ARGYRODINAE
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2004.00120.x |
|
persistent identifier |
https://treatment.plazi.org/id/7E1687E1-422B-6C1C-FC7B-FAF38922FCF8 |
|
treatment provided by |
Diego |
|
scientific name |
ARGYRODINAE |
| status |
|
ARGYRODINAE : CLADE 32
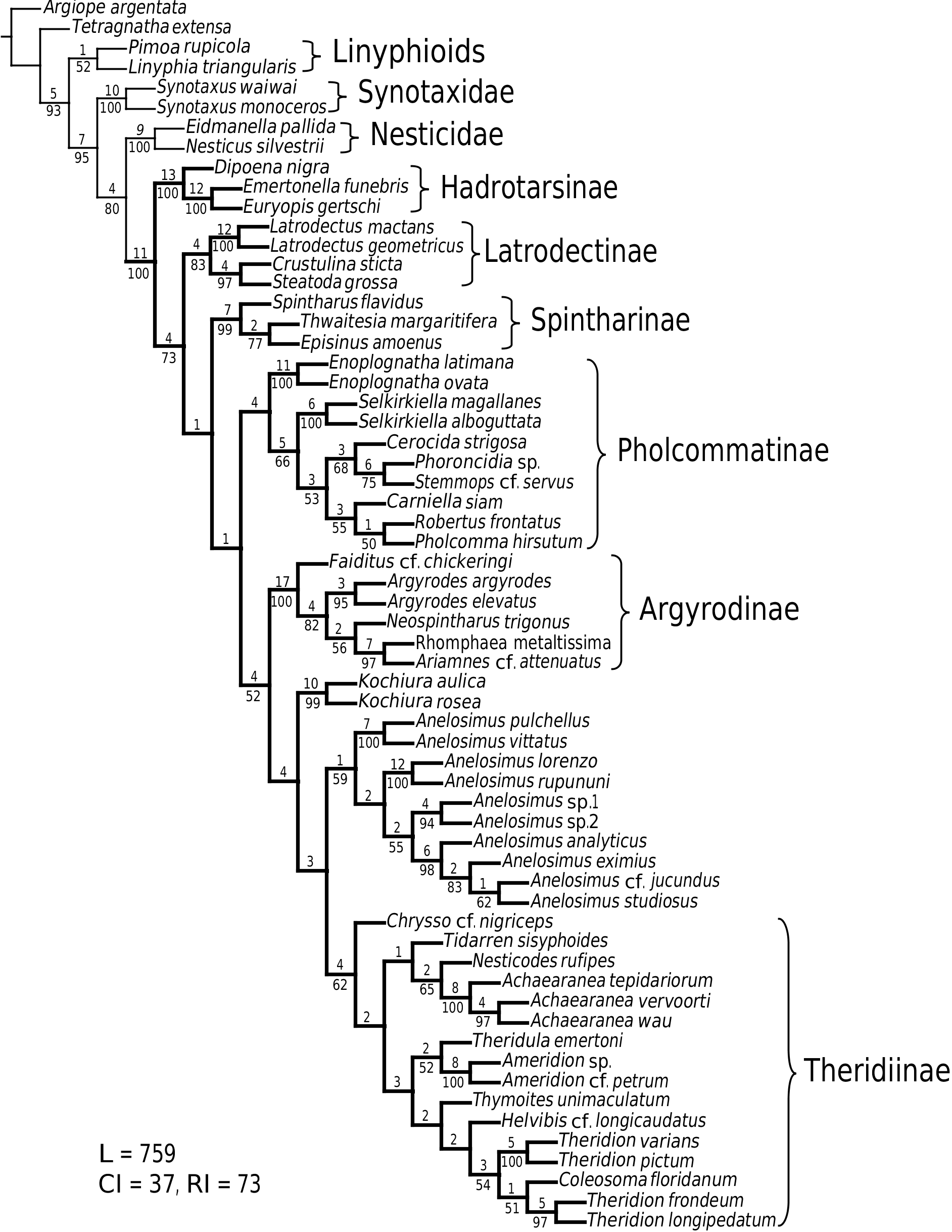
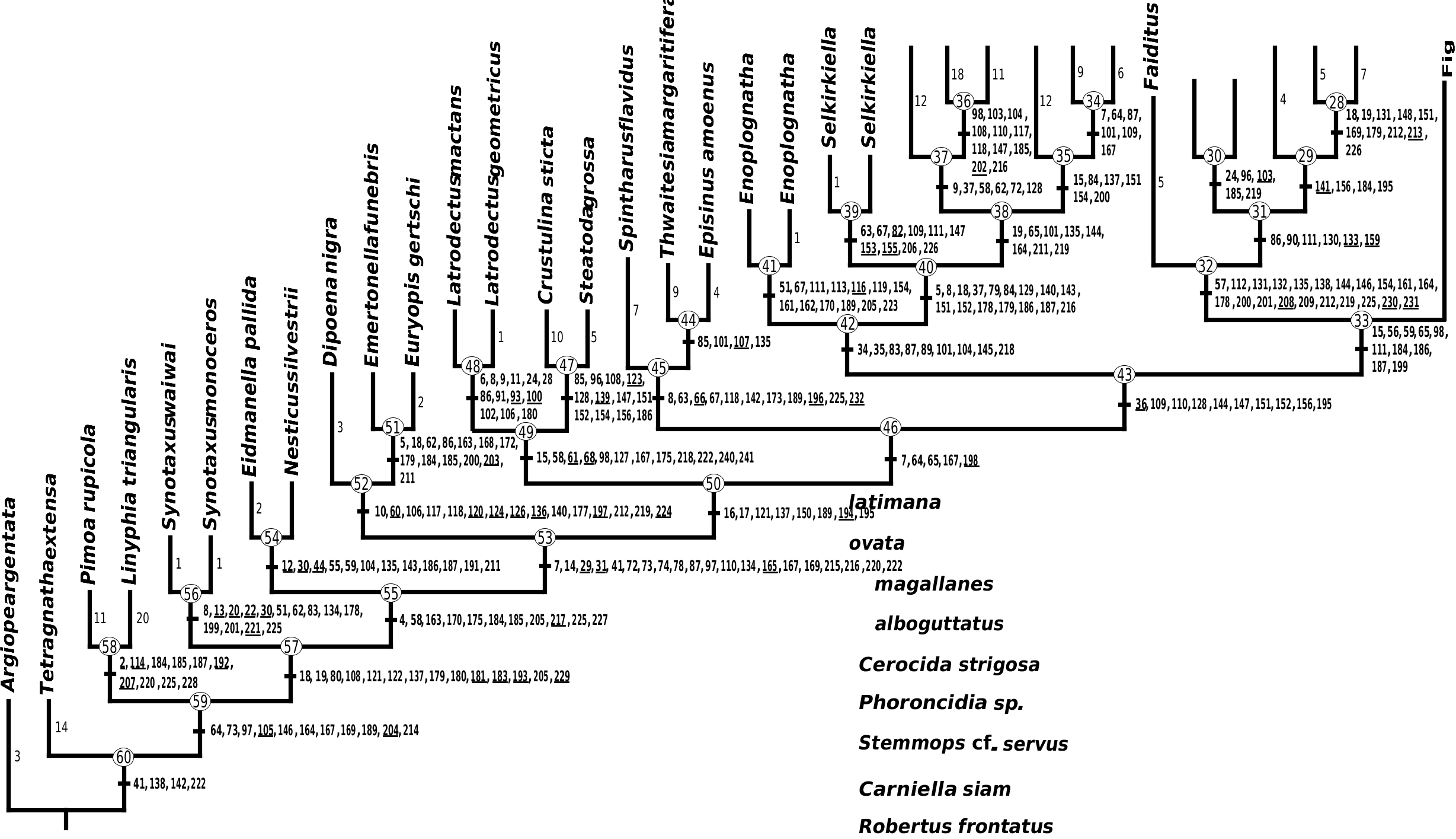
The six species of argyrodines included in this study represent the five of the six species groups of Exline & Levi (1962), including the newly resurrected genera Rhomphaea and Ariamnes (see Yoshida, 2001b). Although all groups differ considerably in morphology and behaviour, they also share numerous peculiarities and argyrodine monophyly is strongly supported ( Fig. 103 View Figure 103 ), as Exline & Levi (1962) hypothesized (see also Arnedo et al., 2004).
However, simply synonymizing all available names in this group with Argyrodes has turned out to be too broad a formulation, in part because species range from free living araneophages to obligatory kleptoparasites (see ‘Kleptoparasitism and araneophagy’ below). Typical biological summaries are thus misleading and confusing: ‘Tropical spiders of the theridiid genus Argyrodes Simon inhabit the webs of other spiders’ ( Vollrath, 1979b: 1149); ‘Spiders of the genus Argyrodes (Theridiidae) are generally known as kleptoparasitic’ ( Tanaka, 1984: 363); ‘Some species of Argyrodes (Theridiidae) can regularly be found in the webs of other spiders… Other Argyrodes are free-living, some feed on spiders’ ( Vollrath, 1984: 70). It has even been suggested that the expression of behaviour (either kleptoparasitism or free living araneophagy) is environmentally controlled, rather than showing a phylogenetic pattern (see Whitehouse et al., 2002).
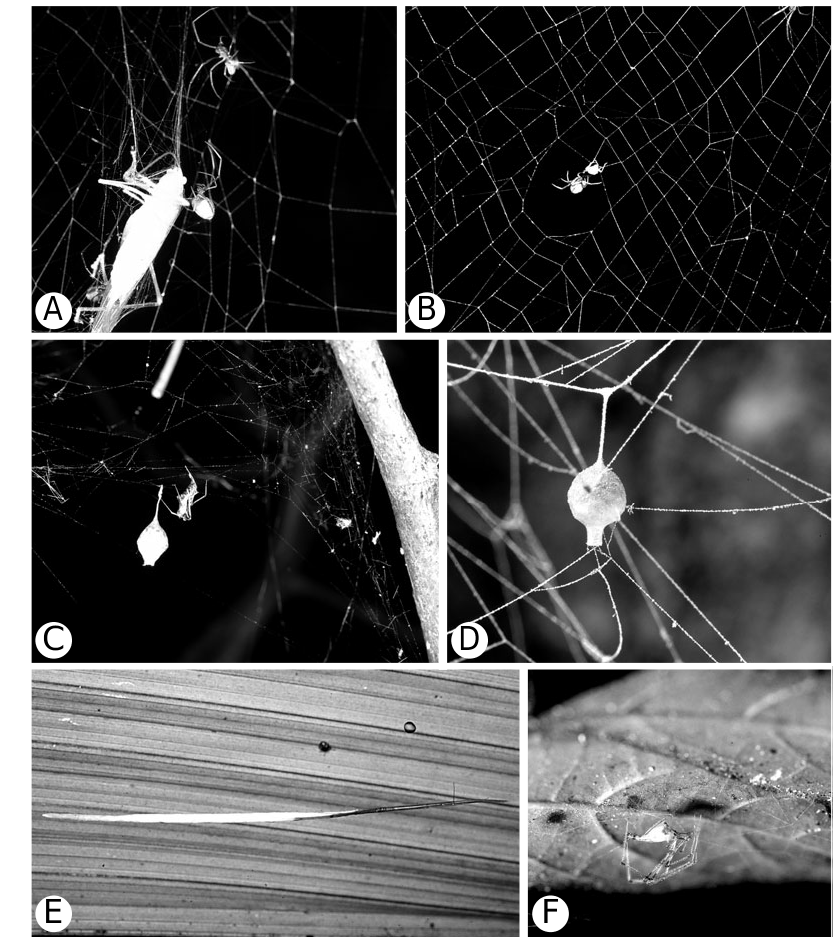
However, morphology and behaviour are coherent within species groups. All studied Ariamnes species have a characteristically elongated abdomen ( Fig. 94A View Figure 94 ), construct simple nonsticky line webs, and specialize on nematocerous flies and wandering male spiders that use the lines to travel (e.g. Ariamnes attenuatus (O. P.-Cambridge) (see Eberhard, 1979), A. colubrinus (Keyserling) (see Clyne, 1979), and A. flagellum (Doleschall) (see Roberts, 1952). All studied ‘ Argyrodes argyrodes group’ (sensu Exline & Levi, 1962) species seem to be obligate kleptoparasites, e.g. A. antipodianus O. P.-Cambridge (see Whitehouse, 1986; Whitehouse & Jackson, 1993; Grostal & Walter, 1997), A. argentatus O. P.-Cambridge (see Robinson & Robinson, 1973), A. argyrodes (Walckenaer) (see Wiehle, 1928; Kullmann, 1959a, b) A. elevatus Taczanowski (see Vollrath, 1977, 1979a, 1984, 1987), and A. nephilae Taczanowski (see Vollrath, 1987).
Most authors do not keep track of informal species group names as they would generic names, and thus these patterns have been obscured. The Linnaean rank system has been criticized on many grounds (e.g. de Queiroz & Cantino, 2001), but prevails, in part due to the information ranks (in particular family and genus) can convey. In order to maximize this information content, the genus rank should, when possible, identify a coherent group, whose morphology and biology can be summarized efficiently based on any of its members. Some authors (e.g. Whitehouse, 1987b; Forster & Forster, 1999) never accepted all of Exline and Levi’s synonymies, and continued treating Ariamnes and Rhomphaea (the most distinct nonkleptoparasitic Argyrodinae ) as valid genera. Tanikawa (1998) added Spherophista to the growing Argyrodes , before Yoshida (2001b) explicitly rejected the synonymies of Argyrodes , Ariamnes , Rhomphaea and Spheropistha , and resurrected the latter three (note that Platnick, 2003, did not follow Yoshida, 2001b). Following Arnedo et al. (2004), I agree with Yoshida’s revalidation of Ariamnes and Rhomphaea , although it is insufficient, as it renders the remaining ‘ Argyrodes ’ paraphyletic ( Fig. 102 View Figure 102 ), and ignores other distinct argyrodine clades. Therefore, Faiditus and Neosphintharus also should be recognized as genera (see Taxonomy).
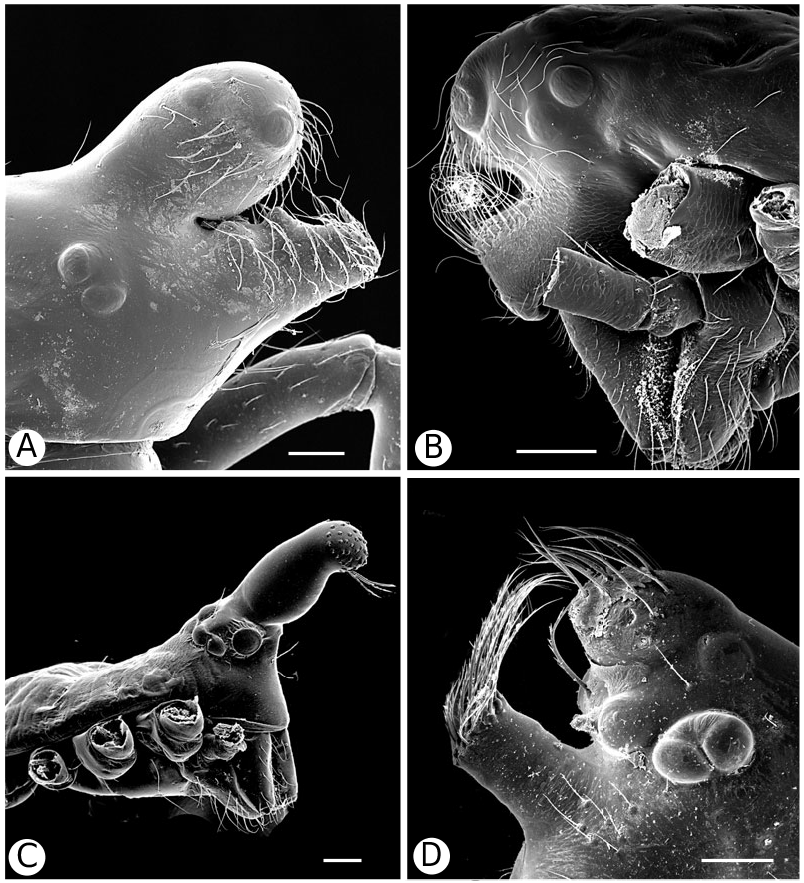
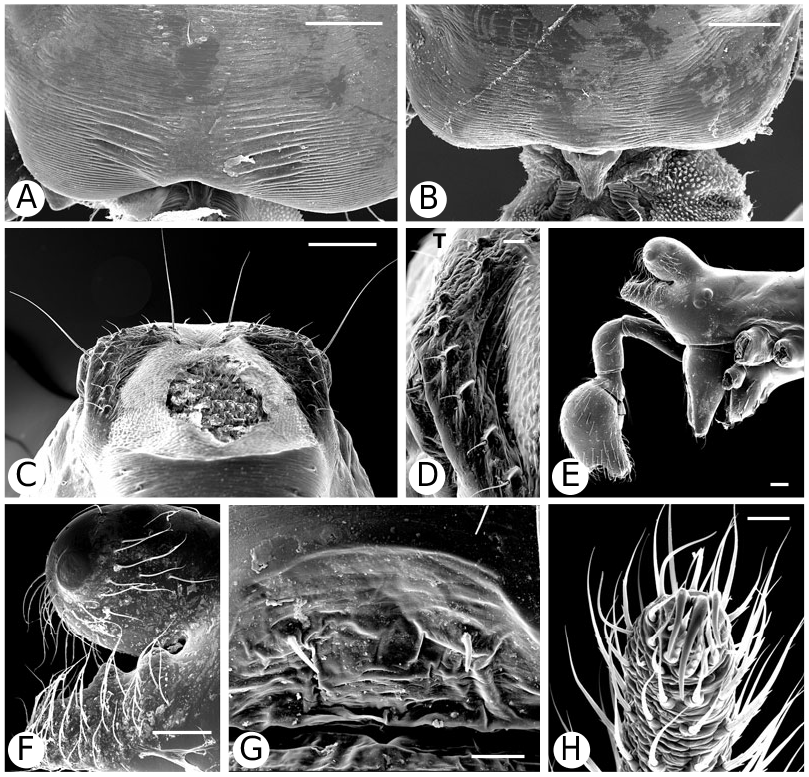
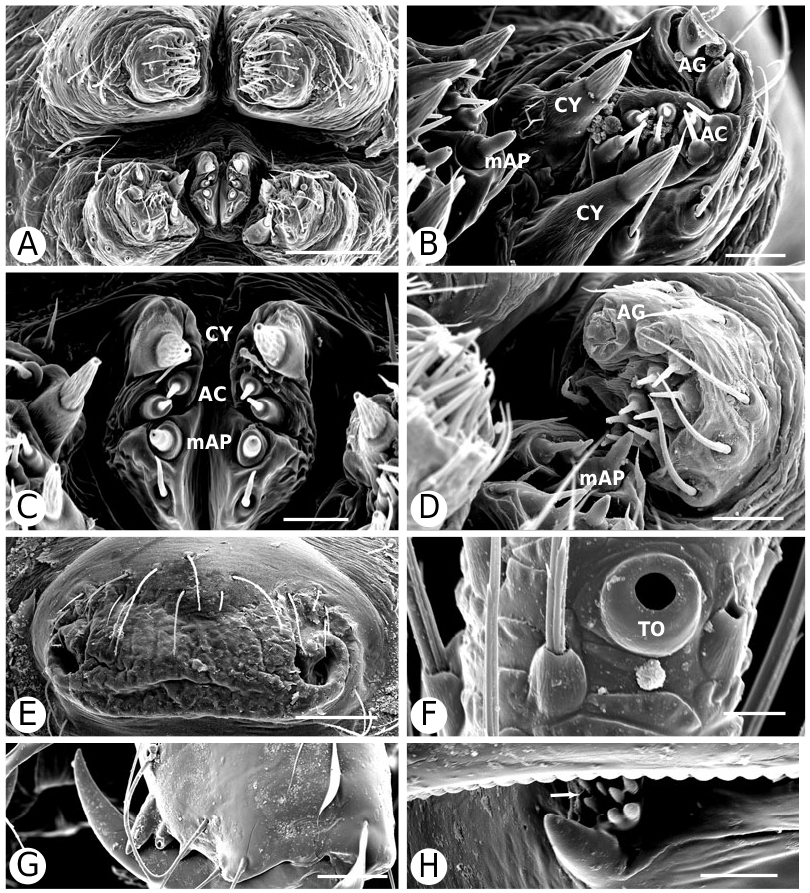
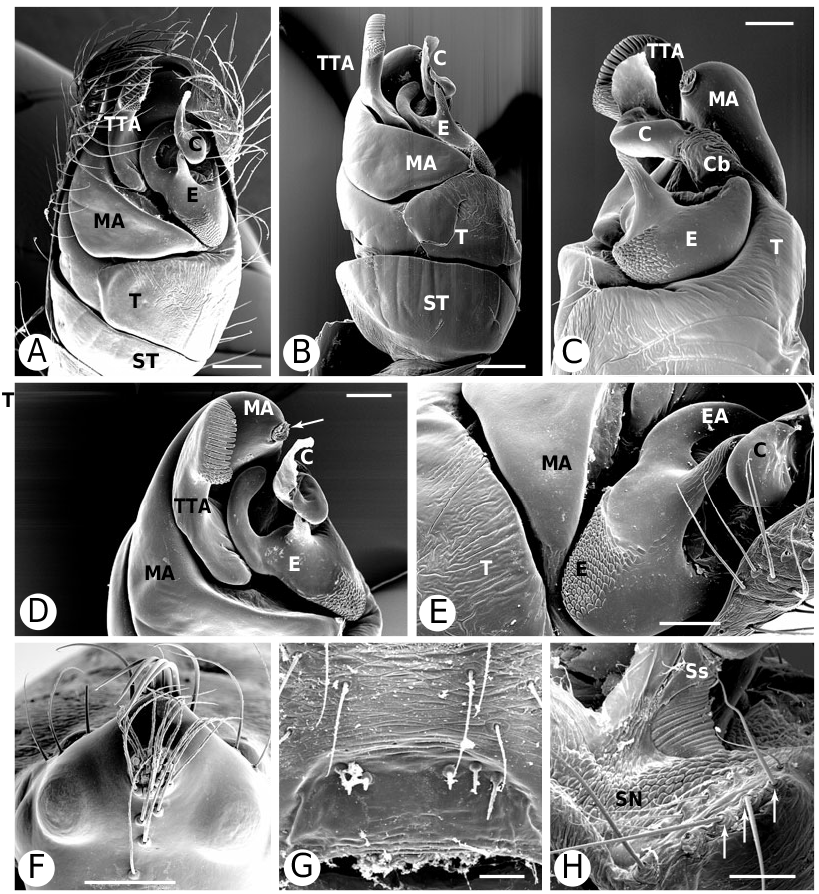
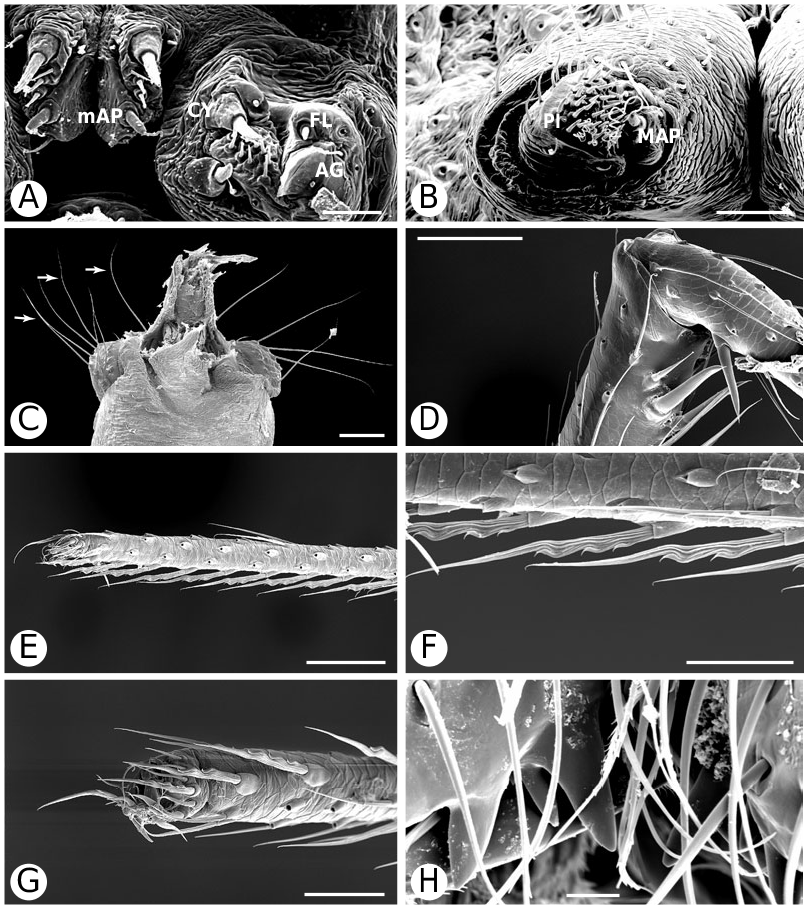
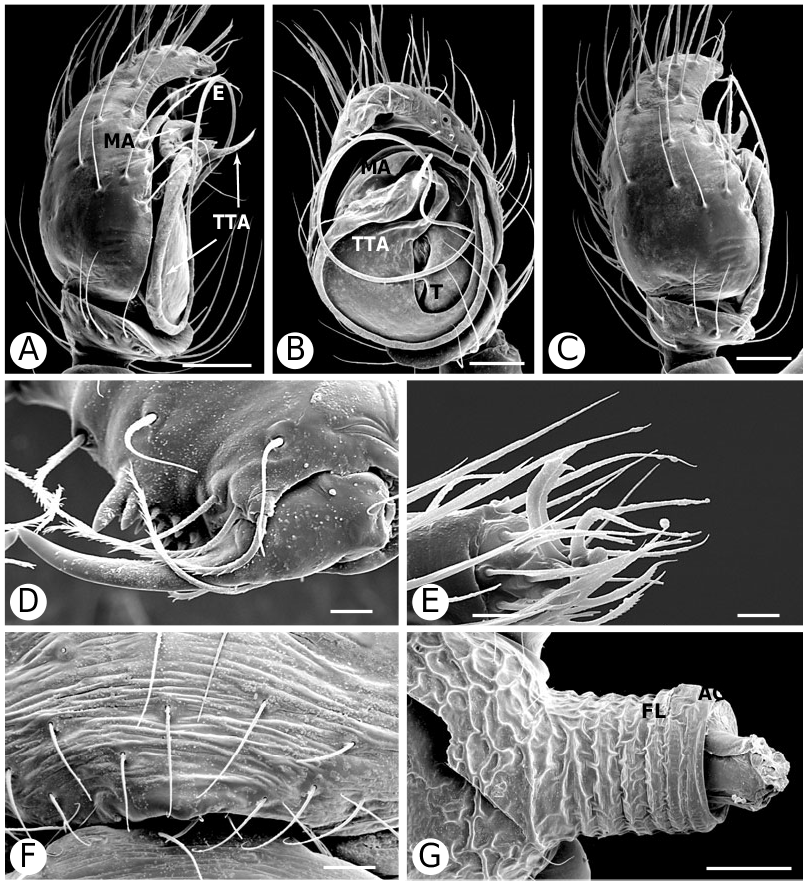
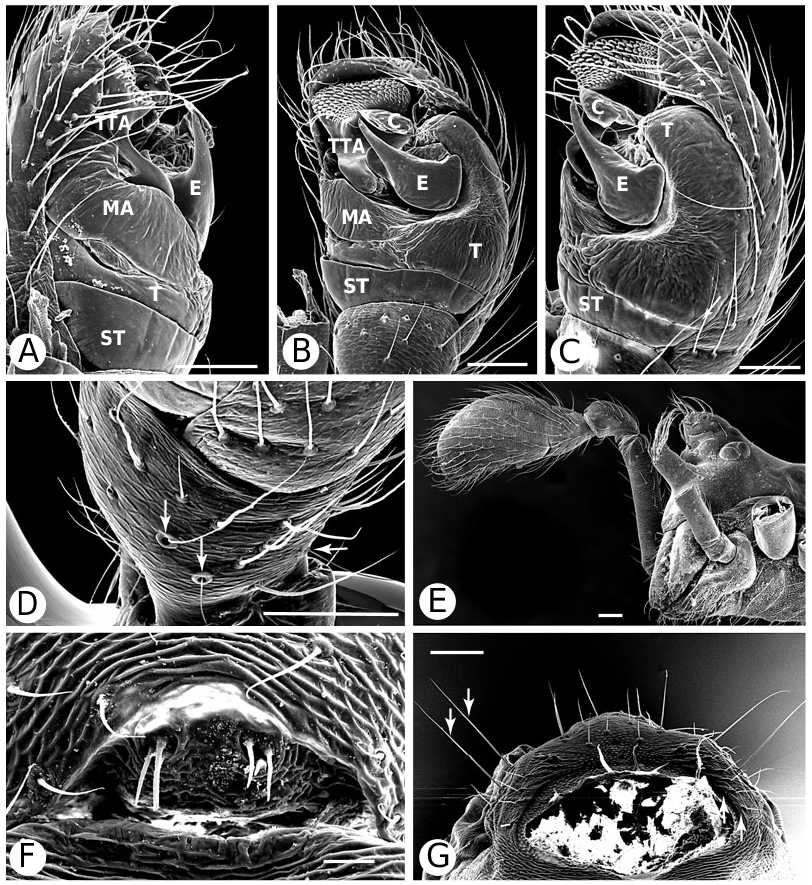
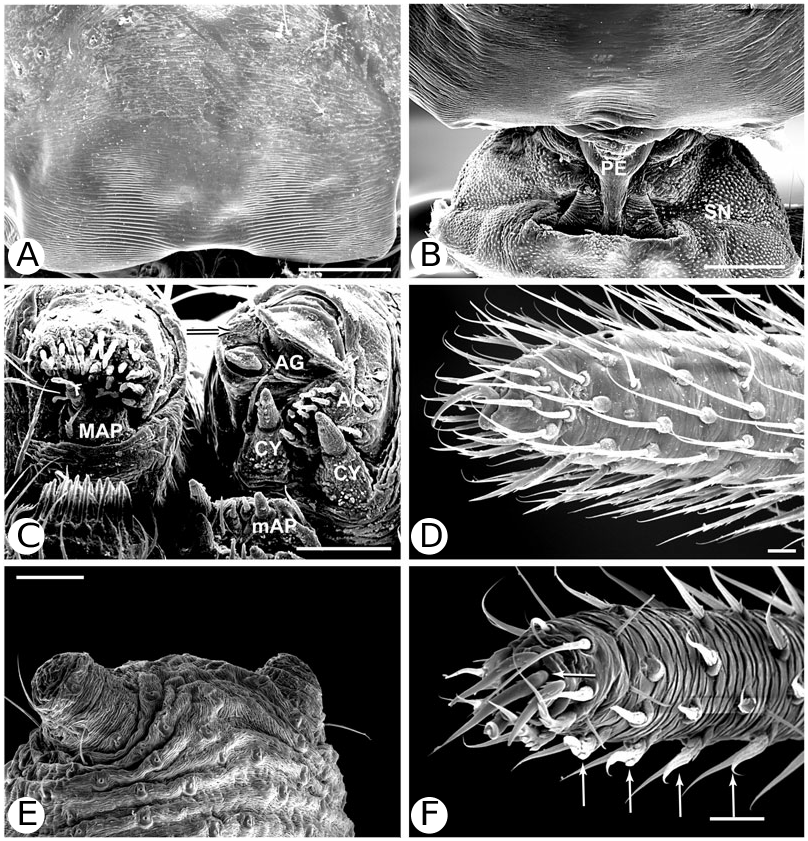
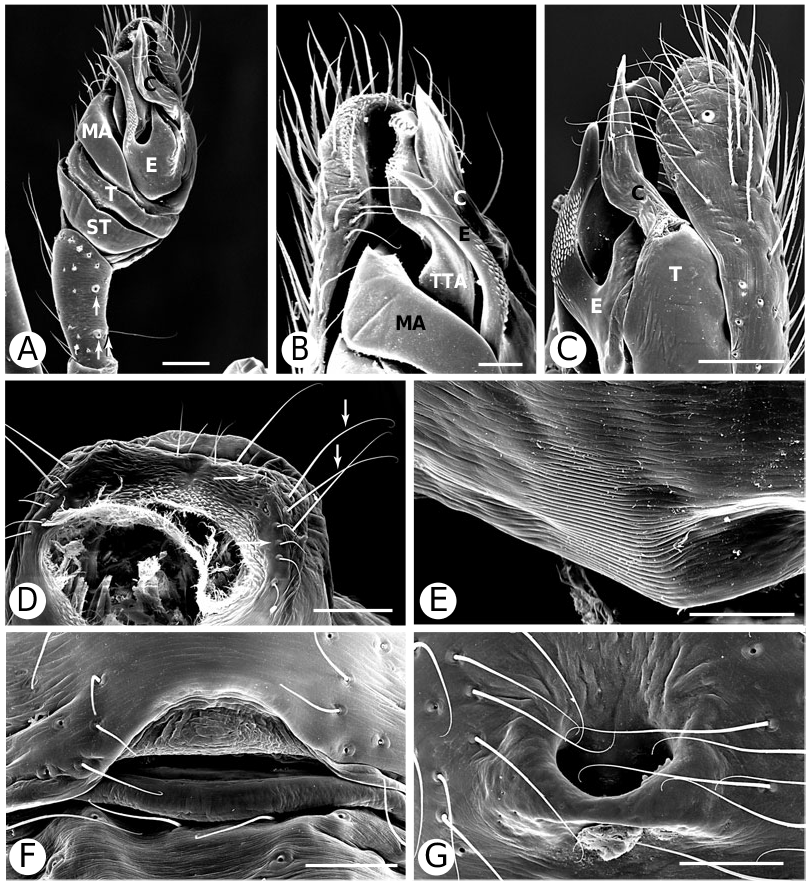
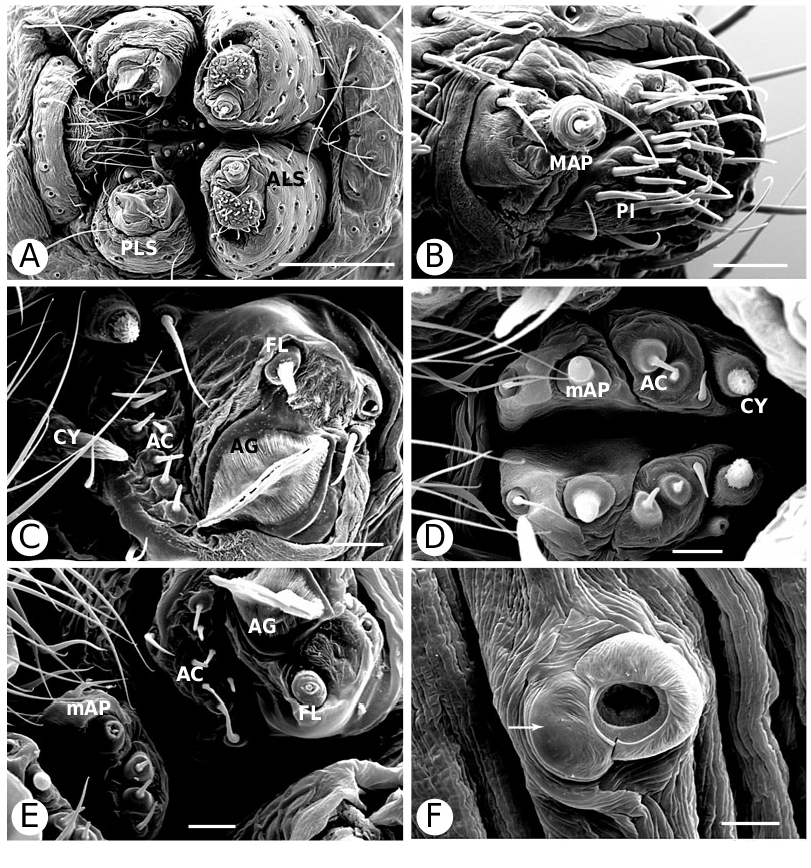
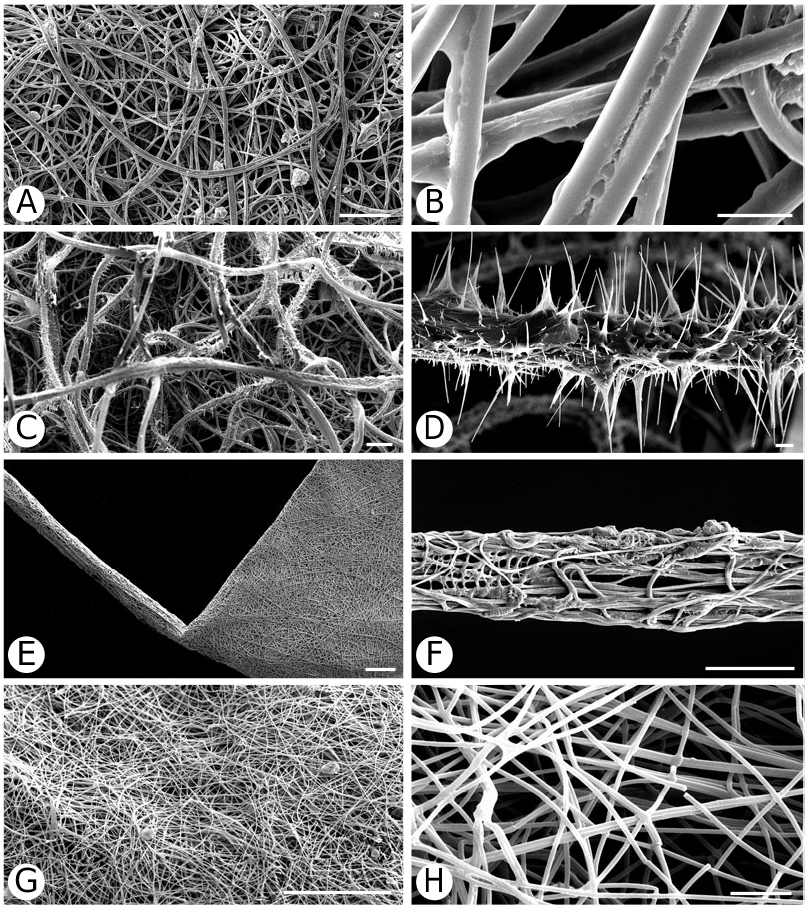
Argyrodinae is supported by an array of synapomorphies, including: sperm duct reverse switchbacks (57, Fig. 93D, E View Figure 93 ), cheliceral furrow denticulated (112, Figs 33H View Figure 33 , 37D View Figure 37 ), clypeal projection (131, Figs 30A–D View Figure 30 , 94B–E View Figure 94 ), dense field of setae in ocular area (132, Figs 30A–D View Figure 30 , 34F View Figure 34 ), silvery dots on abdomen (146), abdominal stridulatory picks on ridge, parallel with pedicel (154, 161, Figs 32C, D View Figure 32 , 56G View Figure 56 , 64D View Figure 64 ), ventrolateral suprapedicellate setal proprioreceptors absent (164, Figs 32C View Figure 32 , 64D View Figure 64 ), female fourth tarsal central claw much longer than laterals (200, Figs 32H View Figure 32 , 57F View Figure 57 ), abdomen extending beyond the spinnerets (201, Figs 94A– E View Figure 94 , 98F View Figure 98 ), huge and elongated, strongly grooved, CY spigots (208, 209, Figs 33B View Figure 33 , 57C View Figure 57 , 65C, D View Figure 65 ), PLS FL absent (212, Fig. 33B View Figure 33 , possibly reversed in Ariamnes ( Fig. 35A View Figure 35 ), Rhomphaea ( Fig. 65C View Figure 65 ), see character description), simple nonsnare webs (225), and egg case stalked and modified (230, 231, Figs 88E, F View Figure 88 , 98C–E View Figure 98 ). Unusually, the AGs are functional in all argyrodine males examined here (19 species) (219, Fig. 48F View Figure 48 ) except Argyrodes ( Fig. 33D View Figure 33 ); this condition is an ambiguous synapomorphy of Argyrodinae (ACCT- RAN). Kovoor & Lopez (1983: 35) claim that argyrodines have larger ampullate silk glands than do other theridiids, and some lack flagelliform glands.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |