics for samples of the H. anselli group (Statistics include the sample mean, standard deviation, observed range, and sample size in parentheses; see Materials and Methods for variable abbreviations).
| H. heinrichorum | H. anselli | H. arcimontensis | H. kerbispeterhansi |
|---|---|---|---|
| Variable Holotype Angolan Highlands | Mbizi Mts | E Usambara Mts | Kenyan Mts |
| TOTL 245.0 231.4 ± 12.5 207–255 (25) | 246.1 ± 7.4 238–264 (12) | 224.4 ± 17.1 186–247 (24) | 219.3 ± 13.3 195–231 (7) |
| HBL 100.0 94.0 ± 6.6 82–106 (25) | 101.6 ± 3.8 95–109 (12) | 88.1 ± 8.3 72–110 (24) | 90.3 ± 7.8 76–100 (7) |
| TL 145.0 137.3 ± 7.4 121–150 (25) | 146.3 ± 5.0 141–159 (12) | 136.5 ± 10.1 116–150 (24) | 129.0 ± 6.8 119–138 (7) |
| HFL 22.0 21.1 ± 1.0 18.5–22 (25) | 21.2 ± 0.7 20–22 (12) | 19.5 ± 1.3 17–22 (24) | 19.1 ± 1.2 17–21 (8) |
| EAR 19.0 18.5 ± 0.9 17–20 (24) | 20.1 ± 0.5 19–21 (12) | 17.8 ± 1.0 15–19 (24) | 18.6 ± 0.8 17–19.5 (8) |
| WT -- -- | 28.5 ± 3.1 22.0–34.5 (12) | 20.2 ± 4.4 12.0–29.0 (24) | 21.5 ± 3.3 18.0–26.0 (6) |
| ONL 27.4 26.7 ± 0.8 25.0–28.1 (14) | 27.4 ± 0.7 26.3–28.6 (12) | 25.2 ± 1.0 23.0–27.0 (24) | 25.5 ± 0.8 24.6–26.9 (6) |
| ZB 13.5 13.0 ± 0.4 12.4–13.8 (14) | 13.5 ± 0.2 12.9–13.7 (12) | 12.5 ± 0.5 11.6–13.7 (24) | 12.7 ± 0.6 12.0–13.5 (6) |
| BBC 11.8 11.7 ± 0.2 11.3–12.1 (14) | 11.9 ± 0.2 11.5–12.3 (12) | 11.2 ± 0.2 10.7–11.8 (24) | 11.3 ± 0.2 10.8–11.5 (6) |
| IOB 4.4 4.5 ± 0.1 4.4–4.7 (14) | 4.6 ± 0.1 4.5–4.8 (12) | 4.3 ± 0.1 4.0–4.7 (24) | 4.3 ± 0.1 4.2–4.6 (6) |
| BOC 6.5 6.2 ± 0.2 6.0–6.5 (13) | 6.2 ± 0.1 5.9–6.5 (12) | 6.0 ± 0.2 5.5–6.5 (24) | 6.0 ± 0.1 5.8–6.2 (6) |
| LR 8.2 8.2 ± 0.5 7.2–8.9 (14) | 8.7 ± 0.3 8.3–9.1 (12) | 7.7 ± 0.4 7.1–8.4 (24) | 7.9 ± 0.2 7.6–8.1 (6) |
| BR 4.7 4.4 ± 0.2 4.1–4.8 (14) | 4.7 ± 0.2 4.5–5.0 (12) | 4.3 ± 0.2 3.9–4.7 (24) | 4.5 ± 0.2 4.3–4.8 (6) |
| PPL 9.4 9.3 ± 0.5 8.6–10.1 (14) | 9.5 ± 0.4 8.9–10.1 (12) | 8.7 ± 0.4 7.6–9.4 (24) | 8.7 ± 0.6 8.0–9.6 (6) |
| HPL 4.7 4.4 ± 0.2 4.0–4.7 (14) | 4.8 ± 0.2 4.5–5.0 (12) | 4.3 ± 0.2 4.0–4.7 (24) | 4.4 ± 0.2 4.2–4.8 (6) |
| LIF 5.9 5.7 ± 0.3 5.2–6.4 (14) | 5.5 ± 0.1 5.3–5.8 (12) | 5.2 ± 0.2 4.6–5.6 (24) | 5.3 ± 0.1 5.1–5.5 (6) |
| LD 7.4 7.4 ± 0.4 6.7–8.1 (14) | 7.7 ± 0.3 7.4–8.4 (12) | 7.0 ± 0.3 6.4–7.7 (24) | 7.5 ± 0.3 7.1–7.9 (6) |
| BBP 5.5 5.3 ± 0.1 5.1–5.5 (14) | 5.2 ± 0.1 5.1–5.4 (12) | 4.9 ± 0.1 4.6–5.1 (24) | 5.0 ± 0.1 4.8–5.2 (6) |
| BZP 2.8 2.5 ± 0.1 2.2–2.8 (14) | 2.4 ± 0.1 2.2–2.6 (12) | 2.3 ± 0.1 2.1–2.6 (24) | 2.3 ± 0.1 2.1–2.5 (6) |
| LAB 4.4 4.4 ± 0.1 4.3–4.6 (14) | 4.5 ± 0.1 4.5–4.7 (12) | 4.1 ± 0.1 3.9–4.3 (24) | 4.4 ± 0.1 4.2–4.6 (6) |
| CLM 4.30 4.21 ± 0.12 4.02–4.39 (14) | 4.16 ± 0.11 3.87–4.28 (12) | 3.70 ± 0.12 3.37–3.89 (24) | 3.80 ± 0.04 3.76–3.89 (6) |
| WM1 1.32 1.29 ± 0.04 1.20–1.34 (14) | 1.24 ± 0.04 1.17–1.29 (12) | 1.13 ± 0.03 1.06–1.18 (24) | 1.17 ± 0.01 1.16–1.19 (6) |
| FMNH |
Field Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Murinae |
|
Genus |
Hylomyscus heinrichorum
| Carleton, Michael D., Banasiak, Rebecca A. & Stanley, William T. 2015 |
Hylomyscus carillus
| Crawford-Cabral 1998: 79 |
Praomys carillus
| Crawford-Cabral 1986: 163 |