Heptodonta melanopyga ( Schaum, 1862 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4875.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:278200CE-E16F-45B4-9A89-60C2052415C7 |
|
DOI |
https://doi.org/10.5281/zenodo.4579791 |
|
persistent identifier |
https://treatment.plazi.org/id/807787F6-B82D-FFCE-49A1-D755FCEE3BED |
|
treatment provided by |
Plazi |
|
scientific name |
Heptodonta melanopyga ( Schaum, 1862 ) |
| status |
|
Heptodonta melanopyga ( Schaum, 1862) View in CoL
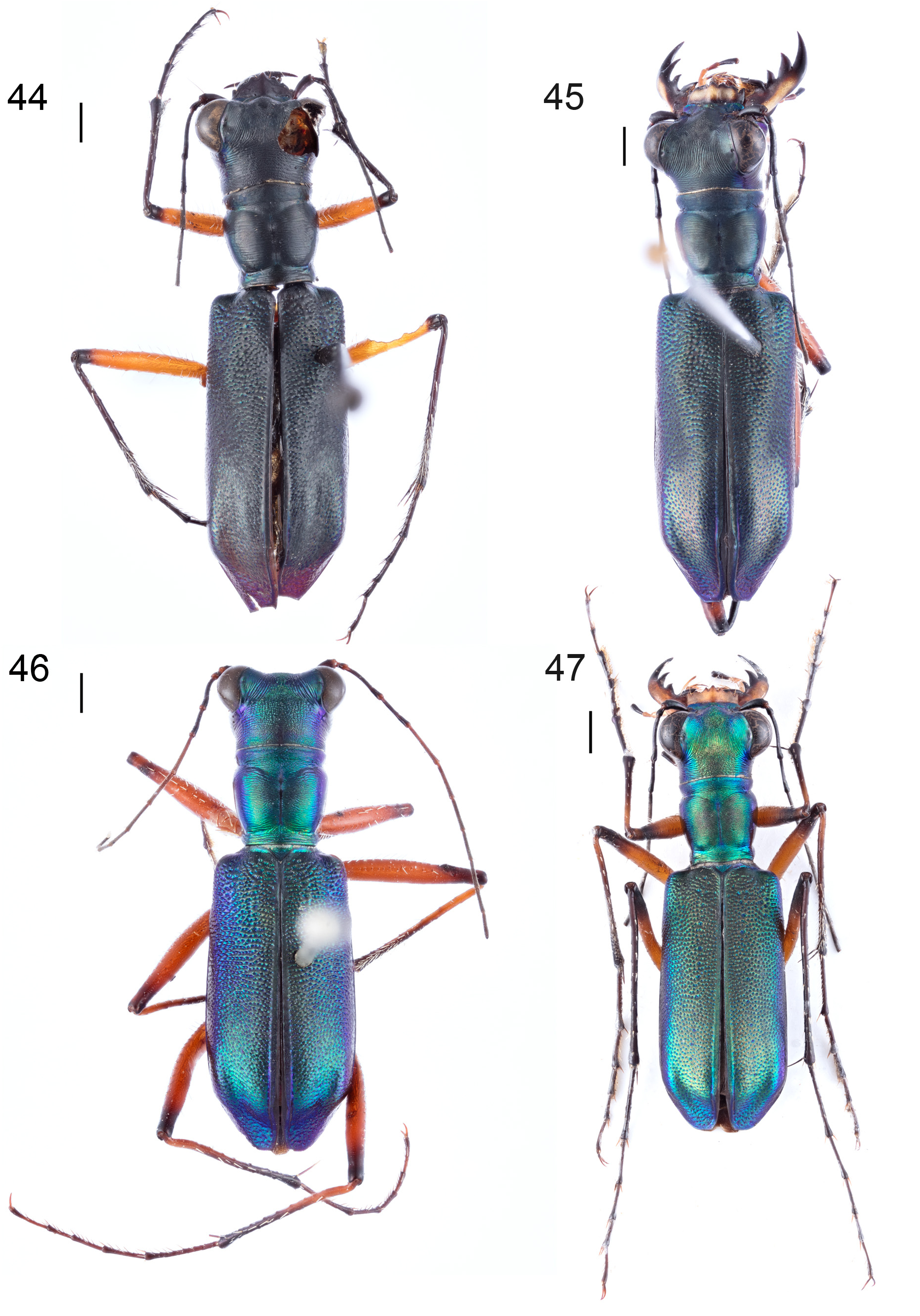
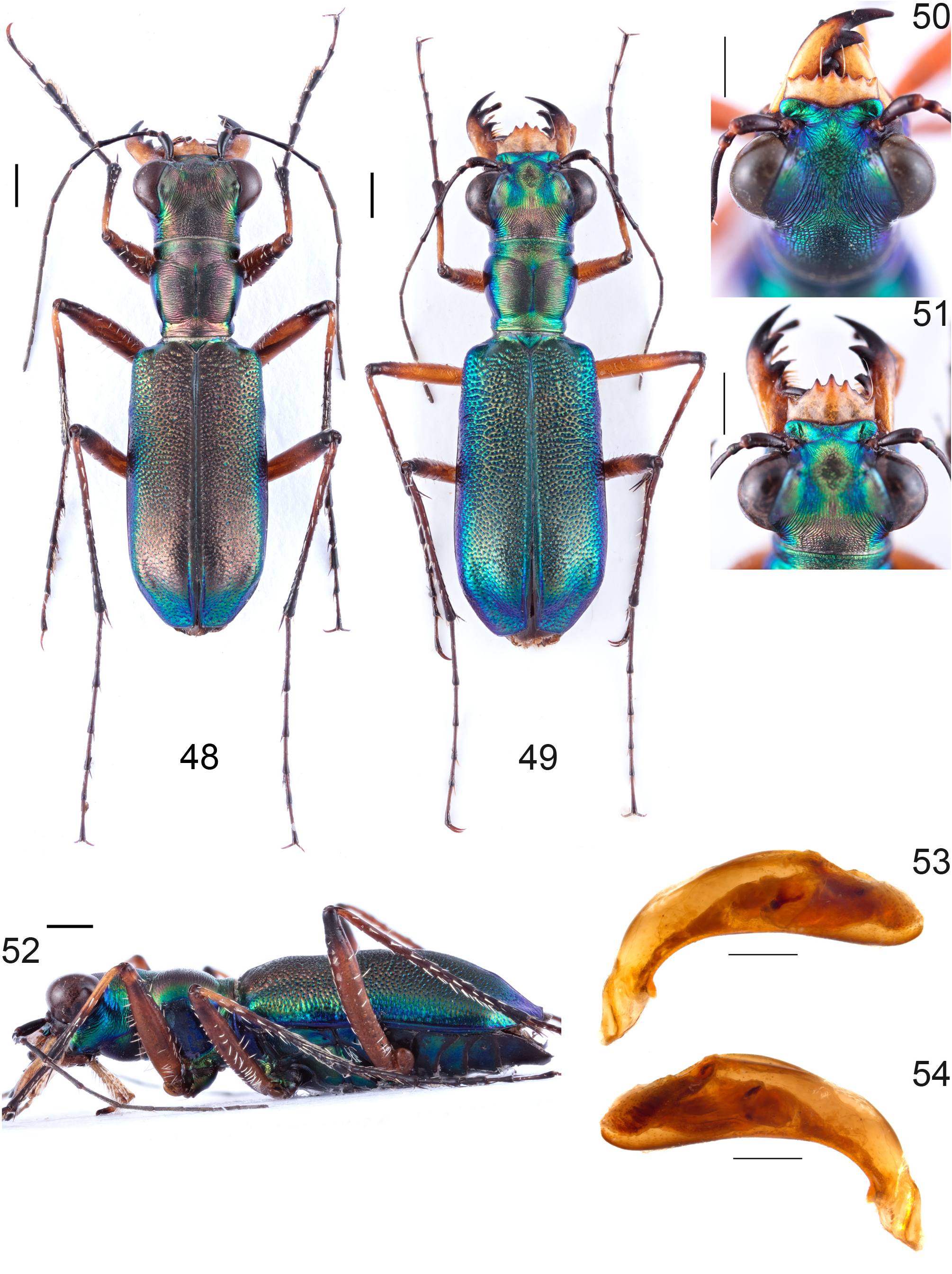
Figs. 1 View FIGURES 1–7 , 46 View FIGURES 44–47 , 48–54 View FIGURES 48–54
Cicindela (Heptadonta) melanopyga Schaum, 1862: 173 .
Heptodonta melanopyga View in CoL . Chaudoir, 1865: 20.
Heptadonta melanopyga . Fleutiaux, 1892: 128.
Odontochila (“group” Heptodonta) melanopyga View in CoL . W. Horn, 1910: 203.
Odontochila (Heptodonta) melanopyga View in CoL . W. Horn, 1926: 123.
Type locality. Philippines: Luzon .
Type material. Lectotype (designated here): ♂ in MNB, labelled: “42450” [printed] // “Hist.-Coll. (Coleop- tera) / Nr. 42450 / Cicindela melanopyga / Schaum* / Luzon, Coll. Schaum / Zool. Mus. Berlin ” [yellow with black frame, printed] // “melanopyga / Schaum.* / Luzon. (Semper)” [yellow with black frame, handwritten]. Paralectotype: 1 ♀ in MNB: “Hist.-Coll. ( Coleoptera ) / Nr. 42450 / Cicindela melanopyga / Schaum* / Luzon, Coll. Schaum / Zool. Mus. Berlin ” [yellow with black frame, printed]. All type specimens labelled: “ SYNTYPE / Cicindela / melanopyga / Schaum, 1862 / labelled by MFNB 2018” [red, printed] // “ LECTOTYPE (or PARALECTOTYPE respectively) / Cicindela / melanopyga / Schaum, 1862 / design. Sebastian Görn 2020” [red, printed] // “ Heptodonta / melanopyga / ( Schaum, 1862) / det. Sebastian Görn 2020” [printed].
Diagnosis. Distinguishable from H. analis , H. abasileia sp. nov., H. halensis sp. nov., and H. nigrosericea by lacking mediolateral and anteroapical elytral impressions. In contrast to continental species, with shiny lateroapical lustre, due to deeper apical impression, round aedeagal tip, short rather rectangular labrum in males, and triangular, usually less elongated labrum in females. Distinguishable from the similar H. mindoroensis , by slightly dilated, more robust elytra, irregular, more fused elytral punctation ( Fig. 1 View FIGURES 1–7 ), and rather orthogonal aedeagus. In contrast to H. wiesneri sp. nov., pronotum with continuously converging lateral margin and relatively coarse transverse striation, aedeagus more elongated.
Redescription. Body size: Length 10.3–13.5 mm (without labrum), width 3.2–4.1 mm ( Figs. 46 View FIGURES 44–47 , 48–49 View FIGURES 48–54 ).
Dorsal surface of head copper or bronze with metallic green areas on vertex and lateral occiput, or metallic bluish-green with iridescent yellowish-bronze areas on frons, vertex, and occiput, plus blue areas on vertex, temples blue. Frons transversely to irregularly grooved, crumpled wrinkles in transition to vertex. Vertex median with parallel longitudinal striation, that converging in transition to the orbital plates. Orbital plates extensively smooth, with fine, convex to parallel converging striae and two setae on each side. Occiput with irregular transverse grooves, anteromedian irregularly rugose. Genae glabrous and shallowly grooved, violet-blue posterior and yellowish-green anterior. Clypeus glabrous, iridescent bronze and/or green, often with two dull dark-blue frontal impressions. Labrum testaceous, with four setae, five apical teeth, and one lateral tooth on each side, three median teeth acute, third apical teeth often reduced, shape variable, male labrum rectangular and short (0.48–0.70 mm long, 1.28–1.73 wide mm, Fig. 50 View FIGURES 48–54 ); female labrum antero-triangular or three median teeth protruding (0.83–1.05 mm long, 1.40–1.63 mm wide, Fig. 51 View FIGURES 48–54 ). Mandibles testaceous, with black teeth. Labial and maxillary palpi testaceous, terminal palpomeres apically to entirely black, pre-terminal maxillary palpomere sometimes apically dark testaceous in females. Antennae slender, extending back over the middle of the elytra, metallic black, scape, pedicel, and antennomeres 3–4 dorsally rarely with a gentle violet lustre, pedicel testaceous at base, antennomeres 5–6 sometimes lightened, scape with a single apical seta, antennomeres 3–4 with few scattered setae, antennomeres 5–11 finely and evenly pubescent.
Thorax entirely glabrous. Pronotum most frequently copper or bronze with green lateral margin, but also green or bluish-green with bronze disc and blue lateral margin, strong median line usually dull blue with green margin, pronotal shape variable, usually slightly longer in small specimens and slightly wider in large specimens (2.10–2.63 mm long, 2.03–2.78 mm wide), pronounced transverse grooves generally irregular on disc, distinct anterior and posterior sulci, anterior lobe wider than posterior, lateral margins of the median lobe moderately to distinctly converging to the base. Episterna violet, pro- and mesepisternum sometimes and metepisternum frequently covered with iridescent bluish-green to yellowish-green lustre. Prosternum, posterior mesosternum, metasternum, and epimera iridescent bluish-green, yellowish-green, or yellowish-bronze.
Elytra elongate, length 6.1–8.4 mm, slightly dilated laterally to almost parallel, maximal width posterior, bronze with green lateral colouration or green with bronze lustre along the sutures and blue lateral colouration, lateral margin violet, apex green to violet, discal and juxtahumeral impression moderate or slightly pronounced, impression on posterior declivity moderate or lacking, apical impression deep, basodiscal convexity and posterior gibbosity moderate, punctures irregularly distributed and decreasing in size to apex ( Fig. 1 View FIGURES 1–7 ), punctures irregularly fused to grooves running more transverse anterior and more converging to the apex posterior.
Coxae dark testaceous with iridescent bluish-green to yellowish-bronze lustre, pro- and mesocoxae anteriorly and metacoxae laterally densely covered with long white setae, mesocoxae posteriorly with few white setae. Trochanters and femora testaceous. Generally, distal femoral metallic black colouration expanded dorsally on profemur, dorsally and posteriorly on mesofemur, and over all sides on metafemur, covering usually one-fifth to one-third of the femora. Tibiae and tarsi metallic black. Proximoapical tibiae testaceous or at least slightly lightened. Claws rufous-testaceous.
Abdominal sternites glabrous, apart from few long setae at posterior margins, some minute single setae on sternites 4–5 of females, and in males some minute setae on posterior margins of sternites 4–5 and on posterolateral margins of sternites 2–5, dark-brown with iridescent violet-blue to yellowish-green reflections; anal sternite posterior without reflections ( Fig. 52 View FIGURES 48–54 ).
Aedeagus angled, length 2.08–2.30 mm, ventral side straight or convex, apex ventrally convex and thus tip pointed rather dorsally, dorsoapically slightly ascending over approximately a third of the aedeagus, dorsomedian convexity moderately and aedeagal base steeply sloping. Inner sac in left lateral aspect median with spoon-shaped diagonal sclerite ( Figs. 53–54 View FIGURES 48–54 ).
Variability. Variable in pronotal shape and colouration.
Distribution. Northern and central PHILIPPINES (Batan, Luzon, Marindugue, Mindoro, Negros, Sibuyan).
Additional material examined. 136 specimens. PHILIPPINES: Batan: Basco , 3.IX.1983 ( ZSM) ; Luzon: Mt. Province , 27.V.1986 ( JWCW) ; Abra Province, 1978 ( JWCW) ; Isabella Province, VI.2012 ( JWCW) ; Imugan ( SNMF, MNB, SDEI) ; North Kayapa , Nueva Vizcaya, VIII.2016 ( PSCH) ; Ifugao, Banaue , VII.2016 ( PSCH, SGCH) ; Zambales, Subic , VII.2016 ( PSCH) ; Luisiana , VII.2016 ( PSCH) ; Mt. Province, VI.1990 ( PSCH) ; Nueva Vizcaya, near Bayombong , VI.2004 ( CKCE) ; Aurora, Sierra Madre , XI.2014 ( JWCW) ; Mt. Banahao , VI.1914 ( MNB) ; Limay , VII.1914 ( MNB) ; Bilucao , La Laguna ( SDEI) ; Mt. Makiling ( SDEI) ; Mt. Limay ( SDEI) ; Quezon Park , 1.VI.1932 ( SDEI) ; Tayabas, Malinao ( SDEI) ; Baguio, Benguet ( SDEI) ; Quezon Park, Tabayas , 16.VI.1931 ( SDEI) ; Marindugue: Marindugue , IX.1984 ( ZSM) ; Mindoro: Mindoro ( SNMF) ; Negros: Negros oriental, VII.1985 ( JWCW) ; Murcia , 28.V.–30.VI.1991 ( SMNS) ; Negros , V.1986 ( ZSM) ; Sibuyan: Mt. Guiting , IV.2015 ( JWCW) ; Espana ( ZSM) ; Sibuyan ( SDEI) .
Biology. According to Medina et al. (2020) “on well-exposed sandy ground near the irrigation dam.”
Remarks. Common in the northern part of the Philippines ( Schaum 1862; Chaudoir 1865; Fleutiaux 1892; Horn 1910, 1926; Wiesner 1980, 1992; Cassola 2000; Cabras et al. 2016; Medina 2020). The co-occurrence with H. mindoroensis on the island Mindoro is unclear; both specimens are old material determined by W. Horn. Also, the existence of H. melanopyga on Mindanao needs to be verified, since the only evidence for this occurrence is an entry in the list of Philippine tiger beetles by Wiesner (1980).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Heptodonta melanopyga ( Schaum, 1862 )
| Görn, Sebastian 2020 |
Odontochila (Heptodonta) melanopyga
| Horn, W. 1926: 123 |
Odontochila (“group” Heptodonta) melanopyga
| Horn, W. 1910: 203 |
Heptadonta melanopyga
| Fleutiaux, E. 1892: 128 |
Heptodonta melanopyga
| Chaudoir, M. 1865: 20 |
Cicindela (Heptadonta) melanopyga
| Schaum, H. 1862: 173 |