Chrysometa, SIMON, 1894
|
publication ID |
https://doi.org/10.1111/j.1096-3642.2011.00692.x |
|
persistent identifier |
https://treatment.plazi.org/id/7D5E87AD-C04A-552C-FCC7-4C01D0C3F96E |
|
treatment provided by |
Valdenar |
|
scientific name |
Chrysometa |
| status |
|
CHRYSOMETA SIMON, 1894 View in CoL View at ENA ( FIGS 3D View Figure 3 , 18–21 View Figure 18 View Figure 19 View Figure 20 View Figure 21 )
Type species: Chrysometa tenuipes ( Keyserling, 1864) . Levi (1986) designated a female lectotype and five female paralectotypes from Bogota ( Colombia), which are deposited at The Natural History Museum , London .
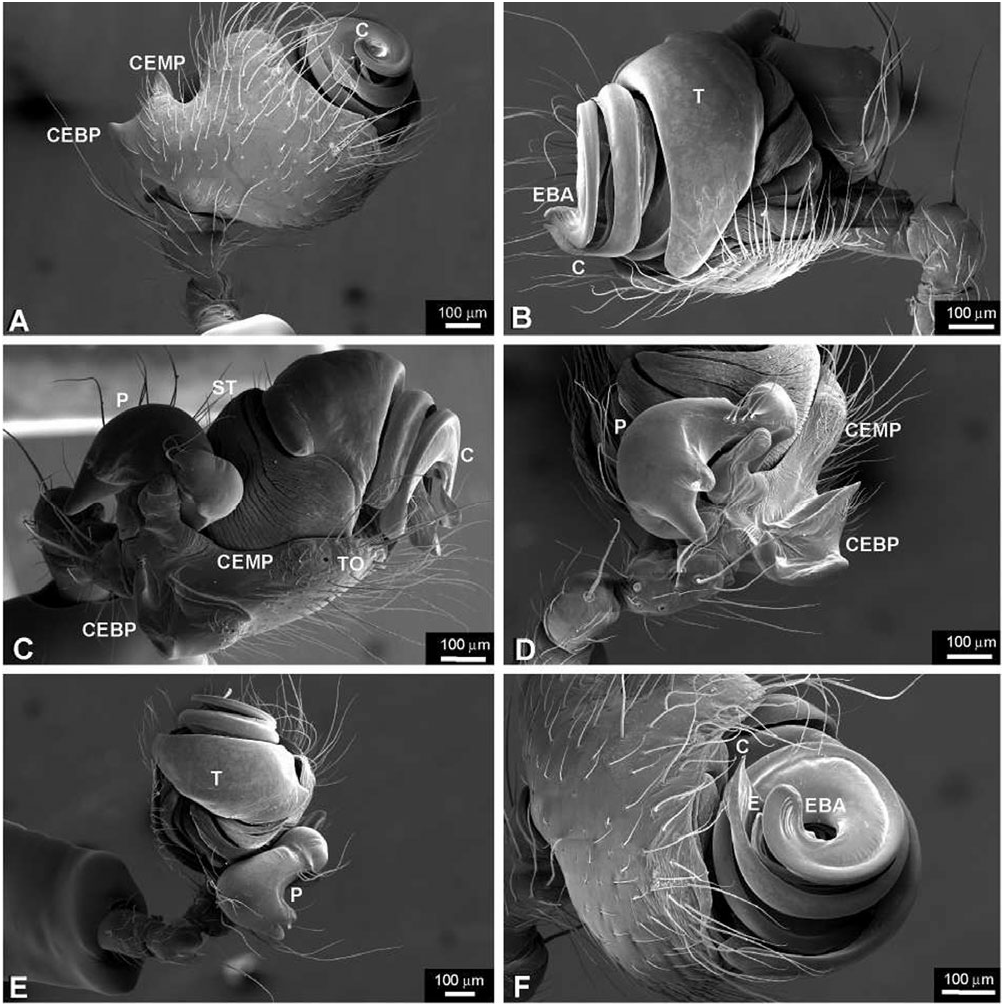
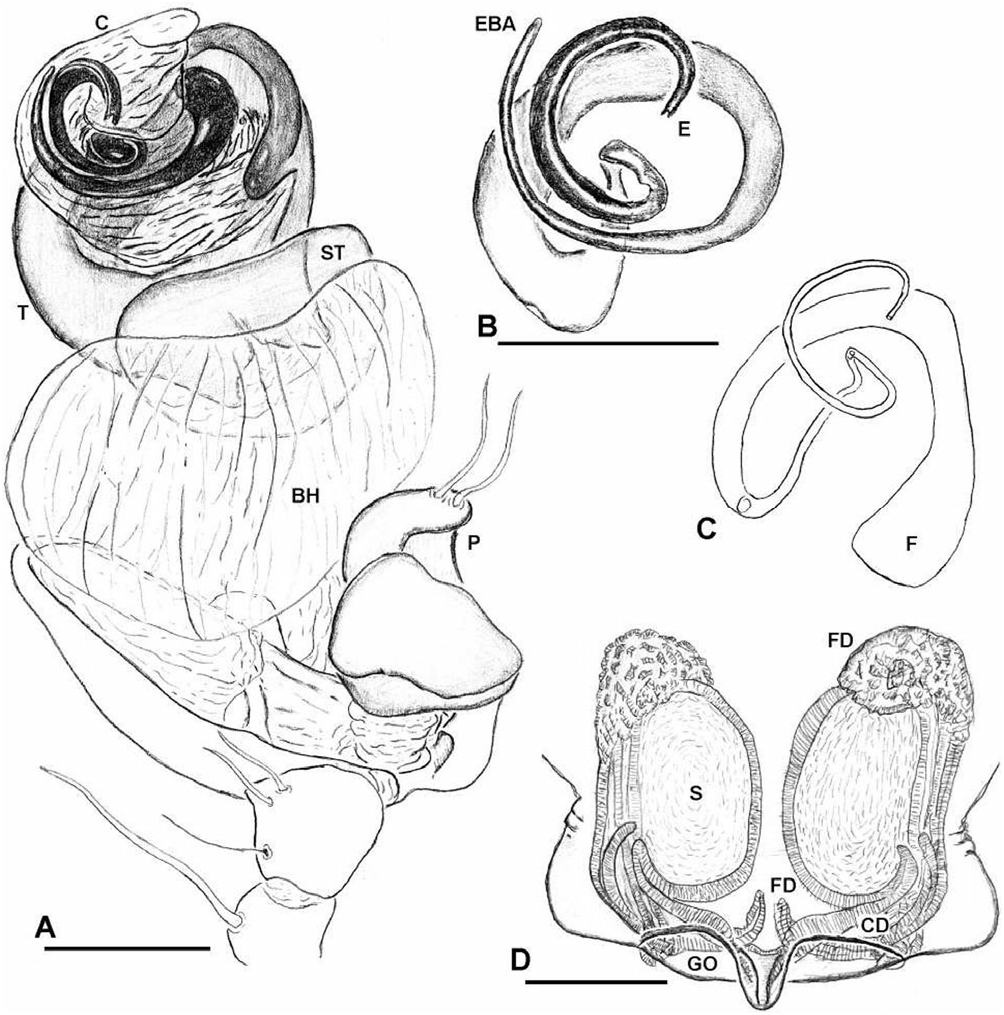
Diagnosis: Chrysometa species can be distinguished from all other tetragnathid genera by the following combination of characters: femora without trichobothria; abdomen covered with silver guanine patches ( Levi, 1986: fig. 651); epigynum flat ( Fig. 20A, B View Figure 20 ); fertilization ducts originating anteriorly and crossing over the spermathecae ( Figs 20E View Figure 20 , 22D View Figure 22 ); male palpal tibial length approximately as long as its widest point; paracymbium articulated and bearing several apophyses located at both extremes ( Fig. 21C, D View Figure 21 ); male cephalic region narrower than in the female, relative to the thoracic region ( Fig. 19A, B View Figure 19 ); and cymbial ectobasal process and cymbial ectomedian process present ( Fig. 21A, C View Figure 21 ).
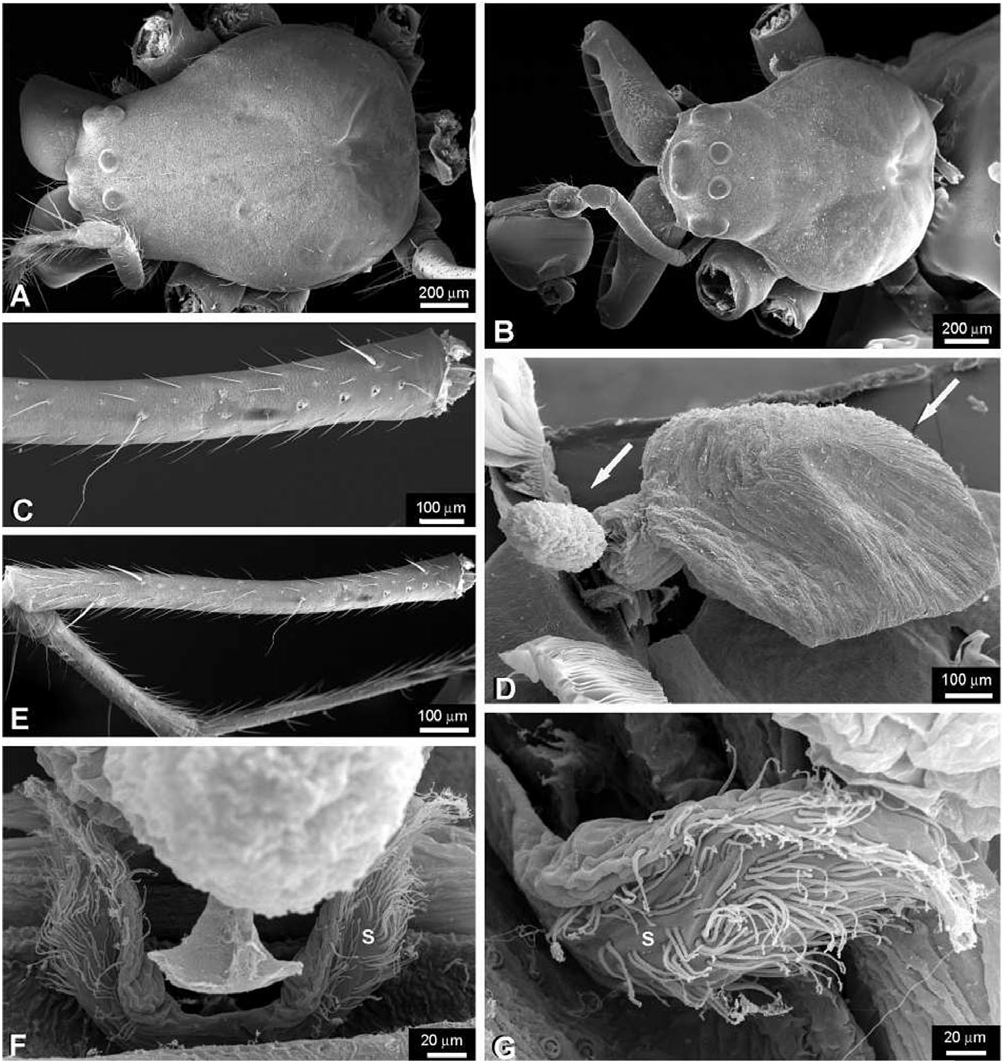
Description: Female: body length c. 10.0 mm. Carapace with a deep transverse m-shaped groove, cephalic region more than 70% of thoracic region width ( Fig. 19A View Figure 19 ). Ocular area higher than the carapace lateral margins ( Fig. 19C View Figure 19 ). Sternum as wide as long, labium trapezoidal and rebordered ( Fig. 19G View Figure 19 ). Anterior surface of chelicerae smooth ( Fig. 19E View Figure 19 ). Cheliceral boss present ( Fig. 19E, F View Figure 19 ). Secondary eyes with canoe-shaped tapetum. Eyes subequal in size, lateral eyes slightly smaller, juxtaposed and on a tubercle. Clypeus higher than one AME diameter. Median tracheae not ramified, with the tips leaf-shaped ( Fig. 18D View Figure 18 ) and less than half the lateral tracheae length ( Fig. 18A View Figure 18 ). Tracheal spiracle near the spinnerets, with fewer than four accessory glands on each junction with the tracheal trunks ( Fig. 18B, C View Figure 18 ). ALS with several piriform spigots ( Hormiga et al., 1995; fig. 16A–D). PMS anterior surface without aciniform spigots ( Fig. 18F View Figure 18 ; Hormiga et al., 1995; Fig. 20C View Figure 20 ). PLS with c. ten aciniform spigots roughly arranged in two parallel lines; aggregate spigots apex not embracing the flagelliform spigot tip ( Fig. 18E View Figure 18 ; Hormiga et al., 1995: Fig. 20D View Figure 20 ). Epigynal plate well sclerotized, slightly protruded ventrally and copulatory openings posteriorly orientated ( Fig. 20A–C View Figure 20 ). Spermathecae well sclerotized; fertilization ducts crossing over the spermatheca and originating anteriorly; accessory glands concentrated at the fertilization ductspermatheca junction and arranged in groups ( Figs 20E View Figure 20 , 22D View Figure 22 ). Copulatory duct walls well sclerotized and slightly longer than half the spermatheca length and fertilization ducts also well sclerotized ( Fig. 22D View Figure 22 ).
Male: body length c. 8.0 mm. Cephalic area less than 70% of thoracic region width, cephalic fovea deep, elongated x-shaped ( Fig. 19B View Figure 19 ). Ocular area is higher than the carapace lateral margins ( Fig. 19C, D View Figure 19 ). Cheliceral anterior-ectal margin rugose ( Fig. 19F View Figure 19 ). PLS aggregate and flagelliform spigots reduced to nubbins. Epiandrous fusules arranged in a transversal line, formed by several spigot clusters that are immersed in pits ( Fig. 18G View Figure 18 ). Male palpal patella with one macroseta ( Fig. 21D View Figure 21 ). Paracymbium with several basal and distal apophyses, with few macrosetae, and attached to the cymbium by a membrane ( Figs 21C–E View Figure 21 , 22A View Figure 22 ). Tegulum cup-shaped, with elevated apical margin ( Figs 21B View Figure 21 , 22A View Figure 22 ). Conductor membranous and originating behind a tegular ectal margin projection ( Fig. 22A View Figure 22 ). Embolus lamelliform and coiled. Embolic metine apophysis present (‘terminal apophysis’ in Levi, 1986), coiled with the embolus and firmly attached to its base ( Fig. 22B View Figure 22 ). Sperm duct spiralled without switchbacks ( Fig. 22C View Figure 22 ).
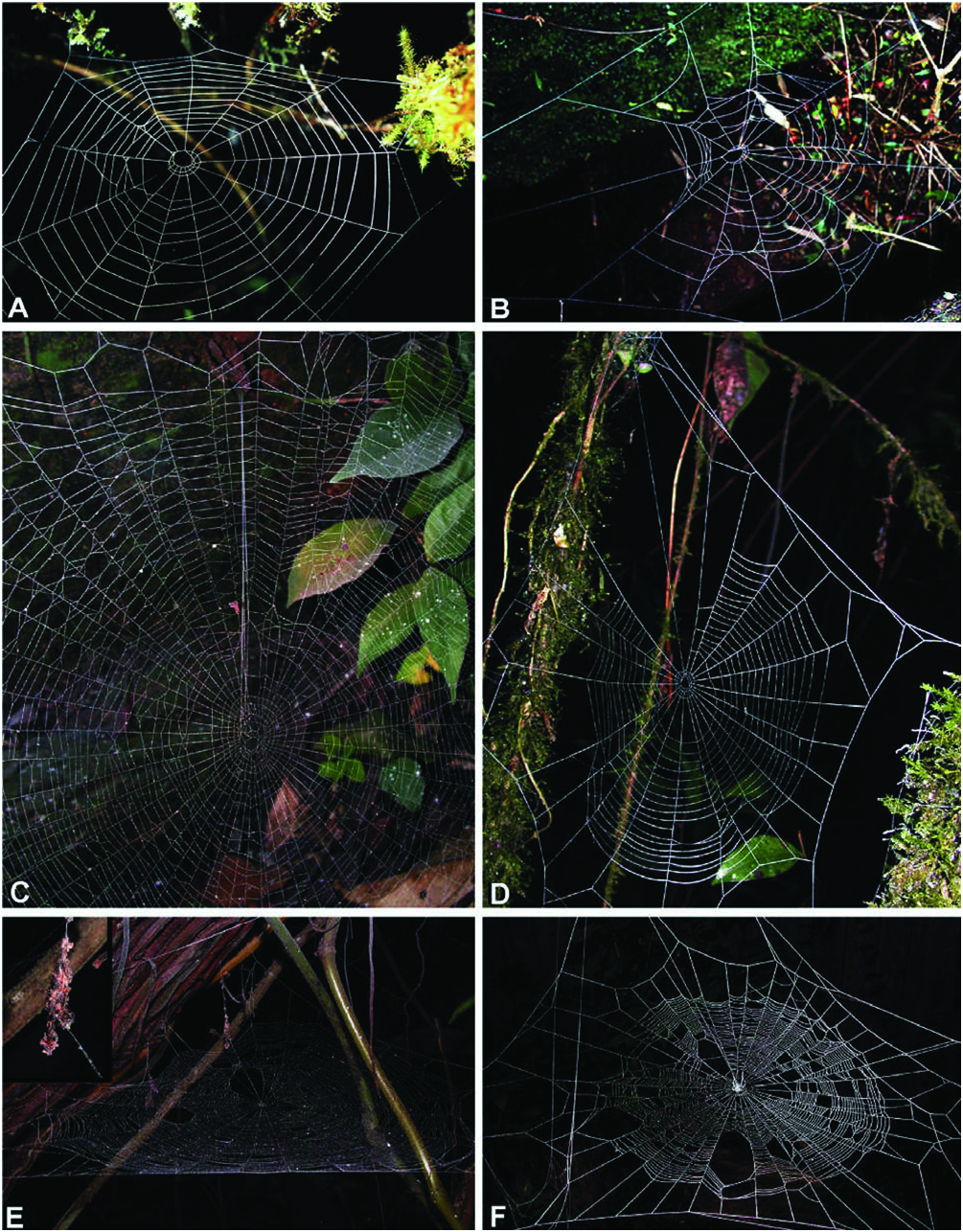
Natural history: Chrysometa is the third most diverse genus of tetragnathids, with 130 species described so far ( Platnick, 2009). These species inhabit the tropical and south temperate regions of the Americas, being more diverse in the tropical areas ( Levi, 1986). These spiders build vertical webs amongst the forest vegetation. Their webs usually have few radii, few spirals (fewer than 30), an open hub, and an open sector at the top of the web ( Fig. 3D View Figure 3 ; Levi, 1980: plates 1–2). This open sector, when present, has a ‘telegraph’ silk line that connects the centre of the web with the spider retreat (outside the web). These spiders are usually found hiding in their retreat holding the end of this line.
Taxonomy: Chrysometa was revised by Levi (1986) who illustrated all 127 species. It seems very likely that many new species of Chrysometa remain to be discovered. Some aspects of the behaviour of Chrysometa have been studied, such as the sequence of leg movements used during web construction ( Eberhard, 1982, 1984, 1987). The diagnosis and description of this genus is based on Levi (1986) plus additional observations of specimens from the following species: Chrysometa nuboso , Chrysometa saladito , Chrysometa acinosa , Chrysometa flava , and other specimens of Chrysometa spp. from Colombia. Some characters in the genus description refer only to Chrysometa alajuela Levi, 1986 (from Costa Rica) because they have only been studied in this species so far. The monophyly of Chrysometa remains to be tested with a large taxonomic sample. The only phylogenetic analysis to date included only six species, and was designed to test whether the two described Chilean Chrysometa species belonged to this genus ( Álvarez-Padilla, 2007). The synapomorphies proposed for Chrysometa in that study must be taken as tentative because of the relatively small taxonomic sample used. C hrysometa has been proposed as sister to Metellina ( Hormiga et al., 1995) or Allende ( Álvarez-Padilla, 2007) . The present phylogenetic analysis based on morphology and behaviour recovered Chrysometa as sister to a large clade of tetragnathids that includes: the Nanometinae , D. spinifera , and Tetragnathinae ( Fig. 143A View Figure 143 ). When these data are combined with the nucleotide sequences Chrysometa is recovered as sister to Diphya and this clade is nested within Metainae ( Fig. 144 View Figure 144 ). We coded specimens of c. alajuela for the phylogenetic analysis.
( FIGS 2A View Figure 2 , 3A View Figure 3 , 23–25 View Figure 23 View Figure 24 View Figure 25 )
Type species: Cyrtognatha nigrovittata Keyserling, 1881a . The holotype of c. nigrovittata is a male specimen from Pumamarca ( Peru) deposited at the Polish Academy of Sciences ( Dimitrov & Hormiga, 2009).
Diagnosis: Cyrtognatha species can be distinguished from all other tetragnathid genera by the following combination of characters: the presence of feathered trichobothria on femur IV ( Fig. 24C, E View Figure 24 ); PLS ectal surface with a straight line of long and robust macrosetae with enlarged bases ( Dimitrov & Hormiga, 2009: fig. 35E, F); spermathecae reduced, the sperm being stored in a specialized membranous sac ( Fig. 24D–G View Figure 24 ; Dimitrov, Álvarez-Padilla & Hormiga, 2007: fig. 3A–F); male chelicerae not projected, wider distally and conspicuously divergent in its distal two thirds ( Fig. 23A, B View Figure 23 ); embolus enlarged and bearing a basal apophysis that can be subdivided ( Dimitrov & Hormiga, 2009: fig. 6A, E); sclerotized paracymbium and cymbium joint ( Dimitrov & Hormiga, 2009: fig. 41D–F); and conductor with several apophyses ( Fig. 25A, B View Figure 25 ; Dimitrov & Hormiga, 2009: fig. 6C).
Description: Female: body length variable, c. 4.5 to 6.0 mm ( Dimitrov & Hormiga, 2009). Cephalothorax fovea transverse ( Fig. 24A View Figure 24 ). Femora IV with both branched and smooth trichobothria ( Fig. 24C–E View Figure 24 ; Dimitrov & Hormiga, 2009: figs 12H, 29F, Cyrtognatha quichua and Cyrtognatha pachygnathoides , respectively). Ocular area lower than carapace lateral margins ( Fig. 23F View Figure 23 ). Labium trapezoidal, wider than long and rebordered. Sternum longer than wide ( Fig. 23C View Figure 23 ). Chelicerae not divergent, anterior surface smooth and cheliceral boss present ( Fig. 23A, D View Figure 23 ). Clypeus less than one AME diameter. Abdomen cylindrical with silver guanine patches and with anterior and caudal tubercles. Booklung cuticle smooth ( Dimitrov & Hormiga, 2009: fig. 36F). Tracheal spiracle located near the spinnerets. ALS have an extensive field of piriform spigots ( Dimitrov & Hormiga, 2009: fig. 35G). PMS anterior surface are without aciniform spigots ( Dimitrov & Hormiga, 2009: fig. 36A). PLS with c. 15 aciniform spigots arranged in two parallel lines, distal end of the aggregate spigots separated from the distal end of the flagelliform spigot ( Dimitrov & Hormiga, 2009: fig. 35E, F). Epigynal plate absent, copulatory opening as a transverse spiracle ( Dimitrov & Hormiga, 2009: fig. 36E). Spermathecae vestigial, presumably not functional ( Fig. 24F, G View Figure 24 ; Dimitrov et al., 2007: fig. 3A–F). Copulatory ducts weakly sclerotized, not membranous, covered with accessory glands. Fertilization ducts absent. Two types of accessory glands are present, long-stem gland ductiles over the copulatory ducts and short-stem gland ductiles over the posterior sac ( Fig. 24D, F, G View Figure 24 , respectively, and Dimitrov et al., 2007: fig. 3B). Accessory glands immersed in individual pits ( Fig. 24G View Figure 24 ).
Male: body length from 3.0 to 4.3 mm ( Dimitrov & Hormiga, 2009). Somatic morphology similar to that of the female, except that the chelicerae are considerably larger, divergent, with the ectal surface rugose and with distal apophyses ( Fig. 23A, B View Figure 23 ). Abdomen without caudal tubercle ( Dimitrov & Hormiga, 2009: fig. 28A–D). PLS triplet reduced to nubbins. Epiandrous fusules area as the surrounding abdominal cuticle; fusules arranged in one transverse line with enlarged bases. Palpal patella with one macroseta. Palpal femur more than four times its width, tibia approximately as long as the widest point of the tibia ( Figs 24B View Figure 24 , 25E View Figure 25 ). Paracymbium cylindrical, longer than half the cymbium length, with few macroseta and without a basal apophysis ( Fig. 25D, F View Figure 25 ). Paracymbium and cymbium attachment with a sclerotized continuous margin. Tegulum spherical ( Fig. 25C View Figure 25 ). Conductor-tegulum attachment membranous, located on the centre of the tegulum ( Fig. 25A View Figure 25 ). Embolus lamelliform, coiled and without basal apophyses ( Fig. 25E View Figure 25 ). Most Cyrtognatha species have basal apophyses on the embolus ( Dimitrov & Hormiga, 2009: fig. 6A, B, D, E). Sperm duct spiralled and considerably enlarged in its middle section ( Dimitrov & Hormiga, 2009: fig. 6A, E).
Natural history: There are 21 Cyrtognatha species described, but most of them are known from only a few museum specimens ( Dimitrov & Hormiga, 2009). Cyrtognatha species live in cloud and rain forests in the Americas, from Mexico to Argentina, the West Indies, and are more diverse in the tropical areas. The wide geographical distribution of the genus and its poor representation in collections suggests that many species of Cyrtognatha remain to be discovered. Cyrtognatha species are found resting on the centre of the web with legs I and II extended, legs I always in contact with radii ( Fig. 2A View Figure 2 ). They usually build horizontal orb webs with open hub and the number of radii and spirals vary considerably amongst species ( Fig. 3A View Figure 3 ; Dimitrov & Hormiga, 2009: fig. 2C, E). The web building behaviours of a Cyrtognatha species were described by Eberhard (1982). The information in the diagnosis and description is based on Dimitrov & Hormiga’s (2009) monograph. We coded specimens of Cyrtognatha espanola ( Bryant, 1945) in this phylogenetic analysis.
Taxonomy: This genus has been recently revised, its monophyly tested, and its phylogenetic relationships studied ( Dimitrov & Hormiga, 2009). The monophyly of Cyrtognatha is well supported with BS values of 13 and JK values of 99. These authors concluded that the sister group of Cyrtognatha is Tetragnatha . The present phylogenetic analysis with morphology and behaviour also recovered Cyrtognatha and Tetragnatha as sister taxa ( Fig. 143A View Figure 143 ); however, when these data were combined with the DNA partition Cyrtognatha was recovered as sister to a clade that including Tetragnatha , Pachygnatha , and Glenognatha ( Fig. 144 View Figure 144 ).
| PMS |
Peabody Essex Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.